Abstract
Introduction
A schwannoma is a benign, slow growing, encapsulated nerve sheath tumour. Presentation of a schwannoma is a diagnostic and management challenge.
Methods
Internet searches of PubMed/MEDLINE® for all articles listing schwannomas of the vagus nerve in the cervical/neck region (1980–2012) were undertaken to ascertain diagnostic pitfalls. The references of all articles were cross-checked to include all pertinent contributions. Further articles were traced through reference lists.
Results
Schwannomas are solitary, well circumscribed and medial to the carotid sheath. Preoperative diagnoses of schwannomas in the lateral part of the neck can cause confusion with its nerve of origin (ie whether it arises from the vagus nerve or a sympathetic chain). Computed tomography and magnetic resonance imaging reveal valuable information regarding the location and origin of the tumour as well as aiding surgical planning. The diagnosis can be confirmed intraoperatively. Postoperative recovery of neurological function is dependent on the type of surgery. Histopathological studies searching for classical features and immunohistochemical staining for S100 also confirm the diagnosis.
Conclusions
Schwannomas should be considered in the differential diagnoses of unusual masses in the neck. Preoperative imaging elicits valuable information regarding the location and origin of schwannomas and histopathology confirms the diagnosis.
Keywords: Cervical, Schwannoma, Vagus nerve, Sympathetic chain, Computed tomography, Magnetic resonance imaging, Diagnosis
A schwannoma is a benign, slow growing, encapsulated nerve sheath tumour comprising Schwann cells in a collagenous matrix that can arise from any cranial, peripheral or autonomic nerve in the body(except the olfactory and optic nerves). About 25–45% of extracranial schwannomas have been reported to lie in the head and neck.1–3 Calcaterra et al reported that the head and neck is the site of origin in more than one-third of all solitary schwannomas and that they occur most often in the lateral part of neck.4 Most extracranial schwannomas are found in the parapharyngeal space and are usually of vagal origin.5–7 Presentation of a schwannoma is a diagnostic and management challenge.
In general, schwannomas are believed to be slow growing neoplasms. Zhang et al 8 and de Araujo et al 9 reported annual rates of tumour growth of 2.75mm and 3mm respectively. Malignant change in schwannomas of the head and neck is rare, with a prevalence of 8–13.9%.10–12
Methods
A systematic search of PubMed/MEDLINE® (1980–2012) was undertaken to determine the diagnostic procedure for schwannomas of the vagus nerve in the cervical region. The keywords employed were ‘neck neurilemmoma’, ‘vagus nerve’, ‘sympathetic chain’, ‘tomography’ and ‘histopathology’, and included all related terms.
The search was carried out independently by the three authors. Abstracts were reviewed and relevant articles identified. Further contributions were identified through the reference lists of relevant articles. After omission of duplicated articles, relevant contributions were retrieved and reviewed. The final results of each article were collated and are described below and in Figure 1.
Figure 1.
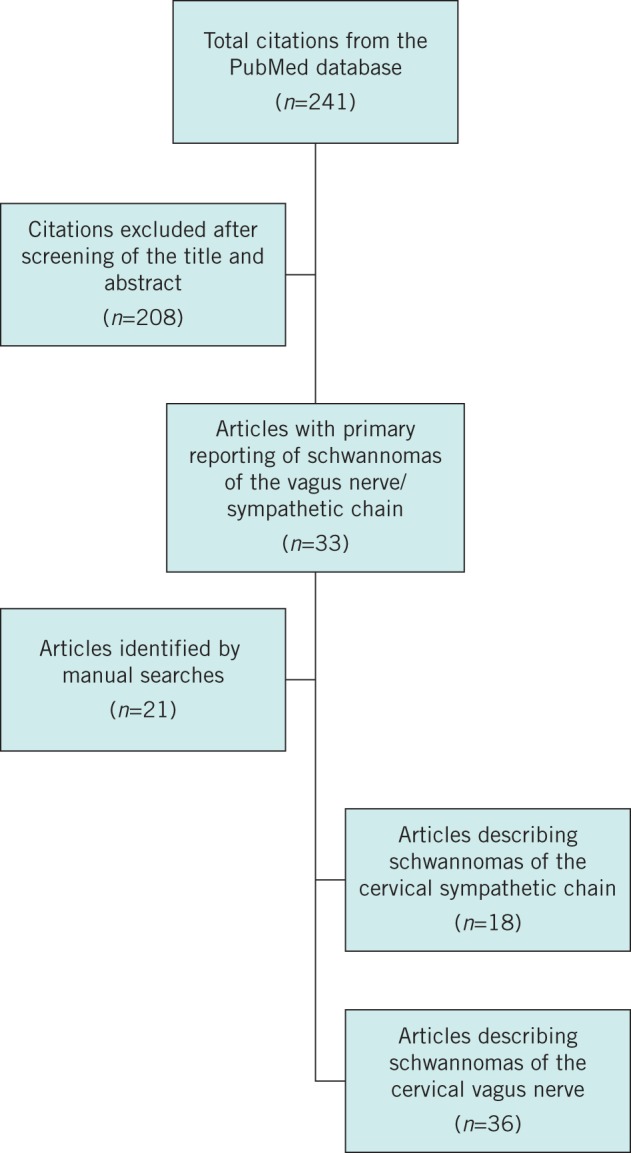
Flow diagram of study selection
Results
Schwannomas of the head and neck can affect any peripheral cranial or autonomic nerve. Signs and symptoms can be varied and are often non-specific. Presentation can also be heavily dependent on the nerve of origin as well as the location and extent of the tumour.
Usually, remarkable signs or symptoms are not seen in the early stages of the disease. A solitary, slow growing mass in the neck is the most common symptom (Fig 2). In later stages, signs and symptoms are related to the size of the schwannoma and the surrounding anatomical structures involved: dysphagia, nasal obstruction, painless swelling of the cheek, dyspnoea in the supine position.13 Presentation with a neural deficit is a controversial issue. Some authors have reported that most patients have neural deficits whereas other scholars have observed neural deficits only in large tumours or when neural compression is present (usually among intracranial or skull base tumours).9,12
Figure 2.
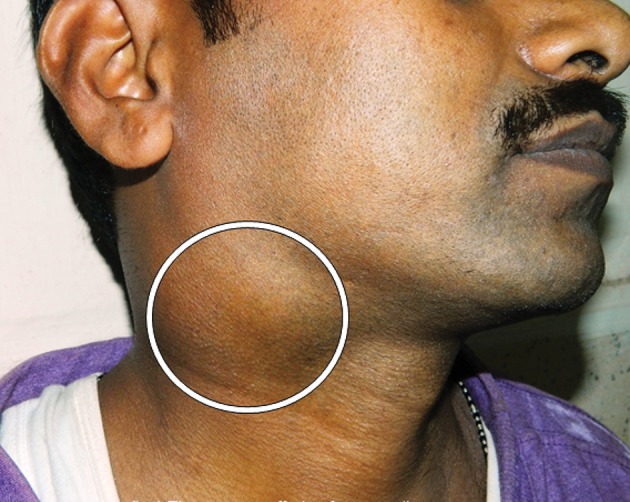
Neck swelling
Tumours arising from sensory nerves may present as pain; those arising from the vagus nerve may present as hoarseness and a globus sensation; those from the facial nerve may present as a facial palsy.14,15 Horner’s syndrome is rarely seen in a schwannoma of the cervical sympathetic chain.
With regard to a schwannoma of the vagus nerve, the most common symptom is hoarseness. Occasionally, a paroxysmal cough may be produced on palpation of the mass. This is unique to a schwannoma of the vagus nerve. This sign (which is associated with a mass located along the medial border of the sternocleidomastoid muscle) should make the clinician suspect a tumour of the sheath of the vagus nerve.16–20
Diagnosis
The diagnosis of a schwannoma is problematic because the medical history and clinical examination are usually non-specific. Fine needle aspiration (FNA), magnetic resonance imaging (MRI) and computed tomography (CT) have, to a certain degree, solved the problem of misdiagnoses.21–24 In addition to facilitating the diagnosis, preoperative imaging provides information on the size, location and extent of the tumour as well as the surrounding anatomy, thereby aiding surgical planning.
Microscopic
The usefulness of FNA is controversial. Most authors do not recommend open biopsy or FNA for these masses.20 Microscopically, schwannomas typically exhibit a biphasic histological pattern termed Antoni A and Antoni B.25,26
Antoni A areas are regions of high cellularity with spindle shaped cells. Cells are often arranged in bundles, palisades or whirls. Groups of compact parallel nuclei are also seen and are known as Verocay bodies. Antoni B areas have fewer cells with additional histopathological features of cystic degeneration or xanthomatous changes and do not exhibit a distinctive pattern (Figs 3 and 4). Schwannomas usually exhibit intense immunostaining for S100 (particularly Antoni A areas), which may help to distinguish neoplasms in the sheaths of peripheral nerves from other tumour types.27,28
Figure 3.
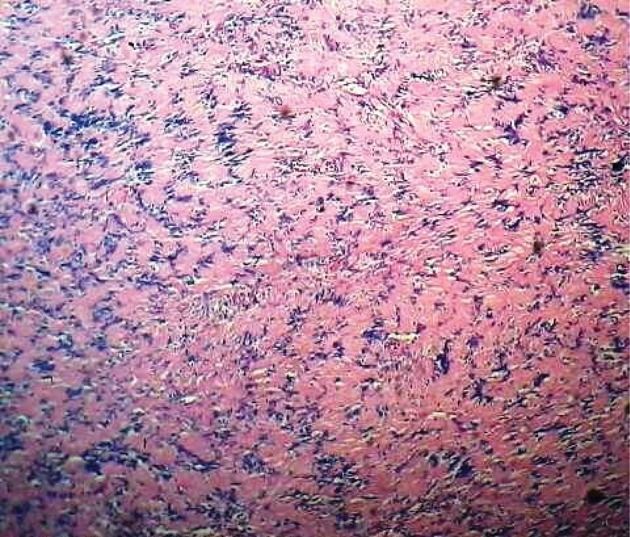
Histopathology of a schwannoma at low magnification
Figure 4.
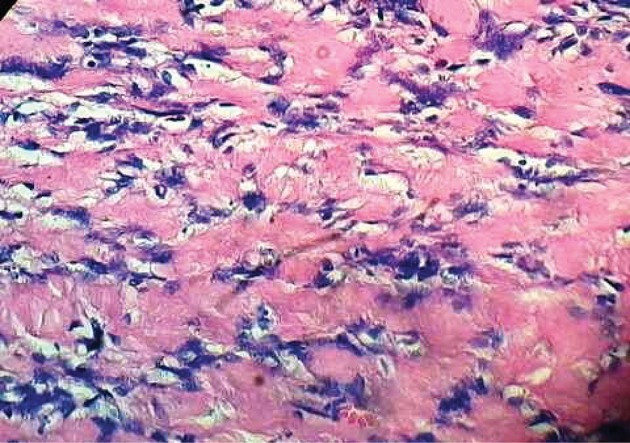
Histopathology of a schwannoma at high magnification
CT versus MRI
On CT, schwannomas appear to be well covered, well defined and fusiform.24,29 They show relatively homogenous enhancement of contrast, with internal cystic changes becoming more prominent as the tumour enlarges. Cystic changes are associated with mucinous degeneration, haemorrhage, necrosis and the formation of microcysts (Fig 5).
Figure 5.
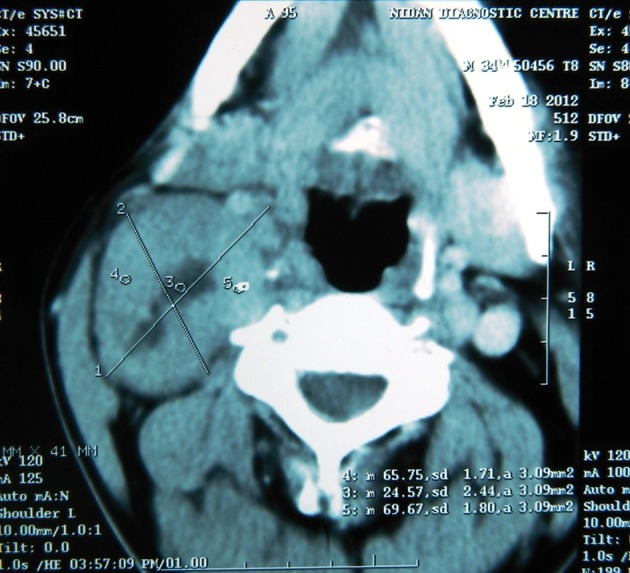
Computed tomography showing a mass displacing the carotid sheath
Kumar et al described two schwannomas that appeared as homogenous, low density masses on CT.30 They ascribed the low density nature of these schwannomas to their lipid content. In addition, Powers et al described a schwannoma that appeared on CT as a cystic mass but this finding was not explained.31
According to Cohen et al, pathological examination of schwannomas shows each tumour to have one or more characteristic histological features.32 First, there are confluent areas of hypocellularity adjacent to more cellular tumour regions. Second, schwannomas undergo cystic degeneration due to vascular thrombosis and subsequent necrosis.25,26 Third, areas of reduced cellularity are seen adjacent to areas of cellularity with dense bundles of collagen. Finally, schwannomas have xanthomatous regions (which are a common feature of these tumours macroscopically).25,26 On microscopic examination, these areas have been described as containing aggregates of lipid laden foam cells. These features (separately or in combination) seem to account for the inhomogeneous appearance of schwannomas on CT examination.
Macroscopically, in contrast to a schwannoma, a pure neurofibroma is a solid tumour. Areas of cystic degeneration, hypocellularity and xanthomatous material are uncommon features of neurofibromas.25,26 Calcifications and bony changes are seen more frequently with neurofibromas than with schwannomas.
It has been suggested that the CT finding of inhomogeneities reflects malignancy in neurogenic tumours, especially in patients with neurofibromatosis.33–35
MRI characteristics36 of schwannomas include specific signs (split fat, fascicular, target) and signal patterns (ie isointense T1 signal relative to skeletal muscle; increased and slightly heterogeneous T2 signals).
Ultrasonography
Ultrasonographic images of schwannomas are characterised by a round or elliptical cross-section with a clear border as well as an internal echo reflective of histology. Patterns may be homogenous-to-heterogeneous and cystic changes may be seen. Ultrasonography has greater diagnostic utility if the diameter of the nerve of origin is large and often the tumour is connected to a clearly delineated nerve.29,36
Nerve of origin
As reported by Furukawa et al, MRI findings are also useful for preoperative estimation of the nerve of origin of schwannomas of the vagus nerve and schwannomas of the cervical sympathetic chain.37 Schwannomas of the vagus nerve displace the internal jugular vein laterally and carotid artery medially; schwannomas from the cervical sympathetic chain displace the carotid artery and jugular vein without separating them.37–41 The cervical sympathetic trunk is posteromedial to the carotid vessels as it runs longitudinally over the longus capitis and longus colli muscles, deep to the prevertebral fascia.5,42 In strict anatomical terms, the sympathetic chain is outside the carotid sheath (which contains the carotid artery, internal jugular vein and vagus nerve) (Fig 6).
Figure 6.
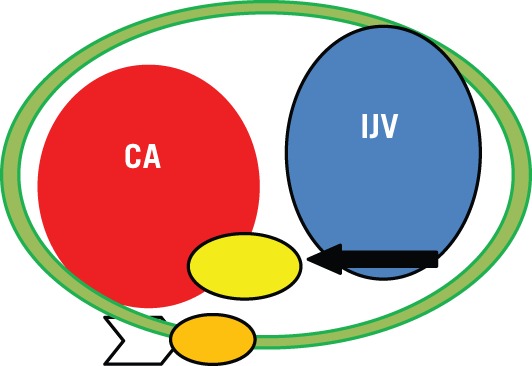
Relationship between the carotid artery (CA), internal jugular vein (IJV), cervical sympathetic chain (arrowhead) and cervical vagus nerve (arrow) in the carotid sheath
In 1996 Furukawa et al undertook imaging studies on nine patients with schwannomas and suggested their neurological origin prior to surgery: they were correct in all cases.37 In 2007 the accuracy of the preoperative diagnosis made by Saito et al in 12 patients with schwannomas was 83%.38 Preoperative diagnoses based on imaging studies offer better understanding of the anatomical correlations between the nerve and vascular structures than intraoperative diagnoses. Preoperatively, surgeons can explain to patients each complication relating to schwannomas of the vagus nerve that may follow the surgical procedure.
Differential diagnoses
Schwannomas of the vagus nerve can be confused with metastatic lymph nodes, paragangliomas and schwannomas of the cervical sympathetic chain. Metastatic lymph nodes are often multiple and the identity of the primary cancer is not known, thereby making thediagnosis relatively straightforward.7 However, a solitary metastatic node from an unknown primary cancer may be difficult to differentiate from a schwannoma in the absence of periadenitis or extranodal extension of disease. Paragangliomas show early arterial enhancement on CT; they are hypervascular lesions (whereas schwannomas are hypovascular) and have certain characteristic MRI appearances such as scattered flow voids (‘salt and pepper’) that usually enable the correct diagnosis.43 The pattern of splaying the carotid bifurcation is also distinct from that of schwannomas of the cervical sympathetic chain (Figs 7 and 8). Usually, separation of the internal jugular vein and internal carotid artery helps to distinguish between schwannomas of the vagus nerve and those of the cervical sympathetic chain.37,38
Figure 7.
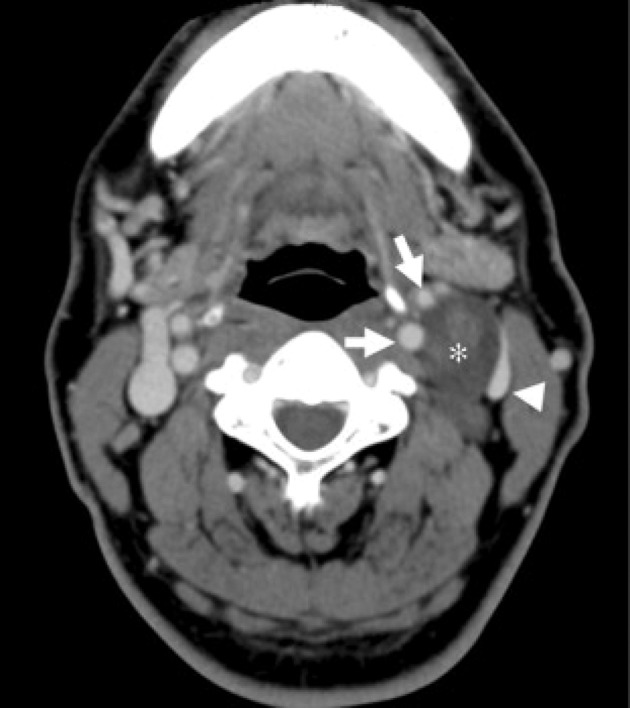
Computed tomography of the neck showing a schwannoma of the vagus nerve. The tumour (asterisk) is separating the common carotid artery (white arrow) anteriorly and internal jugular vein (arrowhead) posteriorly.
Figure 8.
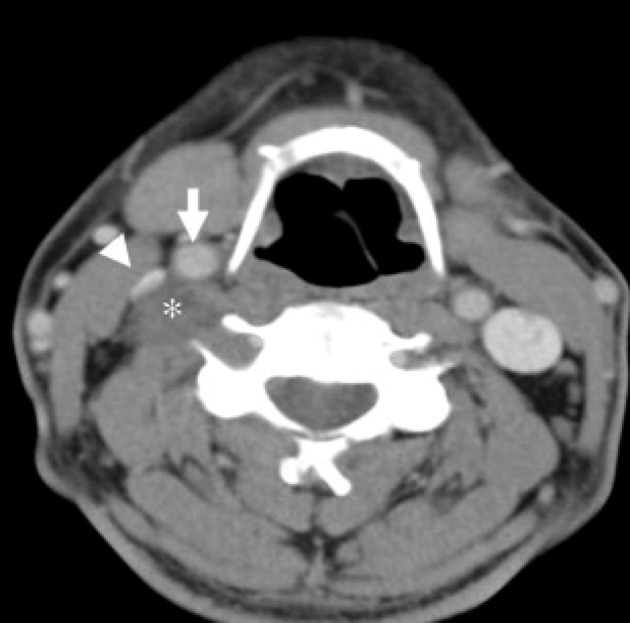
Computed tomography of the neck showing a schwannoma of the sympathetic chain. The tumour (asterisk) is displacing the common carotid artery (white arrow) and internal jugular vein (arrowhead) together anteriorly without separating them.
Role of surgery
Previously, to prevent recurrence of schwannomas, radical dissection involving the neuroprogenitor cells was carried out. Even if recovery is achieved after nerve transplantation or a primary anastomosis, preservation of neurological function is not expected. Most neuroprogenitor fibres do not run through schwannomas: they pass over the tumour capsule.44,45
Most schwannomas are encapsulated. If nerve fibres surround the tumour surface, intracapsular enucleation can be carried out while preserving nerve fibres by making a small longitudinal incision in the capsule (Figs 9 and 10). Valentino et al found that intracapsular enucleation while preserving nerve fibres ensured that nerve fibres retained their function by >30% compared with tumour resection with a primary anastomosis.46 Neurological functions can also be monitored by using a nerve stimulator or a microscope when carrying out intracapsular enucleation.46–48
Figure 9.
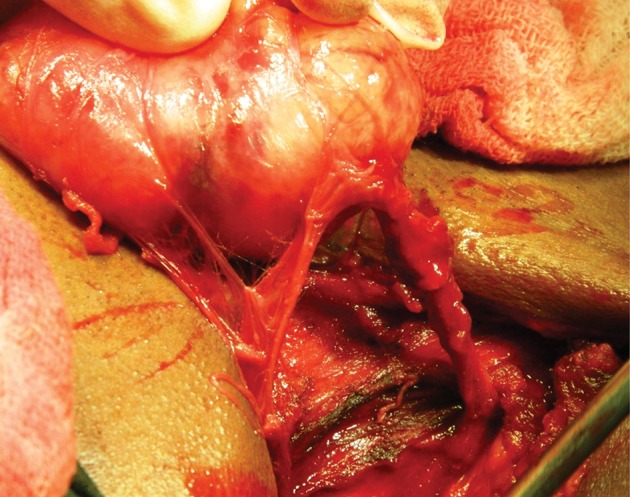
Intraoperative image showing a mass attached to the vagus nerve.
Figure 10.
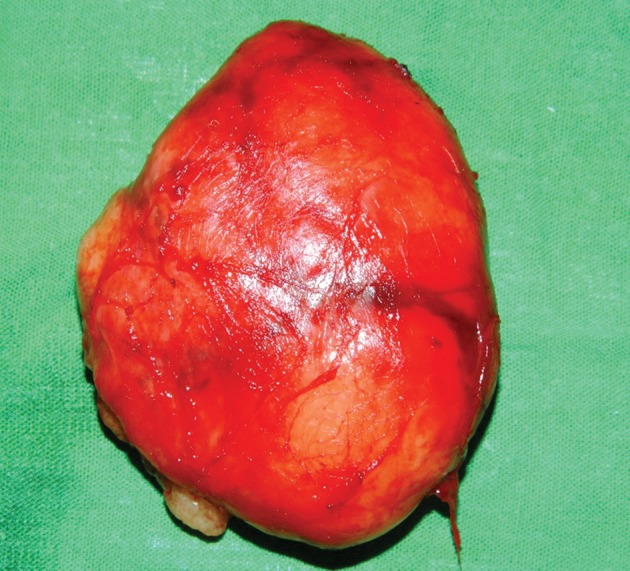
Macroscopic view showing intact mass separated from the attached nerve
There is considerable controversy regarding the prevalence of recurrence of schwannomas between total resection of the tumour including nerve fibres and intracapsular enucleation. Zbren et al found no significant difference in the prevalence of recurrence between these two procedures.49 However, if partial removal of the tumour is carried out, the prevalence of recurrence has been reported to increase.48
Pezzullo et al reported the prevalence of preoperative paralysis of the vocal cord to be 12% but hoarseness is almost always present after surgery of schwannomas originating from the cervical vagus nerve.20 Therefore, preoperative assessment of mobility of the vocal cords is strongly recommended. With respect to postoperative vocal cord palsy, aggressive voice therapy for vocal cord compensation should be started soon after surgery.
Conclusions
Neither the diagnosis nor the treatment of schwannomas of the cervical vagus nerve are straightforward. Only appropriate imaging and preoperative planning ensure successful treatment of schwannomas.
References
- 1. Batsakis JG. Tumors of the Peripheral Nervous System. 2nd edn. Baltimore: Williams & Wilkins; 1979. pp313–333. [Google Scholar]
- 2. Putney FJ, Moran JJ, Thomas GK. Neurogenic tumors of the head and neck. Laryngoscope 1964; 74: 1,037–1,059. [DOI] [PubMed] [Google Scholar]
- 3. Sharma DK, Sohal BS, Parmar TL et al. Schwannomas of head and neck and review of literature. Indian J Otolaryngol Head Neck Surg 2011; 64: 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calcaterra TC, Rich JR, Ward PW. Neurilemmoma of the sphenoid sinus. Arch Otolaryngol 1974; 100: 383–385. [DOI] [PubMed] [Google Scholar]
- 5. Anil G, Tan TY. Imaging characteristics of schwannoma of the cervical sympathetic chain: a review of 12 cases. Am J Neuroradiol 2010; 31: 1,408–1,412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malone JP, Lee WJ, Levin RJ. Clinical characteristics and treatment outcome for nonvestibular schwannomas of the head and neck. Am J Otolaryngol 2005; 26: 108–112. [DOI] [PubMed] [Google Scholar]
- 7. Som PM, Curtin HD. Parapharyngeal Space In: Som PM, Curtin HD. Head and Neck Imaging. Volume 2 3rd edn. Saint Louis: Mosby; 1996. pp915–951. [Google Scholar]
- 8. Zhang H, Cai C, Wang S et al. Extracranial head and neck schwannomas: a clinical analysis of 33 patients. Laryngoscope 2007; 117: 278–281. [DOI] [PubMed] [Google Scholar]
- 9. de Araujo CE, Ramos DM, Moyses RA et al. Neck nerve trunk schwannomas: clinical features and postoperative neurologic outcome. Laryngoscope 2008; 118: 1,579–1,582. [DOI] [PubMed] [Google Scholar]
- 10. Das Gupta TK, Brasfield RD. Solitary schwannoma. Ann Surg 1970; 171: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakayama H, Gobara R, Shimamoto F et al. Ancient schwannoma of oral floor and ventral portion of the tongue: a case report and review of the literature. Jpn J Clin Oncol 1996; 26: 185–188. [DOI] [PubMed] [Google Scholar]
- 12. Kanatas A, Mücke Thomas, Houghton D et al. Schwannomas of the head and neck. Oncol Rev 2009; 3: 107–111. [Google Scholar]
- 13. Zhang H, Cai C, Wang S et al. Extra cranial head and neck schwannomas: a clinical analysis of 33 patients. Laryngoscope 2007; 117: 278–281. [DOI] [PubMed] [Google Scholar]
- 14. Rosen FS, Pou AM, Quinn FB. Obstructive supraglottic schwannoma: a case report and review of the literature. Laryngoscope 2002; 112: 997–1,002. [DOI] [PubMed] [Google Scholar]
- 15. Sherman JD, Dagnew E, Pensak ML et al. Facial nerve neuromas: a report of 10 cases and review of the literature. Neurosurgery 2002; 50: 450–456. [DOI] [PubMed] [Google Scholar]
- 16. Chang SC, Schi YM. Neurilemmoma of the vagus nerve: a case report and brief literature review. Laryngoscope 1984; 94: 946–949. [DOI] [PubMed] [Google Scholar]
- 17. Ford LC, Cruz RM, Rumore GJ et al. Cervical cystic schwannoma of the vagus nerve: diagnostic and surgical challenge. J Otolaryngol 2003; 32: 61–63. [DOI] [PubMed] [Google Scholar]
- 18. Fujino K, Shinohara K, Aoki M et al. Intracapsularenucleation of vagus nerve-originated tumours for preservation of neural function. Otolaryngol Head Neck Surg 2000; 123: 334–336. [DOI] [PubMed] [Google Scholar]
- 19. Gilmer-Hill HS, Kline DG. Neurogenic tumours of the cervical vagus nerve: report of four cases and review of the literature. Neurosurgery 2000; 46: 1,498–1,503. [DOI] [PubMed] [Google Scholar]
- 20. Chiofalo MG, Longo F, Marone U et al. Cervical vagal schwannoma. A case report. Acta Otorhinolaryngol Ital 2009; 29: 33–35. [PMC free article] [PubMed] [Google Scholar]
- 21. Colreavy MP, Lacy PD, Hughes J et al. Head and neck schwannomas – a 10 year review. J Laryngol Otol 2000; 114: 119–124. [DOI] [PubMed] [Google Scholar]
- 22. Moukarbel RV, Sabri AN. Current management of head and neck schwannomas. Curr Opin Otolaryngol Head Neck Surg 2005; 13: 117–122. [DOI] [PubMed] [Google Scholar]
- 23. Al-Ghamdi S, Black MJ, Lafond G. Extracranial head and neck schwannomas. J Otolaryngol 1992; 21: 186–188. [PubMed] [Google Scholar]
- 24. Kang GC, Soo KC, Lim DT. Extracranial non-vestibular head and neck schwannomas: a ten-year experience. Ann Acad Med Singapore 2007; 36: 233–240. [PubMed] [Google Scholar]
- 25. Russell DS, Rubenstein LJ. Pathology of Tumors of the Nervous System. 4th edn. Baltimore: Williams & Wilkins; 1977. [Google Scholar]
- 26. Harkin JC, Reed RJ. Atlas of Tumor Pathology – Second Series, Fascicle 3: Tumors of the Peripheral Nervous System. Washington, DC: Armed Forces Institute of Pathology; 1969. [Google Scholar]
- 27. Hu J, Bao YY, Cheng KJ et al. Computed tomography and pathological findings of five nasal neurilemmomas. Head Neck Oncol. 2012; 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruan LX, Zhou SH, Wang SQ. Palatine tonsil schwannoma: correlation between clinicopathology and computed tomography features. J Int Med Res 2008; 36: 1,140–1,147. [DOI] [PubMed] [Google Scholar]
- 29. Yamazaki H, Kaneko A, Ota Y et al. Schwannoma of the mental nerve: usefulness of preoperative imaging: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 97: 122–126. [DOI] [PubMed] [Google Scholar]
- 30. Kumar AJ, Kuhajda FP, Martinez CR et al. Computed tomography of extracranial nerve sheath tumors with pathologic correlation. J Comput Assist Tomogr 1983; 7: 857–865. [DOI] [PubMed] [Google Scholar]
- 31. Powers SK, Norman D, Edwards MS. Computerized tomography of peripheral nerve lesions. J Neurosurg 1983; 59: 131–136. [DOI] [PubMed] [Google Scholar]
- 32. Cohen LM, Schwartz AM, Rockoff SD. Benign schwannomas: pathologic basis for CT inhomogeneities. Am J Roentgenol 1986; 147: 141–143. [DOI] [PubMed] [Google Scholar]
- 33. Coleman BG, Arger PH, Dalinka MK et al. CT of sarcomatous degeneration in neurofibromatosis. Am J Roentgenol 1983; 140: 383–387. [DOI] [PubMed] [Google Scholar]
- 34. Patel YD, Morehouse HT. Neurofibrosarcomas in neurofibromatosis: role of CT scanning and angiography. Clin Radiol 1982; 33: 555–560. [DOI] [PubMed] [Google Scholar]
- 35. Vieta JO, Pack GT. Malignant neurilemmomas of peripheral nerves. Am J Surg 1951; 82: 416–431. [DOI] [PubMed] [Google Scholar]
- 36. Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic–pathologic correlation. Radiographics 2004; 24: 1,477–1,481. [DOI] [PubMed] [Google Scholar]
- 37. Furukawa M, Furukawa MK, Katoh K et al. Differentiation between schwannoma of the vagus nerve and schwannoma of the cervical sympathetic chain by imaging diagnosis. Laryngoscope 1996; 106: 1,548–1,552. [DOI] [PubMed] [Google Scholar]
- 38. Saito DM, Glastonbury CM, El-Sayed I et al. Parapharyngeal space schwannoma. Preoperative imaging determination of the nerve of origin. Arch Otolaryngol Head Neck Surg 2007; 133: 662–667. [DOI] [PubMed] [Google Scholar]
- 39. Green JD, Olsen KD, De Santo LW et al. Neoplasm of the vagus nerve. Laryngoscope 1988; 98: 648–654. [DOI] [PubMed] [Google Scholar]
- 40. Leu YS, Chang KC. Extracranial head and neck schwannomas: areview of 8 years experience. Acta Otolarngol 2002; 122: 435–437. [DOI] [PubMed] [Google Scholar]
- 41. Park CS, Suh KW, Kim CK. Neurilemmomas of cervical vagus nerve. Head Neck 1991; 15: 439–441. [DOI] [PubMed] [Google Scholar]
- 42. Civelek E, Karasu A, Cansever T et al. Surgical anatomy of cervical sympathetic trunk during anterolateral approach to cervical spine. Eur Spine J 2008; 17: 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rao AB, Koeller KK, Adair CF. Paragangliomas of the head and neck: radiologic–pathologic correlation. Radiographics 1999; 19: 1,605–1,632. [DOI] [PubMed] [Google Scholar]
- 44. Chang SC, Schi YM. Neurilemmoma of the vagus nerve. A case report and brief literature review. Laryngoscope 1984; 94: 946–949. [DOI] [PubMed] [Google Scholar]
- 45. Saydam L, Kizilay A, Kalcioglu T et al. Ancient cervical vagal neurilemmoma: a case report. Am J Otolaryngol 2000; 21: 61–64. [DOI] [PubMed] [Google Scholar]
- 46. Valentino J, Boggess MA, Ellis JL et al. Expected neurologic outcomes for surgical treatment of cervical neurilemmomas. Laryngoscope 1998; 108: 1,009–1,013. [DOI] [PubMed] [Google Scholar]
- 47. Charles D, Yingling CD. Intraoperative Monitoring of Cranial Nerves in Neurotologic Surgery In: Cummings CW, Fredrickson JM, Harker LA et al. Otolaryngology: Head and Neck Surgery. Saint Louis: Mosby; 1996. pp3,331–3,355. [Google Scholar]
- 48. Si HK, Na HK, Kyung RK et al. Schwannoma in head and neck: preoperative imaging study and intracapsularenucleation for functional nerve preservation. Yonsei Med J 2010; 51: 938–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zbren P, Markwalder R. Schwannoma of the true vocal cords. Otolaryngol Head Neck Surg 1999; 121: 837–839. [DOI] [PubMed] [Google Scholar]


