Abstract
Disrupted protein translation is prevalent in tumours. Eukaryotic translation initiation factors (eIFs) were found to play an important role in various tumours. However, the involvement of eIFs in glioma remains to be elucidated. The present study explored the expression and the role of eIF 3, subunit C (eIF3c) in human glioma. The expression of eIF3c in glioma tissues was evaluated by immunohistochemistry. The impact of eIF3c inhibition on U-87 MG was explored in vitro and in vivo by lentivirus-mediated siRNA targeting eIF3c. The results revealed that overexpression of eIF3c was present in glioma tissues. Knockdown of eIF3c significantly impaired cell proliferation and colony formation, further induced cell cycle arrest and apoptosis in the U-87 MG cell line. Furthermore, tumoursphere formation in the U-87 MG glioma xenograft model was blocked by eIF3c knockdown. The involvement of eIF3c in the tumorigenesis of glioma was confirmed, suggesting eIF3c may be a promising therapy target in human glioma.
Keywords: protein translation, eukaryotic translation initiation factor 3, subunit 3, glioma, cell proliferation, apoptosis
Introduction
Glioma is one of the most common and lethal cancers due to the high incidence, mortality rate and disability rate associated with the disease, which accounts for 32–45% of all primary brain tumours (1,2) and 70–80% of all malignant brain tumours (2–4). Although microsurgery, chemotherapy and radiotherapy have been developed as treatments for glioma, the majority of patients succumb to the disease less than two years subsequent to diagnosis (5). Therefore, there is an urgent requirement for novel efficient glioma therapies. To achieve this aim, it is necessary to explore the molecular abnormalities underlying glioma formation and malignant progression. In addition, reversing such aberrant changes to evaluate their influence on glioma development in vitro and in vivo may provide notable insights into the functions of these glioma-associated molecules, paving the way for their potential clinical applications.
Protein synthesis is a major step in gene expression and translational regulation is critical for homeostasis and normal cell physiology (6). It is not unexpected that aberrant protein synthesis markedly contributes to tumorigenesis and malignant progression, as abnormalities in protein synthesis lead to the interrupted translation of either general or specific mRNAs, which are essential for cell survival, migration or angiogenesis (7). Numerous studies have described the abnormalities of translational processes underlying various types of cancers, and these results indicated the involvement of translation factors in tumorigenesis (6,8–10). Out of these processes, translation initiation is the rate-limiting step of protein translation, and close associations have been identified between multiple cancer types and eukaryotic translation initiation factors (eIFs), which are critical components in translation initiation (8). Therefore, probing the changes and functions of eIFs in glioma progression is necessary, and may enable the development of novel therapies for the treatment of glioma. Among eIFs, the eIF3 complex is particularly important as it serves as a scaffold to mediate translation initiation and start codon recognition. Additionally, eukaryotic translation initiation factor 3, subunit C (eIF3c), a component of the eIF3 complex, is essential for the assembly of the eIF3 complex and the translational initiation complex (11). Although the association between eIF3c and tumorigenesis has been investigated in several studies (11–14), the expression pattern and function of eIF3c in glioma has yet to be elucidated.
The present study aimed to clarify the association between eIF3c and glioma. The expression profiles of eIF3c in human glioma tissues were analysed, and the expression of eIF3c was then manipulated using RNA interference (RNAi) in glioma U-87 MG cells to investigate the function of eIF3c in tumour proliferation and apoptosis. A glioma xenograft model was also established in nude mice and the impact of eIF3c inhibition on tumorigenesis was investigated, suggesting that eIF3c is a potential target for glioma treatment.
Materials and methods
Preparation of clinical specimens and immunohistochemical (IHC) analysis
Written informed consent was obtained from all patients involved in the present study or from the patient's family, and the study was approved by the Ethics Committee of The Second Hospital of Hebei Medical University (Shijiazhuang, Hebei, China). A total of 83 formalin-fixed paraffin-embedded (FFPE) glioma samples were obtained from patients who underwent surgery at The Second Hospital of Hebei Medical University from January 2008 to December 2013 with a protocol approved by the same institution, and the detailed information is summarised in Table II. In total, 25 FFPE normal brain tissues from traumatic brain injury patients were collected and used as control samples. To probe the eIF3c protein expression pattern, eIF3c IHC staining was performed in glioma tissues and normal brain tissues as previously described (15). eIF3c immunoreactivity was then determined independently by two pathologists, and the eIF3c staining was scored as follows: absent, <5% eIF3c-positive nuclei; and present, 6–100% eIF3c-positive nuclei.
Table II.
Assessment of the association between eukaryotic translation initiation factor 3, subunit C expression and clinical pathological parameters by immunohistochemical staining.
| Clinical pathological parameters | n | eIF3c expression | Positive percentage, % | χ2 | P-value | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Age, years | ||||||
| <50 | 49 | 8 | 41 | 83.67 | 0.0250 | 0.8745 |
| ≥50 | 34 | 6 | 28 | 82.35 | ||
| Gender | ||||||
| Male | 44 | 7 | 37 | 84.09 | 0.0613 | 0.8044 |
| Female | 39 | 7 | 32 | 82.05 | ||
| Tumour diameter, cm | ||||||
| ≥3 | 50 | 9 | 41 | 82.00 | 0.1150 | 0.7332 |
| <3 | 33 | 5 | 28 | 84.85 | ||
| Glioma grades | ||||||
| I+II | 27 | 9 | 18 | 66.67 | 6.0950 | 0.0136 |
| III+IV | 56 | 5 | 51 | 91.07 | ||
Preparation of eIF3c small interfering RNA (si)RNA lentivirus
A lentiviral system and siRNA were employed to manipulate eIF3c expression, as previously described (16). Briefly, candidate siRNA specifically targeting human eIF3c was designed for the target sequence GACCATCCGTAATGC CATGAA using online tools, and the target sequence for the negative control siRNA was TTCTCCGAACGTGTCACGT. Stem-loop DNA oligonucleotides were then synthesised and inserted into the lentiviral pGCSIL-GFP vector (GeneChem Co., Ltd., Shanghai, China). eIF3c siRNA-expressing lentivirus was prepared using Lentivector Expression Systems (GeneChem Co., Ltd., Shanghai, China), and the U-87 MG cell line was infected with associated lentivirus to determine the knockdown efficiency using western blot analysis and reverse transcription polymerase chain reaction (PCR).
Cell proliferation assay using 5-bromo-2-deoxyuridine (BrdU)
U-87 MG cells infected with eIF3c or scrambled siRNA-expressing lentivirus were cultured for 48 h. These cells were then resuspended and seeded at the proper density into 96-well plates and cultured in a 5% CO2 incubator at 37°C for another one or four days. During the final 2–24 h, the BrdU reagent was diluted at a ratio of 1:100 and then added to 96-well plates (10 µl/well). Subsequent to 2–24 h of incubation, BrdU signals and cell proliferation were analysed using a BrdU cell proliferation ELISA (catalogue number, 11647229001; Roche Applied Science, Penzberg, Germany), according to the manufacturer's instructions. Briefly, the cells were fixed using 200 µl FixDenat for each well for 30 min, and the cells were then blocked with 5–10% bovine serum albumin for an additional 30 min at room temperature. Anti-BrdU monoclonal antibody from mouse-mouse hybrid cells (clone BMG 6H8,Fab fragment) conjugated with peroxidase; this antibody was a component of BrdU cell proliferation ELISA kit (catalogue no. 11647229001; Roche Applied Sciences, Penzberg, Germany) was diluted, added and incubated for 90 min at room temperature. Subsequent to washing three times using washing buffer, substrate solution was added and the cells were incubated for 5–30 min in the dark. Colour was developed with 10% H2SO4 for 30 min, and the BrdU density was determined at 450 nm.
Cell cycle analysis
Cell cycle analysis was performed according to previously described methods (17). Briefly, U-87 MG cells were infected with eIF3c or scrambled siRNA-expressing lentivirus and incubated for 96 h. Then, the cells were resuspended and seeded in 6 cm dishes until ∼80% coverage was achieved. The cells were then harvested and fixed with ice-cold 70% ethanol for at least 1 h. Subsequent to washing with phosphate-buffered saline (PBS) buffer, the cells were treated with staining solution that was prepared using 2 mg/ml 40X propidium iodide stock solution, 10 mg/ml 100X RNase stock solution and 1X PBS buffer at a dilution of 25:10:1,000. The cell cycle profiles were then analysed with FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA). In total, ≥1×106 cells per sample were prepared for cell cycle analysis, and the experiments were performed in triplicate.
Apoptosis assay
Cell apoptosis was analysed using Annexin V-allophycocyanin (APC) staining. Briefly, U-87 MG cells were infected with eIF3c or scrambled siRNA-expressing lentivirus and incubated for 96 h. These cells were collected and washed with PBS buffer. The cells were resuspended at a final density of 1×106-1×107/ml in staining buffer and 100 µl cell suspension was mixed with 5 µl Annexin V-APC and incubated at room temperature in the dark for 10–15 min. Cell apoptosis was analysed using FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA).
Colony formation assay
As previously described (18), the U-87 MG cells transfected with eIF3c or scrambled siRNA-expressing lentivirus were cultured for 48 h and were collected in the logarithmic phase of growth. Subsequent to the counting of the cells using a haemocytometer, the cells were seeded in triplicate into six-well plates at a density of 800 cells/well and cultured for 14 days at 37°C in an incubator with a 5% CO2 atmosphere. The cells were then fixed with paraformaldehyde for 30–60 min and stained with Giemsa stain for 20 min. Subsequent to washing with double-distilled H2O several times, images of the cell plates were obtained using Micropublisher 3.3RTV (Olympus, Tokyo, Japan) and the cell colonies were counted.
Cell proliferation assay using cell counting methods
Briefly, U-87 MG cells infected with eIF3c or scrambled siRNA-expressing lentivirus were cultured for 48 h and were collected in the logarithmic phase of growth. The cells were then resuspended and seeded in triplicate in 96-well plates, at a density of 2,000 cells/well, and then cultured at 37°C in an incubator with a 5% CO2 atmosphere. After 24 h, images of the cell plates were captured and the images were quantified using Cellomics ArrayScan VTI (Thermo Fisher Scientific, Waltham, MA, USA) once a day for five days, and then a cell growth curve was generated based on data obtained using the Cellomics ArrayScan VTI.
RNA extraction and reverse transcription PCR
Total RNA from cultured tumour cells was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. In total, 2 µg RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega, Madison, WI, USA) and Oligo(dT) primers (Sangon Biotech Co., Ltd., Shanghai, China) to produce cDNA. To quantify the expression of eIF3c in the U-87 MG cells infected with eIF3c siRNA-expressing lentivirus and reverse transcription PCR was performed using the TP800 Real-Time PCR system (Takara Bio, Inc., Otsu, Japan), with GAPDH acting as an internal control. The primers used for reverse transcription PCR were as follows: GAPDH forward, 5′-TGACTTCAACAG CGACACCCA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCC AAA-3′; and eIF3c forward, 5′-AGATGAGGATGAGGATGA GGAC-3′ and reverse, 5′-GGAATCGGAAGATGTGGA ACC-3′. The expression of eIF3c was normalised to GAPDH expression, and data analysis was conducted using the comparative CT method.
Western blot analysis
To determine the knockdown efficiency of the eIF3c siRNA-expressing lentivirus in U-87 MG cells, western blot analysis was performed to analyse the protein levels of eIF3c. Briefly, the U-87 MG cells transfected with eIF3c or scrambled siRNA-expressing lentivirus were cultured for 96 h. The cells were then harvested, lysed with modified RIPA buffer, boiled in 1X SDS loading buffer and resolved by 10% SDS-PAGE. The gel was transferred to polyvinylidene fluoride membranes (Amersham, Uppsala, Sweden), and the membranes were blocked with 5% milk in Tris-buffered saline with Tween-20 (TBST) for 1 h. The membranes were then incubated overnight at 4°C with primary rabbit polyclonal anti-eIF3c antibody (catalog no. ab170841,Abcam, Cambridge, MA, USA) and Synthetic peptide within human eIF3s8 aa 126–155 (N-terminal) conjugated to keyhole limpet haemocyanin (Immunogen, Waltham, MA, USA) at a 1:50 dilution, washed three times in TBST, and the signals from a horse radish peroxidase reaction were detected using the SuperSignal chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, IL, USA).
Tumorigenicity analysis in nude mice
The protocols that required nude mice were approved by the Institutional Animal Care and Use Committee of The Second Hospital of Hebei Medical University (Shijiazhuang, Hebei, China) and the animals were fed according to institution guidelines. A glioma xenograft model in nude mice was established as previously described, with certain modifications (19), as the untreated U-87 MG cells and the U-87 MG cells transfected with eIF3c or scrambled siRNA-expressing lentivirus were cultured for 48 h. The cells were then collected and resuspended with PBS buffer at a density of 1–2×107 cells/ml, and the nude mice were subcutaneously injected with ∼2×105 cells. The mice were allocated to three groups, as follows: Negative control group injected with untreated U-87 MG cells; control group injected with scrambled siRNA-treated U-87 MG cells; and knockdown group injected with eIF3 siRNA-treated U-87 MG cells. The nude mice were fed and the diameter and weight of the tumours were measured every other day from day 10 for 18 days.
Statistical analysis
The present data were analysed with GraphPad Prism 6 software, and all experiments were repeated at least three times. Differences between the groups were analysed for statistical significance using Student's two-tailed t-test. P<0.05 was considered to indicate a statistically significant difference. The data reported in Tables I and II was analysed using Cochran-Mantel-Haenszel statistics and SAS software (SAS Institute, Inc., Cary, NC, USA).
Table I.
Assessment of the expression of eukaryotic translation initiation factor 3, subunit C in human glioma and normal brain samples by immunohistochemical staining.
| Group | n | eIF3c expression | Positive percentage, % | χ2 | P-value | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Glioma tissues | 83 | 14 | 69 | 83.13 | 55.0385 | <0.0001 |
| Normal brain tissue | 27 | 26 | 1 | 3.70 | ||
Results
High eIF3c expression is correlated with malignant phenotypes in human glioma
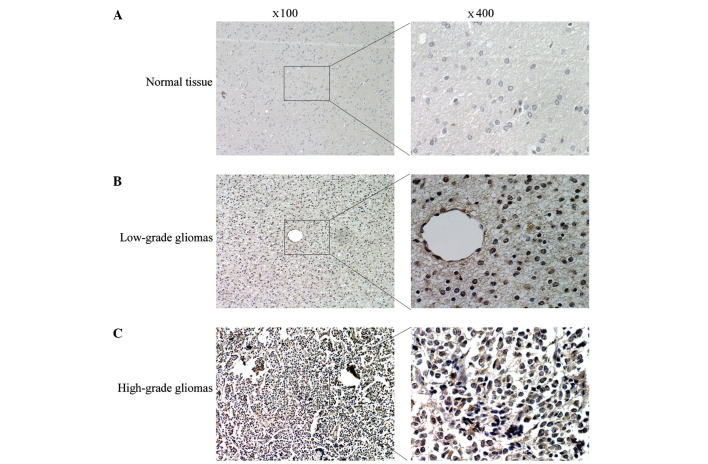
Aberrant eIF3c expression has been observed in several human cancer types. In the present study, the expression pattern of eIF3c was evaluated in 83 FFPE glioma tissues and 27 normal brain tissues using IHC staining. The IHC results revealed that eIF3c expression was significantly increased in glioma tissues compared with normal brain samples, with positive staining of eIF3c in 69 out of 83 (83.13%) glioma tissues and in 1 out of 27 (3.70%) normal brain tissues (Table I). Furthermore, the association between eIF3c expression and clinicopathological parameters were analysed in the present study. No significant association was observed between eIF3c expression and the patient age or gender, or the tumour volume (Table II). However, a strong correlation has been observed between eIF3c expression and glioma grades, with signals for eIF3c expression being detected in 91.07% of high-grade glioma samples (grades III and IV; n=56) and in only 66.67% of low-grade glioma tissue samples (n=27) (Table II). Similarly, Fig. 1 demonstrates that eIF3c expression was increased in high-grade glioma compared with low-grade glioma and was barely detected in normal tissues. These results suggested that increased eIF3c expression is associated with a high grade in glioma.
Figure 1.
High eukaryotic translation initiation factor 3 subunit 3 expression in human malignant glioma. Representative images of immunohistological staining for (A) normal tissue, (B) low-grade glioma and (C) high-grade glioma are shown. The images of low- and high-grade glioma tissue were representative results from one World Health Organization (WHO) grade II glioma specimen and one WHO grade IV glioma specimens, respectively. The original magnifications were ×100 and ×400 for the images on the left and those on the right, respectively..
Lentiviral-mediated siRNA inhibits eIF3c expression efficiently in human glioma cell lines
The significantly increased expression of eIF3c in human malignant glioma tissues compared with normal brain tissues prompted the exploration of its biological roles in glioma in vitro and in vivo. An RNA interference (RNAi) strategy based on lentivirus-mediated siRNA was used to deplete eIF3c expression. To determine the knockdown efficiency, the malignant human glioma cell line U-87 MG was infected with eIF3c or scrambled siRNA-expressing lentivirus. The eIF3c mRNA and protein levels were determined using quantitative fluorescent PCR and western blot analysis, respectively. Treatment with eIF3c siRNA led to a ∼95% reduction in the expression of eIF3c mRNA and evident depletion of the expression of eIF3c protein (Fig. 2A and B). Therefore, the present results demonstrated that a highly efficient knockdown of eIF3c expression at the mRNA and protein levels was achieved using the lentivirus-mediated RNAi strategy.
Figure 2.
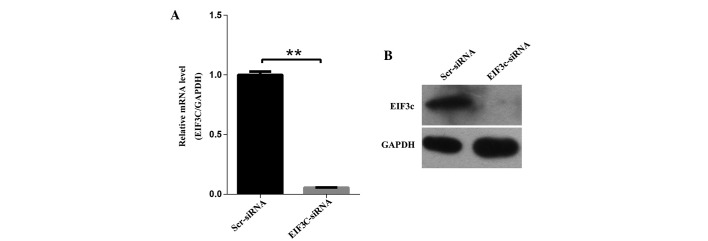
Expression of eIF3c in human malignant glioma cell lines and eIF3c knock-down in human malignant glioma cell line U-87 MG by lentivirus-based siRNA strategy. (A) Efficient knock-down of eIF3c at the mRNA level was achieved by lentivirus expressing eIF3c siRNA in the human malignant glioma cell line U-87 MG and normalised to GAPDH mRNA. Data shown are the mean ± SD of three independent reverse transcript quantitive PCR experiments (**P<0.01). (B) Western blot analysis showed efficient knock-down of eIF3c at the protein level achieved by lentivirus expressing eIF3c siRNA in the human malignant glioma cell line U-87 MG, and GAPDH was used as an internal reference. eIF3c, eukaryotic translation initiation factor 3 subunit 3
eIF3c knockdown inhibits the proliferation of U-87 MG cells
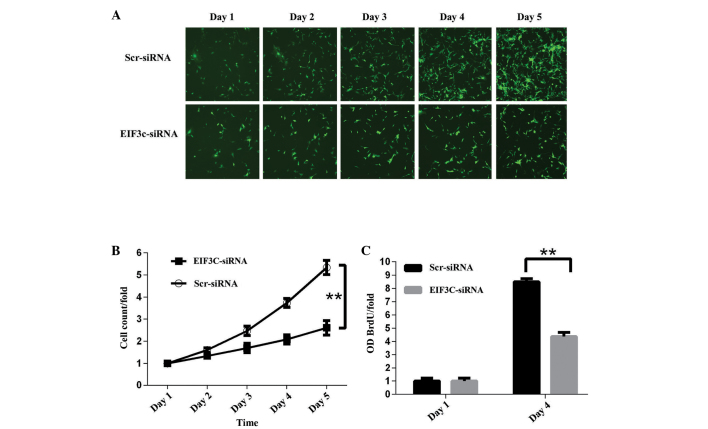
Tumour cells are characterised by uncontrolled division and proliferation. To evaluate the role of eIF3c in the proliferation of U-87 MG cells, the malignant human glioma cell line U-87 MG was infected with eIF3c or scrambled siRNA-expressing lentivirus. Cell numbers were monitored using Cellomics ArrayScan VTI every day for five days, and significant proliferation inhibition was observed in U-87 MG cells after 24 h of infection. Furthermore, the inhibitory effect of eIF3c knockdown on cell proliferation had increased four days subsequent to transfection, indicating a positive association between proliferation suppression and the duration of cell culture (Fig. 3A and B). To further confirm the suppressive effect of eIF3c RNAi on the proliferation of U-87 MG cells, a BrdU incorporation assay was performed to assess cell proliferation states in various treatment conditions. In this assay, cell proliferation was evaluated by DNA synthesis, which is quantified by the BrdU incorporation ratio. Initially, the BrdU incorporation ratio was determined 24 h after the cells were plated and normalised to one. Consistent with the result of cell counting, BrdU incorporation after four days of cell plating was significantly suppressed in U-87 MG cells by eIF3c knockdown compared with controls, with average incorporation changes of 8.5-fold and 4.4-fold, respectively (Fig. 3C). These data suggested that eIF3c knockdown markedly suppressed the proliferation of the malignant human glioma U-87 MG cell line.
Figure 3.
Knock-down of eIF3c expression inhibits proliferation of human malignant glioma cell line U-87 MG. (A) Representative images of U-87 MG cells infected with lentivirus expressing scrambled siRNA or eIF3c siRNA at various time points subsequent to lentivirus transfection. (B) Proliferation of U-87 MG cells was monitored by Cellomics ArrayScan VTI. Proliferation data are shown as fold-changes of cell numbers. The results are expressed as the mean ± standard deviation of six separate experiments (**P<0.01). (C) Proliferation of U-87 MG cells treated with eIF3c siRNA analysed by BrdU incorporation assay. The BrdU incorporation ratio is represented as fold-changes of absorbance at 450 nm (ODBrdU/fold). The results represent the mean ± SD of four separate experiments (**P<0.01). eIF3c, eukaryotic translation initiation factor 3 subunit 3.
eIF3c knockdown impairs the ability of U-87 MG cells to form colonies
In addition to uncontrolled cell proliferation, increased clonogenic capacity is a characteristic of tumour cells that underlines malignant tumour progression. Therefore, a colony formation assay was employed to determine the impact of eIF3c knockdown on the colony formation ability of the malignant human glioma U-87 MG cell line. The results revealed that a reduction in the expression of eIF3c in U-87 MG cells impaired colony formation ability, leading to a ∼34.8% reduction in colony number formation, with an average of 23 clones in cells treated with scrambled lentiviral siRNA, and only 15 clones in cells treated with eIF3c lentiviral siRNA.
eIF3c knockdown induces cell cycle arrest
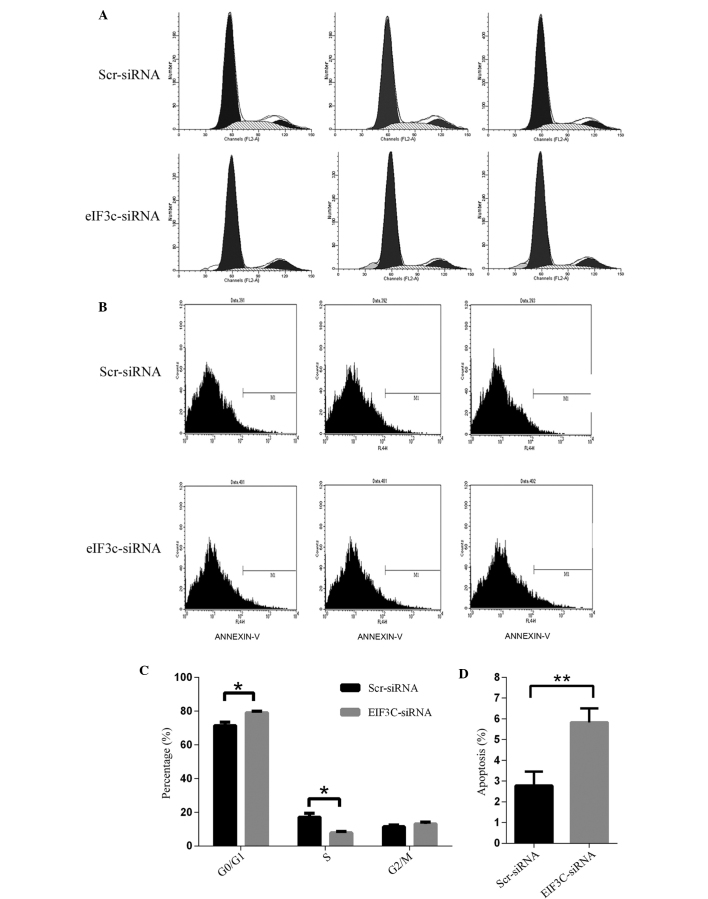
To explore the underlying mechanisms of the inhibitory effect of eIF3c knockdown on U-87 MG cell growth, the impact of eIF3c knockdown on cell cycle distribution of U-87 MG cells was analysed using fluorescence-activated cell sorting. It was revealed that eIF3c knockdown induced a significant G0/G1 phase arrest in the U-87 MG cells, with a corresponding reduction in the percentage of S-phase cells. The proportion of cells in the G0/G1 phase increased between 71.42 and 79.05% in the eIF3c siRNA treatment group, while the proportion in the S phase decreased between 17.09 and 7.83% (Fig. 5A and B). Therefore, the present results indicated that eIF3c knockdown led to inhibition of the G1-S transition.
Figure 5.
Downregulation of eIF3c results in cell cycle arrest and induces cell apoptosis in human malignant glioma cell line U-87 MG. The Scr-siRNA group consists of U-87 MG cells infected with lentivirus expressing scrambled siRNA. EIF3C-siRNA represents U-87 MG cells infected with lentivirus expressing eIF3c siRNA. (A) Cell cycle distribution of U-87 MG cells infected with lentivirus expressing scrambled siRNA (Scr-siRNA) or eIF3c siRNA (EIF3C-siRNA). (B) Cell apoptosis by Annexin-V staining was analysed with FACS. (C) Cell cycle distribution in scr-siRNA or eIF3c-siRNA-treated cells. The graph expresses the mean ± standard deviation of cell proportion in the G0/G1 phase, S phase and G2/M phase from three separate experiments (*P=0.011 in G0/G1 phase and P=0.014 in S phase). (D) Cell apoptosis in cells treated with eIF3c siRNA. The graph expresses the mean ± standard deviation of cell percentage in apoptosis from three separate experiments (**P<0.01). eIF3c, eukaryotic translation initiation factor 3 subunit 3.
eIF3c knockdown promotes apoptosis in U-87 MG cells
In addition to unlimited proliferation and elevated clonogenic capacity, evading apoptosis is also essential for tumour initiation and development (20,21). As eIF3c knockdown led to a reduction in cell number (Fig. 3B), the present study attempted to investigate the involvement of apoptosis in this process. U-87 MG cells were treated with eIF3c lentiviral siRNA or scrambled lentiviral siRNA and an Annexin V-APC assay was employed to evaluate the status of cell apoptosis. Apoptosis was observed in 5.83% of U-87 MG cells treated with eIF3c siRNA, while only being observed in 2.78% of U-87 MG cells treated with scrambled siRNA (Fig. 5C and D). It was suggested that eIF3c knockdown may induce cell apoptosis in the malignant human glioma U-87 MG cell line.
Depletion of eIF3c delays tumoursphere formation in vivo
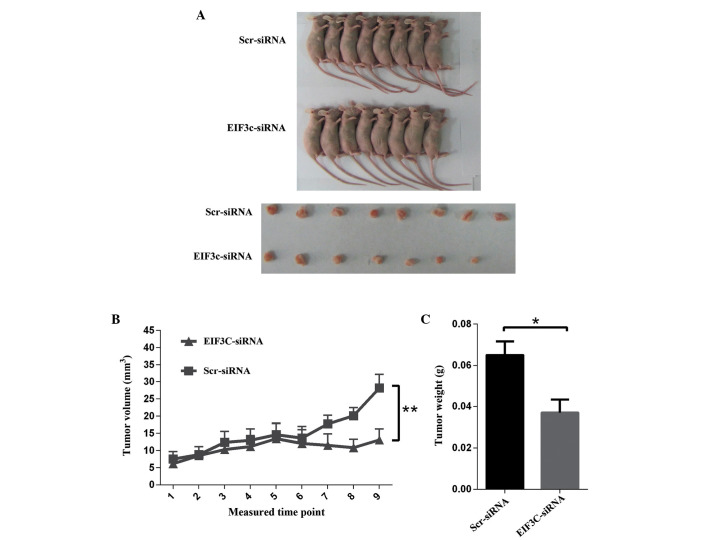
To investigate the role of eIF3c in glioma in vivo, a glioma xenograft model was established in nude mice. The mice were injected subcutaneously with ∼2×106 U-87 MG cells pre-transfected with eIF3c or scrambled siRNA-expressing lentivirus for 48 h. The tumour growth in nude mice was subsequently estimated by measuring tumour volume every other day after day 10 for 18 days. In the initial 11 days of measurement, no significant difference was observed between the various groups. However, after day 13, the tumour growth was markedly suppressed in the group treated with lentiviral eIF3c siRNA, and the suppression effect was more discernible with an increasing time after tumor implantation (Fig. 6A and B). Nude mice were sacrificed after 27 days of feeding, and the isolated tumours were weighed. These results demonstrated that tumour weight was significantly reduced by 43% subsequent to treatment with eIF3c siRNA (Fig. 6B and C).
Figure 6.
Tumour growth in nude mice is inhibited by eIF3c depletion. (A) Representative images of nude mice and tumours. The Scr-siRNA group consists of nude mice injected with U-87 MG cells infected with lentivirus expressing scrambled siRNA. The EIF3C-siRNA group consists of mice injected with U-87 MG cells infected with lentivirus expressing eIF3c siRNA. (B) Regulation of tumour volume by eIF3c depletion in nude mice. The graph demonstrates the tumour growth from day 10 after tumor transplantation for 18 days. In total, 16 nude mice were divided randomly into two groups (n=8 in each group). The Scr-siRNA group was injected with U-87 MG cells infected with lentivirus expressing scrambled siRNA, and the EIF3C-siRNA group was injected with U-87 MG cells infected with lentivirus expressing eIF3c siRNA. (**P<0.01). (C) Tumour weight in EIF3C-siRNA group compared with the Scr-siRNA group. The graph demonstrates the tumour weight measured subsequent to the mice being sacrificed (**P<0.01). eIF3c, eukaryotic translation initiation factor 3 subunit 3
Discussion
Overexpression of eIFs is common in numerous types of cancer and the aberrant expression of several eIFs, including eIF3b (22), eIF4e (23) and eIF5a (24), has also been observed in tissues obtained from patients with glioma, emphasizing the critical role of eIFs in glioma. The upregulation of eIF3c, a component of the eIF3 complex, has been reported in several tumour types, including colon cancer (14), testicular seminoma (12) and meningioma (25). Furthermore, the overexpression of individual eIF3 subunits, including eIF3a, −3b, −3c, −3h and −3i, may promote the malignant transformation of NIH3T3 cells. These findings suggest a causal association between the expression of components of the eIF3 complex and tumour progression (13). However, prior to the current study, the role of eIF3c in glioma remained elusive. To the best of our knowledge, the present study is the first to uncover the specific overexpression of eIF3c in human glioma. In normal brain tissue, eIF3c expression was barely detected, while eIF3c expression was markedly increased in glioma tissue. These results suggested that the role of eIF3c in glioma is specific and essential. Additionally, an increased expression of eIF3c is strongly associated with an increased glioma grade, which provides additional evidence for the involvement of eIF3c in the development of glioma.
In support of the critical functions of eIF3c in glioma development and progression, it was found that eIF3c knockdown inhibited the proliferation and colony formation ability (Figs. 3 and 4) of the human malignant glioma U-87 MG cell line. The present study also demonstrated that such suppression may be due to cell cycle arrest in the G0/G1 phase and apoptosis induced by eIF3c depletion.
Figure 4.
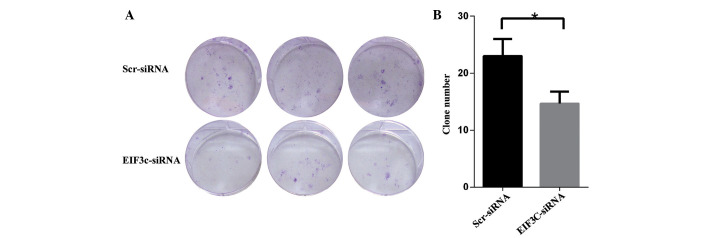
Silencing of eIF3c using lentivirus-mediated eIF3c siRNA suppresses colony formation in the human malignant glioma U-87 MG cell line. (A) Representative images of colony formation of U-87 MG cells in six-well plates. The Scr-siRNA group consists of the U-87 MG cells infected with lentivirus expressing scrambled siRNA. The EIF3C-siRNA groups consists of the U-87 MG cells infected with lentivirus expressing eIF3c siRNA. (B) Colony formation of U-87 MG cells treated with eIF3c siRNA. The results are expressed as the mean ± standard deviation of three separate experiments (*P=0.02). eIF3c, eukaryotic translation initiation factor 3 subunit 3.
As a component of the eIF3 complex, eIF3c mainly functions through the eIF3 complex, which is composed of 13 subunits, with an aggregate molecular weight of ∼700 kDa, and the eIFs that orchestrate translation initiation, the rate-limiting step of protein translation (26). Thus, the upregulation of translational initiation factors may enhance the initiation of protein translation, leading to acceleration of the protein synthesis involved in tumour initiation and progression (27). It has been demonstrated that the eIF3 complex serves as a dynamic scaffold for mammalian target of rapamycin complex 1 (mTORC1) recruitment and that eIF3c is critical for the assembly of the eIF3 complex (28). Therefore, eIF3c upregulation may enhance mTORC1-mediated translation upregulation, ultimately leading to aberrant increased protein synthesis and tumour progression, while eIF3c depletion may impair the assembly of the eIF3 complex, leading to robust reduction of protein synthesis. Consistent with this hypothesis, previous studies have revealed that eIF3c knockdown induced similar changes in human bladder cell lines to eIF3b knockdown, leading to the significant inhibition of novel protein synthesis and cell growth (29,7). It was also noted that the effect of eIF3c and eIF3b on the cell cycle process is similar in tumour cells, as depletion of either eIF3c or eIF3b leads to cell cycle arrest at the G0/G1 phase. It has previously been reported that cell cycle arrest caused by eIF3b depletion may be due to the suppression of cyclin expression, the elevation of p27Kip1 levels and the hypophosphorylation of Rb proteins (23). In consideration of the similarity between eIF3b and eIF3c, it is possible that eIF3c depletion induces cell cycle arrest through similar mechanisms.
Overall, the present study focused on the functions of eIF3c in the development of tumours in vitro and in vivo. The present results indicated that eIF3c is involved in multiple processes of glioma growth and survival. Furthermore, the inhibitory effect of eIF3c knockdown on glioma growth was validated in a nude mouse xenograft model. The results suggested that eIF3c may be a potential target for glioma treatment. However, the underlying mechanisms, including the influence of eIF3c knockdown on tumour proliferation and apoptosis, remain to be explored. As eIFs are composed of multiple factors, the systematic evaluation of the changes of all of the components in various tumour types to determine their specific association with distinct tumour types and grades is essential, and manipulating the expression of specific eIFs in various cell lines to identify potential gene targets may reveal promising cancer biomarkers and drug targets for tumour treatment.
References
- 1.Wakabayashi T. Clinical trial updates for malignant brain tumors. [(In Japanese)]. Rinsho Shinkeigaku. 2011;51:853–856. doi: 10.5692/clinicalneurol.51.853. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15:ii1–ii56. doi: 10.1093/neuonc/not151. (Suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 5.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. NABTT CNS Consortium: Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong J, Lasko P. Translational control in cellular and developmental processes. Nature Reviews Genetics. 2012;13:383–394. doi: 10.1038/nrg3184. [DOI] [PubMed] [Google Scholar]
- 7.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nature Reviews Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 8.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandin V, Miluzio A, Barbieri AM, et al. Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature. 2008;455:684–688. doi: 10.1038/nature07267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmanuel R, Weinstein S, Landesman-Milo D, Peer D. eIF3c: A potential therapeutic target for cancer. Cancer Lett. 2013;336:158–166. doi: 10.1016/j.canlet.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Rothe M, Ko Y, Albers P, Wernert N. Eukaryotic initiation factor 3 p110 mRNA is overexpressed in testicular seminomas. Am J Pathol. 2000;157:1597–1604. doi: 10.1016/S0002-9440(10)64797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Pan X, Hershey JWB. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J Biol Chem. 2007;282:5790–5800. doi: 10.1074/jbc.M606284200. [DOI] [PubMed] [Google Scholar]
- 14.Song N, Wang Y, Gu XD, Chen ZY, Shi LB. Effect of siRNA-mediated knockdown of eIF3c gene on survival of colon cancer cells. J Zhejiang Univ Sci B. 2013;14:451–459. doi: 10.1631/jzus.B1200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haapa-Paananen S, Kiviluoto S, et al. HES6 gene is selectively overexpressed in glioma and represents an important transcriptional regulator of glioma proliferation. Oncogene. 2012;31:1299–1310. doi: 10.1038/onc.2011.316. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JF, Xie F, Zhang LJ, Jiang WG. iASPP is over-expressed in human non-small cell lung cancer and regulates the proliferation of lung cancer cells through a p53 associated pathway. BMC Cancer. 2010;10:694. doi: 10.1186/1471-2407-10-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seely BL, Samimi G, Webster NJ. Retroviral expression of a kinase-defective IGF-I receptor suppresses growth and causes apoptosis of CHO and U87 cells in vivo. BMC Cancer. 2002;2:15. doi: 10.1186/1471-2407-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enderling H, Hahnfeldt P. Cancer stem cells in solid tumors: Is ‘evading apoptosis’ a hallmark of cancer? Prog Biophys Mol Biol. 2011;106:391–399. doi: 10.1016/j.pbiomolbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620–633. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang H, Ding X, Zhou C, Zhang Y, Xu M, Zhang C, Xu L. Knockdown of eukaryotic translation initiation factors 3B (EIF3B) inhibits proliferation and promotes apoptosis in glioblastoma cells. Neurol Sci. 2012;33:1057–1062. doi: 10.1007/s10072-011-0894-8. [DOI] [PubMed] [Google Scholar]
- 23.Tejada S, Lobo MV, Garcia-Villanueva M, et al. Eukaryotic initiation factors (eIF) 2alpha and 4E expression, localization and phosphorylation in brain tumors. J Histochem Cytochem. 2009;57:503–512. doi: 10.1369/jhc.2009.952929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preukschas M, Hagel C, Schulte A, et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: implications for new targeted therapies. PloS One. 2012;7:e43468. doi: 10.1371/journal.pone.0043468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scoles DR, Yong WH, Qin Y, Wawrowsky K, Pulst SM. Schwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit c (eIF3c) Hum Mol Genet. 2006;15:1059–1070. doi: 10.1093/hmg/ddl021. [DOI] [PubMed] [Google Scholar]
- 26.Emmanuel R, Weinstein S, Landesman-Milo D, Peer D. eIF3c: A potential therapeutic target for cancer. Cancer Lett. 2013;336:158–166. doi: 10.1016/j.canlet.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Silvera D, Arju R, Darvishian F, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nature Cell Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 28.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Ru Y, Sanchez-Carbayo M, Wang X, Kieft JS, Theodorescu D. Translation initiation factor eIF3b expression in human cancer and its role in tumor growth and lung colonization. Clin Cancer Res. 2013;19:2850–2860. doi: 10.1158/1078-0432.CCR-12-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]