Abstract
Introduction
Patient reported outcome measures (PROMs) were used to evaluate outcomes of the artificial urinary sphincter (AUS) and the AdVance™ (American Medical Systems, Minnetonka, MN, US) male sling system (AVMS) for the symptomatic management of male stress urinary incontinence.
Methods
All male patients with stress urinary incontinence referred to our specialist clinic over a two-year period completed the ICIQ-UI SF (International Consultation on Incontinence Questionnaire on Urinary Incontinence Short Form) and the ICIQ-MLUTS LF (International Consultation on Incontinence Questionnaire on Male Lower Urinary Tract Symptoms Long Form) at consultation as well as at subsequent follow-up appointments. The Wilcoxon signed-rank test for non-parametric paired data was used for pre and postoperative comparisons. The chi-squared test was used for categorical variables.
Results
Thirty-seven patients (forty surgical cases) completed a preoperative and at least one follow-up questionnaire. There was a statistically significant improvement in PROMs postoperatively, regardless of mode of surgery (p<0.01). Analysis of the ICIQ-MLUTS LF showed that patients with higher preoperative scores (>25) had greater improvement with an AUS than with the AVMS (p<0.01).
Conclusions
This prospective study shows that completion and collection of PROMs as part of routine clinical practice is achievable and useful in the assessment of male stress incontinence surgery. PROMs are important instruments to assess effectiveness of healthcare intervention and they are useful adjuncts in surgical studies.
Keywords: Patient reported outcome measurements, Artificial urinary sphincter, Male sling, Stress incontinence
The quality of surgical care is commonly assessed by objective indicators of operative success such as perioperative morbidity and mortality, intraoperative complications, length of hospital stay and readmission rates. While these are fundamentally important and useful markers of surgical performance, the need for better qualitative, subjective assessment of health and care delivery from the patient's own perspective has led to increased interest in patient reported outcome measures (PROMs).1,2 Indeed, PROMs are deemed useful and important to healthcare policy makers in prioritisation decisions, to benchmark quality and compare outcomes between institutions.3
Moreover, there is often variability between surgeon reported outcomes and patient reported outcomes. For example, a meta-analysis of studies investigating surgeon measured urinary continence recovery following robotic radical prostatectomy in a total of 3,808 patients reported highly variable incontinence rates of between 4% and 31% (depending on definition of incontinence), with a mean of 16% using a ‘no pad’ definition at 12 months.4 However, a large study of 1,005 robotic prostatectomy patients using specific patient responses and a strict definition of ‘leak free, pad free’ continence reported a more alarming incontinence rate of 76% at 12 months.5 PROMs can therefore provide valuable insights into the quality and effectiveness of surgical intervention for patients, and should be considered as an important component of outcome measures in clinical audit.
The artificial urinary sphincter (AUS) has historically been considered the gold standard treatment of severe stress urinary incontinence due to intrinsic sphincter deficiency.6 The three main components comprise a cuff (bulbar urethra or bladder neck), a pressure regulating balloon that is usually sited in the retropubic space and a control pump that is placed in the scrotum.
The AUS was first introduced in 1973 by American Medical Systems (AMS) (Minnetonka, MN, US) and, following modifications, it has largely been unchanged technically since 1987 with the release of the narrow back cuff AMS 800® urinary control system.6 Although there are alternative AUS devices available, it is estimated that the vast majority of the more than 150,000 patients worldwide implanted with an AUS have the AMS 800®.7 Over the last 30 years, the AMS 800® has been implanted in more than 94,000 men with stress urinary incontinence secondary to prostatectomy. These figures are all the more important given that an increasing number of men in the UK are undergoing radical prostatectomy.
The AdVance™ male sling (AVMS) system is also manufactured by AMS. It is a tape made from type 1 polypropylene monofilament mesh, which is placed via a transobturator route under the bulbar urethra to provide elevation. It has been available since 2006. In the UK in 2012–2013, there were 156 recorded cases of AVMS insertion compared with 287 AUS cases.8 Long-term data are not yet available but surgical insertion of the AVMS is less invasive than for the AUS, the operation and inpatient stay are shorter, and as it does not have the mechanical components of the AUS, it has fewer associated complications.
To date, published data on surgeon reported outcomes exist for up to three years with the AVMS, with cure rates in the region of 40% in the severely incontinent group, and up to 58% in the mild and moderate groups.9 In contrast, the AUS has higher surgeon reported cure rates, in the region of 80–90%.5,10 Qualitative studies have reported high patient satisfaction following AVMS or AUS insertion but these studies have been of retrospective design so they are limited by possible recall bias among participants.11,12
In this prospective study, we investigated the utility of PROMs to assess and compare the outcomes of the AUS and AVMS for the symptomatic management of urodynamically proven male stress incontinence. To our knowledge, following a literature search on PubMed, no comparative study is available and this is the first prospective study to be conducted comparing the two surgical modalities.
Methods
All male patients with stress incontinence referred to our specialist clinic were invited to complete the ICIQ-UI SF (International Consultation on Incontinence Questionnaire on Urinary Incontinence Short Form) and the ICIQ-MLUTS LF (International Consultation on Incontinence Questionnaire on Male Lower Urinary Tract Symptoms Long Form) at initial consultation, three months postoperatively and at subsequent follow-up appointments. The ICIQ-MLUTS LF is a questionnaire completed by patients for evaluating male lower urinary tract symptoms and impact on quality of life (QoL). There are 23 scored items, giving an overall score of between 1 and 84, with greater values indicating greater impact of symptoms for the patients. The ICIQ-UI SF also assesses the severity and impact on QoL of patients but consists of only three scored items, giving an overall score of between 1 and 21, and a fourth non-scored self-diagnostic item.13,14
All patients requiring surgical intervention had a routine clinical evaluation consisting of a full medical history and a focused examination, video urodynamics and flexible cystoscopy, to determine bladder and sphincter function. The video urodynamics and flexible cystoscopy investigations were performed by the operating surgeons, who then counselled patients on types of male incontinence therapy. All surgical treatment decisions were offered in an unbiased way, regardless of severity of incontinence, enabling the patient to make an informed choice on which procedure to have after weighing up the advantages and disadvantages of each operation.
Patients were made aware of published outcomes on success, available follow-up data, and the complexities of surgery and potential complications, also provided in the patient information leaflet. Patients were told that there is currently insufficient evidence to guide clinicians or patients on which type of procedure to have based on type of prostate surgery or severity of incontinence. This specific issue will be addressed by the MASTER trial (male synthetic sling versus artificial urinary sphincter) in the UK but results are not expected until 2020.15 Patients were also made aware that the AUS could be offered if the AVMS failed to treat their incontinence adequately.16
Surgical procedure
All patients underwent either insertion of the AVMS or implantation of the AMS 800® using standard operative techniques and AMS kits. The procedures were performed by two surgeons only. Standard postoperative care was followed with patients either discharged the following day after AVMS or after two days following AUS implantation. AVMS patients were advised against heavy lifting, bending or other stresses for the first six weeks to ensure consistent sling fixation during healing. The AUS was left deactivated for the first six weeks following surgery.
Follow-up period
The AUS was activated in clinic six weeks postoperatively. Patients were reviewed electively at three months and at one year postoperatively unless otherwise clinically indicated. Patients who had the AVMS were reviewed at 3, 6 and 12 months postoperatively unless otherwise clinically indicated.
Statistical analysis
The Wilcoxon signed-rank test for non-parametric paired data was used for comparisons prior to and following the procedures as the samples were not independent. The chi-squared test was used for categorical variables. Cases for which a preoperative and at least one follow-up questionnaire had been completed were included for further comparative analyses. The patient group was dichotomised using the median preoperative ICIQ-MLUTS LF score to assess the effect of preoperative symptom severity on postoperative outcomes as initial inspection of the data indicated differences in preoperative symptom severity. The small numbers and large variations in follow-up times precluded using a panel approach without duplicates. Kaplan–Meier survival curves were therefore plotted to explore differences in outcome between treatments, using answers to question 5 of the ICIQ-MLUTS LF (‘Does urine leak before you get to the toilet?’) as the outcome (‘no’ (score 0) = cure, ‘yes’ (score 1–4) = no cure).
Results
At the time of analysis, over a 2-year period (February 2010 – February 2012), 69 men were referred for management of postoperative stress urinary incontinence. Four patients declined further investigation and management, nineteen were awaiting investigations or surgery, twenty-two underwent AUS insertion and twenty-seven underwent AVMS insertion. Three patients had secondary surgery (revision of AUS balloon [n=1], replacement of AUS following failure due to inadvertent urethral catheterisation [n=1] and AUS insertion following failed treatment with AVMS [n=1]). Thirty-seven patients (forty surgical cases) had completed a preoperative (T0) and at least one follow-up questionnaire.
Overall, the median time interval to the first follow-up visit (FU1) was 85.5 days (interquartile range [IQR]: 59–135 days) and it was 288 days (IQR: 189–400 days) for the second follow-up visit (FU2). For AUS patients, the median interval was 135 days (IQR: 114–184 days) to FU1 and 466 days (IQR: 371–697 days) to FU2. For AVMS patients, the median intervals were 60 days (IQR: 57–70 days) and 189 days (IQR: 178–383 days) respectively.
There were no statistical differences between the two groups in terms of patient age, symptoms or surgical procedure (Table 1). A summary analysis of all patients regardless of mode of surgery showed a statistically significant improvement in incontinence symptoms postoperatively at FU1 and FU2 (p<0.01 for both instruments) (Table 2). Nevertheless, there was little change in symptom scores between the two postoperative visits although there was some evidence to suggest that patients experienced a symptomatic deterioration detected on the ICIQ-UI SF only (ICIQ-MLUTS LF p=0.8, ICIQ-UI SF p=0.01).
Table 1.
Descriptive analysis of the two study cohorts
| AUS (n=17) | AVMS (n=23) | Total (n=40) | p-value* | |
| Median age | 72 (IQR: 66–75) | 69 (IQR: 65–73) | 0.45 | |
| Causative procedure | 0.76 | |||
| Open prostatectomy | 8 (47.1%) | 9 (39.1%) | 17 (42.5%) | |
| Robotic prostatectomy | 4 (23.5%) | 11 (47.8%) | 15 (37.5%) | |
| HoLEP | 2 (11.8%) | 3 (13.0%) | 5 (12.5%) | |
| TURP | 2 (11.8%) | 0 (0%) | 2 (5.0%) | |
| Other | 1 (5.9%) | 0 (0%) | 1 (2.5%) | |
| Symptoms | 0.07 | |||
| Stress incontinence | 11 (68.8%) | 21 (91.3%) | 32 (82.1%) | |
| Urge incontinence | 1 (6.3%) | 1 (4.3%) | 2 (5.1%) | |
| Mixed incontinence | 4 (25.0%) | 1 (4.3%) | 5 (12.8%) |
AUS = artificial urinary sphincter; AVMS = AdVance™ male sling; IQR = interquartile range; HoLEP = holmium laser enucleation of the prostate; TURP = transurethral resection of the prostate
Wilcoxon signed-rank test for continuous variables and chi-squared test for categorical variables
Table 2.
Collective summary of scores in all 40 patients, assessed preoperatively and at 2 follow-up visits
| Median ICIQ-MLUTS LF score* (IQR) | Median ICIQ-UI SF score** (IQR) | |
| T0 (n=40) | 24.5 (18–35) | 16 (13–18) |
| FU1 (n=40) | 16 (10.5–23.5) | 3.5 (0–7) |
| FU2 (n=25) | 19 (13–22) | 7 (3–11) |
ICIQ-MLUTS LF = International Consultation on Incontinence Questionnaire on Male Lower Urinary Tract Symptoms Long Form; ICIQ-UI SF = International Consultation on Incontinence Questionnaire on Urinary Incontinence Short Form; IQR = interquartile range; T0 = preoperative; FU = follow-up
max score 44
max score 21
There was a significant difference between T0 versus FU1 for both the AUS and AVMS groups indicated by the ICIQ-UI SF (p<0.01) and ICIQ-MLUTS LF (p=0.02). Similarly, there was evidence of a statistical difference between T0 and FU2 for both AUS and AVMS patients indicated in the ICIQ-UI SF and ICIQ-MLUTS LF scores (p<0.05).
Comparison of FU1 versus FU2 ICIQ-MLUTS LF scores yielded no significant difference between procedures (AUS p=0.4, AVMS p=0.6). However, there was a significant difference in ICIQ-UI SF scores at FU2 compared with FU1 in patients who had an AUS (p<0.01) but not an AVMS (p=0.4) (Table 3).
Table 3.
Summary of the scores in 40 patients, assessed preoperatively and at 2 follow-up visits
| Median ICIQ-MLUTS LF score (IQR) | Median ICIQ-UI SF score (IQR) | |||||
| AUS | AVMS | p-value* | AUS | AVMS | p-value* | |
| T0 (n=40) | 30 (13–36) | 23 (18–23) | 0.6 | 15 (8–19) | 16 (14–18) | 0.4 |
| FU1 (n=40) | 12 (8–17) | 21 (13–25) | 0.03 | 2 (0–4) | 4 (0–10) | 0.08 |
| FU2 (n=25) | 20.5 (14–22) | 18 (12–20) | 0.4 | 6 (1–11) | 7 (4–11) | 0.9 |
ICIQ-MLUTS LF = International Consultation on Incontinence Questionnaire on Male Lower Urinary Tract Symptoms Long Form;
ICIQ-UI SF = International Consultation on Incontinence Questionnaire on Urinary Incontinence Short Form; AUS = artificial urinary sphincter; AVMS = AdVance™ male sling; IQR = interquartile range; T0 = preoperative; FU = follow-up
Wilcoxon signed-rank test comparing scores between procedures
Further analysis of ICIQ-MLUTS LF results showed that patients who reported high scores of 25 (the median) or over preoperatively had a greater, significant reduction in scores and improvement in QoL (Table 4). This suggests that patients with worse symptoms prior to surgery stood to gain greater improvement with AUS than with AVMS insertion.
Table 4.
Summary of patient reported improvement in outcomes as indicated by the ICIQ-MLUTS LF. Positive values represent an improvement in outcomes.
| Median difference (IQR) | p-value* | ||
| AUS | AVMS | ||
| Reduction in score at FU1 | 20 (1–23) | 6 (-2–10) | 0.03 |
| T0 score ≤25 | -4 (-12–6) | 4.5 (-2–10) | 0.2 |
| T0 score >25 | 22.5 (20–26) | 8 (5–12) | <0.01 |
| Reduction in score at FU2 | 7.5 (3–19) | 6 (-1–15) | 0.6 |
| T0 score ≤25 | -1 (-9.5–4.5) | 16.5 (8–22) | 0.2 |
| T0 score >25 | 5 (-1–12) | 13.5 (6–18) | 0.3 |
ICIQ-MLUTS LF = International Consultation on Incontinence Questionnaire on Male Lower Urinary Tract Symptoms Long Form; IQR = interquartile range; AUS = artificial urinary sphincter; AVMS = AdVance™ male sling; FU = follow-up; T0 = preoperative
Wilcoxon signed-rank test comparing scores between procedures
Examining differences in responses to individual ICIQ-MLUTS LF questions between the AUS and AVMS groups, it was found that patients reported differences in particular to question 5 (‘Does urine leak before you get to the toilet?’), question 8 (‘Do you ever leak with no obvious reason and without feeling you want to go?’) and question 13 (strength of urinary stream) but, interestingly, not question 15 (illustration of strength of stream) or any of the other questions. The differences in the patients’ report of incontinence cure (cure = no leak) were explored using a Kaplan–Meier curve (Fig 1). The graph suggests more reported cures at an earlier follow-up time with the AUS than with the AVMS although this was not statistically significant (p=0.4).
Figure 1.
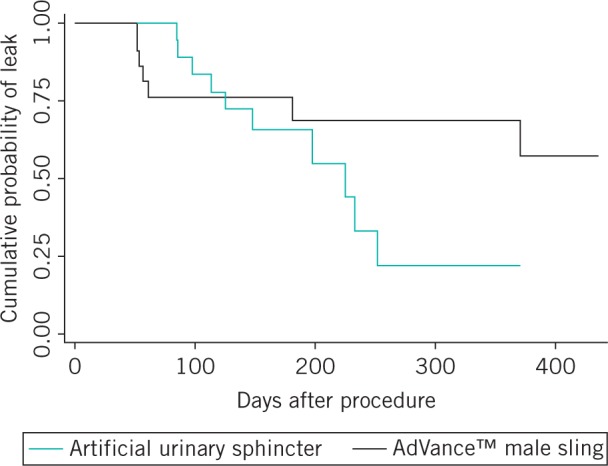
Kaplan–Meier plot showing probability of a leak following surgery
Discussion
Berwick stated eloquently that ‘the ultimate measure by which to judge the quality of a medical effort is whether it helps patients (and their families) as they see it. Anything done in health care that does not help a patient or family, is by definition, waste, whether or not the professions and their associations traditionally hallow it’.17 At a time when the National Health Service (NHS) is facing immense financial pressures, PROMs can play an important role in the paradigm shift of assessing healthcare productivity from output to outcome. Since 2009, the Department of Health has required the routine measurement of PROMs for all NHS patients in England before and after receiving certain types of surgery. Data from four surgical procedures (hernia repair, hip and knee replacement, and varicose vein surgery) are collected nationally and there is considerable interest for extending measurements to other surgical specialties, indeed to encompass all elective surgery.
In this paper, it has been shown that by using established and validated incontinence PROM instruments, we can quantify qualitative outcomes to compare surgical intervention in male incontinence therapy. Statistically significant differences are reported between pre and postoperative scores with both the AUS and AVMS, indicating an improved QoL from the patient’s perspective after surgical intervention. Both the ICIQ-UI SF and ICIQ-MLUTS LF are therefore sensitive to change following anti-incontinence surgery.
Significant differences were found in postoperative patient reported outcomes in individuals with worse symptoms preoperatively. In particular, patients reporting more severe incontinence symptoms (ICIQ-MLUTS LF score >25) seem to have a greater reduction in scores (suggesting a greater improvement in QoL) with the AUS than with the AVMS, especially at early follow-up visits. The results should be interpreted with caution, however, owing to the small patient numbers and further study is required in a larger population. We also accept that although we used well validated instruments (ICIQ-MLUTS LF and ICIQ-UI SF) to measure incontinence, we have not used (and are not aware of) any procedure specific PROMs; use of such PROMs may have yielded different results.
The increased scores and perceived deterioration in outcomes at FU2 was unexpected. This may be due to selection bias as patients who were satisfied with their outcomes could be less likely to reattend clinic.
Study limitations
There are some limitations to our paper. First, the small number of patients may have reduced the power of the study and the reliability of the results. However, we have demonstrated that the ICIQ-MLUTS LF and ICIQ-UI SF can detect symptomatic improvement in urinary incontinence following surgery. Furthermore, there is also some evidence to show preoperative symptom severity may serve as a useful method of assessing postoperative outcomes for the AUS and AVMS. This can potentially be used to guide patient choice and improve patient care.
Second, owing to the pragmatic nature of the study, there were inconsistent intervals between the follow-up patient assessments. Follow-up for AVMS patients was initially more rigorous than for the AUS group because of the novel nature of the AVMS and the desire to determine whether the PROM changed over the year following surgery for the AVMS. Since this study, the PROMs have been delivered in a more consistent and comparable fashion.
The small numbers and large variations in follow-up times precluded using a panel approach without duplicates. Nevertheless, the Kaplan–Meier curves show differences in ‘cure’ rates between treatments and this accounts for the differences in follow-up times. This analysis also does not take into account patients who may regress symptomatically after experiencing no leak. Furthermore, the pragmatic nature of this study could lead to selection bias, where patients with good outcomes are less likely to reattend over longer follow-up periods.
Conclusions
PROMs are widely accepted as appropriate instruments to assess the effectiveness of healthcare intervention. However, there is currently underutilisation in surgical reports. In this paper, we show that completion and collection of PROMs as part of routine clinical practice is achievable and can be used to detect symptomatic improvement in urinary incontinence following surgery. Importantly, with further larger studies, preoperative symptom severity as indicated by an ICIQ-MLUTS LF score of >25 may potentially serve as a useful method of assessing postoperative outcomes for AUS and AVMS patients.
References
- 1.Devlin NJ, Parkin D, Browne J. Patient-reported outcome measures in the NHS: new methods for analysing and reporting EQ-5D data. Health Econ 2010; 19: 886–905. [DOI] [PubMed] [Google Scholar]
- 2.Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167. [DOI] [PubMed] [Google Scholar]
- 3.Jackson MJ, N'Dow J, Pickard R. The importance of patient-reported outcome measures in reconstructive urology. Curr Opin Urol 2010; 20: 495–499. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Rosen RC et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012; 62: 405–417. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds WS, Shikanov SA, Katz MH et al. Analysis of continence rates following robot-assisted radical prostatectomy: strict leak-free and pad-free continence. Urology 2010; 75: 431–436. [DOI] [PubMed] [Google Scholar]
- 6.Van der Aa F, Drake MJ, Kasyan GR et al. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol 2013; 63: 681–689. [DOI] [PubMed] [Google Scholar]
- 7.Lucas MG, Bosch RJ, Burkhard FC et al. EAU guidelines on surgical treatment of urinary incontinence. Eur Urol 2012; 62: 1,118–1,129. [DOI] [PubMed] [Google Scholar]
- 8.Hospital Episode Statistics, Admitted Patient Care – England, 2012. –2013. Procedures and Interventions. Health and Social Care Information Centre; http://www.hscic.gov.uk/catalogue/PUB12566 (cited July 2014). [Google Scholar]
- 9.Rehder P, Haab F, Cornu JN et al. Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur Urol 2012; 62: 140–145. [DOI] [PubMed] [Google Scholar]
- 10.Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol 2000; 164(3 Pt 1): 702–706. [DOI] [PubMed] [Google Scholar]
- 11.Kahlon B, Baverstock RJ, Carlson KV. Quality of life and patient satisfaction after artificial urinary sphincter. Can Urol Assoc J 2011; 5: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Gill BC, Nowacki AS et al. Therapeutic durability of the male transobturator sling: midterm patient reported outcomes. J Urol 2012; 187: 1,331–1,335. [DOI] [PubMed] [Google Scholar]
- 13.Avery K, Donovan J, Peters TJ et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004; 23: 322–330. [DOI] [PubMed] [Google Scholar]
- 14.Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: the ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn 2009; 28: 411–415. [DOI] [PubMed] [Google Scholar]
- 15.Male Synthetic Sling Versus Artificial Urinary Sphincter Trial for Men with Urodynamic Stress Incontinence after Prostate Surgery. ISRCTN Register; http://www.controlled-trials.com/ISRCTN49212975 (cited July 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lentz AC, Peterson AC, Webster GD. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J Urol 2012; 187: 2,149–2,153. [DOI] [PubMed] [Google Scholar]
- 17.Berwick D. Medical associations: guilds or leaders? BMJ 1997; 314: 1,564–1,565. [DOI] [PMC free article] [PubMed] [Google Scholar]


