Abstract
This study aimed to compare the therapeutic effects and adverse events of the multikinase inhibitors sorafenib, sunitinib, pazopanib and axitinib in advanced renal cell carcinoma (RCC). A meta-analysis of randomized controlled trials was performed to assess the effects of multikinase inhibitors among patients with advanced RCC. The data of median progression-free survival (PFS), median overall survival (OS), progressive disease rate (PDR), objective response rate (ORR) and grade 3/4 adverse events were extracted to assess therapeutic effects and toxicity, respectively. It was found that multikinase inhibitors are more effective in extending PFS [hazard ratio (HR)=0.58; 95% confidence interval (CI): 0.45–0.74; P<0.0001), controlling tumor progression [relative risk (RR)=0.67; 95% CI: 0.55–0.83; P=0.0002) and ORR (RR=2.93; 95% CI: 1.40–6.14; P=0.004) compared with placebo or interferon-α. Patients treated with multikinase inhibitors had significantly higher rates of grade 3 or 4 hypertension (RR=6.00; 95% CI: 3.36–10.69; P<0.00001), diarrhea (RR=5.84; 95% CI: 3.06–11.16; P<0.00001), nausea (RR=2.30; 95% CI: 1.16–4.54; P=0.02), vomiting (RR=1.84; 95% CI: 1.00–3.41; P=0.05) and hand-foot skin reaction (RR=11.78; 95% CI: 5.16–26.93; P<0.00001). Multikinase inhibitors can significantly control disease progress and improve the ORR. However, they are also associated with a higher risk of grade 3 and 4 hypertension and gastrointestinal events. Proper management of these events is necessary to improve patient quality of life.
Keywords: multikinase inhibitors, renal cell carcinoma, meta-analysis
Introduction
Renal cell carcinoma (RCC) is one of the most progressive urological cancers among men and women (1,2), and is considered as one of the most difficult cancers to diagnose and to treat (3). Advanced RCC is highly resistant to radiotherapy and chemotherapy. Approximately 25–30% of RCC patients suffer metastatic or advanced disease, with a 5-year survival rate <10% (4). Prior to recent advancements in understanding of the molecular mechanism of RCC and the development of angiogenesis inhibitors, cytokine-based therapy such as interleukin-2 (IL-2) and interferon (IFN) was the main therapy used to treat RCC (5). However, the therapeutic effect of these treatments is quite limited, as there is only a 4–6% combined response rate for most single agent or combination regimens (6).
Therapeutic options for patients have significantly expanded due to the successful development and adaptation of several targeting agents in the first-line treatment of advanced RCC, including multikinase inhibitors sorafenib (7), sunitinib (8), pazopanib (9) and axitinib (10); the combination of the anti-vascular endothelial growth factor (VEGF) agent bevacizumab with IFN-α (11); and mammalian target of rapamycin (mTOR) inhibitors temsirolimus (12) and everolimus (13). However, considering the poor physical condition, such as cachexia, that is common among patients with advanced RCC (14), selection of treatment should not only consider the therapeutic effect, but also adverse events. In order to make a more rational choice of treatment for individual patients with advanced RCC, it is necessary to identify the level of adverse effects of these options. In this scenario, a meta-analysis of randomized trials was performed to evaluate the therapeutic and adverse effects of the multikinase inhibitors sorafenib, sunitinib, pazopanib and axitinib.
Materials and methods
Study design
This study was a meta-analysis based on data collected from previous randomized controlled trials (RCTs) of first-line chemotherapies for patients with advanced RCC. Two reviewers (QT and YL) selected and reviewed the evidence independently. Disagreements were handled through group discussion.
Search strategy
Studies were search among PubMed/Medline, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) databases using the following terms and strategy: (‘sorafenib’ or ‘sunitinib’ or ‘pazopanib’ or ‘axitinib’) and (‘renal cell carcinoma’ or ‘RCC’ or ‘metastatic renal cell carcinoma’ or ‘advanced RCC’) and (‘randomized trials’ or ‘random*’ or ‘RCT’) in the abstract. The databases were searched for studies published up to February 2014. Only trials published in English were considered. Reference lists of related articles were manually checked to search for additional eligible publications. All references of relevant articles were scanned and all additional studies of potential interest were retrieved for further analysis.
Selection criteria
Eligible trials were required to meet the following criteria: Patients involved were diagnosed with advanced RCC through cytologic diagnosis or pathological diagnosis; RCTs evaluated multikinase inhibitors individually with a control intervention as the sole treatment; patients did not undergo surgery or other non-antiangiogenic treatment; and ≥100 patients were enrolled. Animal studies, non-randomized trials and pharmacokinetic studies were excluded.
The bias risk of included publications was evaluated based on the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.0 (15). The major quality components include: i) Sequence generation of the allocation; ii) allocation concealment; iii) blinding of participants, personnel and outcome assessors; iv) incomplete outcome data; v) selective outcome reporting; and vi) other sources of bias (15). Trials were classified into three levels according to the bias risk. Trials with appropriate and sufficient support of index of outcome assessment that have minimal risk of bias were classified into level A; trials with one or more high or unclear risks for bias among the quality components and with a moderate level risk of bias were in level B; trials with three or more high or unclear risks for bias among the quality components and with the highest level of bias were in level C.
Data extraction
Two reviewers (QT and YL) separately and independently extracted data from the trials. Disagreements were handled by consensus. All data were checked for internal consistency. The trials were identified with the first author and the year of publication. For the trials that did not report the required data to determine the outcomes, the reviewers contacted the authors to obtain required information. The baseline information of patients and details of intervention of each trial were extracted to assess the heterogeneity. The outcomes assessed included tumor progression, objective response rate (ORR) and progressive disease rate (PDR). Toxicity data reported commonly in the trials involved were retrieved, extracted and assessed respectively.
Statistic analysis
Meta-analysis was conducted with Review Manager 5.2 (RevMan 5.2; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Risk ratio (RR) was used for evaluation and the 95% confidence interval (CI) was calculated for each estimate. P≤0.05 was used to denote statistical significance. Heterogeneity of the results of the trials was assessed with the I2 statistic using the χ2 test at α=0.1 (15). Primary assessment was conducted with a fixed model. When P≥0.05 and I2≤50%, it was considered that the trials were without heterogeneity and a fixed-effect model was used to perform the meta-analysis. When P<0.05 and I2>50%, it was considered that the trials had significant heterogeneity (16). The source of the heterogeneity was further analyzed. If there was no significant clinical heterogeneity, a secondary confirmatory analysis was performed with a random-effect model. Otherwise, descriptive analysis was performed. Where necessary, sensitivity analyses were performed to test the stability of identified outcomes.
Results
Literature search
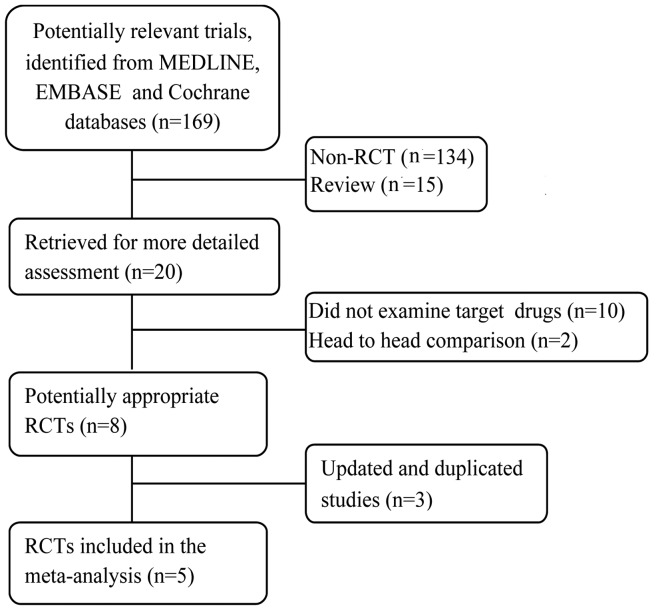
The whole search process was as described in the flowchart in Fig. 1. The primary literature search identified 169 studies. Of these, 149 were excluded since they were not RCTs or were review studies. The remaining 20 studies were reviewed in full-length. Among them, 10 were excluded because they did not examine the target drugs, 2 were excluded since they were head-to-head comparisons, and 3 were excluded since the studies were based on the same patients and the same trial. Finally, 5 RCTs remained for inclusion in this meta-analysis. No patients in the involved studies had received previous systemic therapy. The 5 trials are randomized, multicenter, controlled and phase II or III trials. Among them, two assessed sorafenib (17,18), one evaluated sunitinib (19), one evaluated pazopanib (20) and one evaluated axitinib (21). The methodological details relevant to bias and the treatment arms of the selected studies are presented in Table I.
Figure 1.
Flowchart of the literature search. RCT, randomized controlled trial.
Table I.
Summary of the included trials.
| Trial (ref.) | N | Intervention | Control | Quality components | Quality level |
|---|---|---|---|---|---|
| Escudier 2007 (17) | 903 | Sorafenib (400 mg, twice daily, 6 week cycles) | Placebo | R; S and RPB; C; BR; F; ITT | B |
| Escudier 2009 (18) | 189 | Sorafenib (twice daily; 400 mg, period 1; 600 mg, period 2) | IFN-α | R; S; C; BR; F; ITT | B |
| Motzer 2009 (22) | 750 | Sunitinib (50 mg once daily, with a 4 weeks on and 2 weeks off schedule) | IFN-α | R; S and RPB; C; NB; F; ITT | B |
| Sternberg 2010 (20) | 435 | Pazopanib (800 mg, once daily) | Placebo | R; S and RPB; C; DB; F; ITT | A |
| Rini 2013 (21) | 112 | Axitinib (5 mg + 2 mg, twice daily) | Placebo | R; S and RPB; C; B R; F; ITT | B |
All studies investigated patients with metastatic clear-cell renal cell carcinoma. R, randomized; S, stratification; RPB, random permuted blocks; BR, blind reviewer; DB, double blind; NB, non-blind; F, follow up; C, controlled; ITT, intent-to-treat.
Meta-analysis of the therapeutic efficiency of multikinase inhibitors
Progression free survival (PFS) and overall survival (OS)
All studies reported PFS data and two of the studies reported OS data. Detailed information about the included trials is presented in Table II. The pooled HR of PFS is presented in Fig. 2. This shows that, compared with controls, multikinase inhibitors contributed to significantly longer PFS [hazard ratio (HR)=0.58; 95% confidence interval (CI): 0.45–0.74; P<0.0001]. Of the two studies that reported OS data, one study (17) reported a significantly longer OS for the multikinase inhibitor compared with the control (19.3 vs. 15.9 months, P=0.02), while the other (22) did not find a significant difference (26.4 vs. 21.8 months, not significant).
Table II.
Progression-free survival (PFS) and overall survival (OS) in the included trials.
| Trials (ref.) | N | Median PFSa | HR (95% CI) | P-value | Median OS (months) | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|
| Escudier 2007 (17) | 903 | 5.5 vs. 2.8 | 0.44 (0.35–0.55) | 0.01 | 19.3 vs. 15.9 | 0.72 (0.54–0.94) | 0.02 |
| Escudier 2009 (18) | 189 | 5.7 vs. 5.6 | 0.88 (0.61–1.27) | 0.50 | N/A | – | – |
| Motzer 2009 (22) | 750 | 11.0 vs. 5.0 | 0.54 (0.45–0.64) | 0.01 | 26.4 vs. 21.8 | 0.82 (0.64–1.00) | NS |
| Sternberg 2010 (20) | 435 | 9.2 vs. 4.2 | 0.46 (0.34–0.62) | 0.001 | N/A | – | – |
| Rini 2013 (21) | 112 | 14.5 vs. 15.7 | 0·85 (0.54–1.35) | 0.24 | N/A | – | – |
Invervention vs. control. HR, hazard ratio; CI, confidence interval; N/A, not available; NS, not significant.
Figure 2.
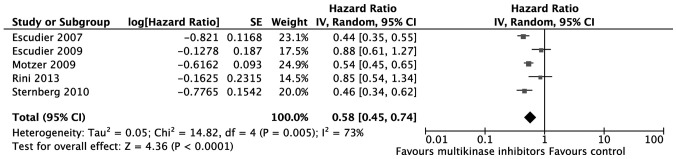
Pooled hazard ratio of progression-free survival. SE, standard error; IV, inverse variance; CI, confidence interval.
PDR and ORR
The number of patients with progressive disease was extracted to assess tumor progression. According to the meta-analysis, multikinase inhibitors are more effective in controlling tumor progression compared with placebo or IFN-α [relative risk (RR)=0.67; 95% CI: 0.55–0.83; P=0.0002; Fig. 3). As to ORR, the effect of multikinase inhibitors was also superior to that of placebo or IFN-α (RR=2.93; 95% CI: 1.40–6.14; P=0.004; Fig. 4).
Figure 3.
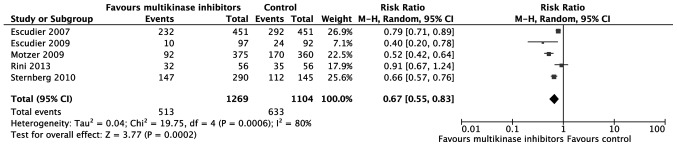
Meta-analysis of progressive disease rate. CI, confidence interval; M-H, Mantel-Haenszel.
Figure 4.

Meta-analysis of objective response rate. CI, confidence interval; M-H, Mantel-Haenszel.
Adverse effects
The reported adverse effects associated with drug administration in the 5 trials were extracted and are summarized in Table III. Six types of adverse effect, including cardiac (hypertension), constitutional (fatigue) and gastrointestinal (diarrhea, anorexia and vomiting) effects and pain (abdominal) were reported. Only adverse effects reported in ≥4 studies were pooled for RR evaluation. According to the meta-analysis, compared with IFN or placebo, multikinase inhibitors were associated with significantly higher rates of grade 3 or 4 hypertension (RR=6.00; 95% CI: 3.36–10.69; P<0.00001), diarrhea (RR=5.84; 95% CI: 3.06–11.16; P<0.00001), nausea (RR=2.30; 95% CI: 1.16–4.54; P=0.02), vomiting (RR=1.84; 95% CI: 1.00–3.41; P=0.05) and hand-foot skin reaction (RR=11.78; 95% CI: 5.16–26.93; P<0.00001; Table III). However, the difference in fatigue between the multikinase inhibitor group and the IFN or placebo group was not significant (RR=0.93; 95% CI: 0.69–1.25; P=0.61; Table III).
Table III.
Meta-analysis of adverse effects.
| Escudier 2007 | Escudier 2009 | Motzer 2009 | Rini 2013 | Sternberg 2010 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | I | C | I | C | I | C | I | C | I | C | Pooled RR (95% CI) | I2 (%) | P-H | P-value |
| No. of patients | 451 | 451 | 97 | 90 | 375 | 360 | 56 | 56 | 290 | 145 | ||||
| Cardiac, hypertension | 16 | 2 | 2 | 1 | 45 | 4 | 10 | 5 | 13 | 1 | 6.00 (3.36, 10.69) | 42 | 0.14 | <0.00001 |
| Hematologic, decreased Hb | 12 | 20 | – | – | – | – | – | – | – | – | ||||
| Constitutional | ||||||||||||||
| Fatigue | 22 | 16 | 5 | 9 | 41 | 47 | 3 | 2 | 7 | 4 | 0.93 (0.69, 1.25) | 0 | 0.52 | 0.61 |
| Weight loss | 3 | 0 | 2 | 1 | – | – | 4 | 3 | – | – | ||||
| Asthenia | – | – | – | – | 26 | 14 | – | – | 8 | 0 | ||||
| Other symptoms | 6 | 6 | 0 | 0 | – | – | – | – | ||||||
| Gastrointestinal | ||||||||||||||
| Diarrhea | 11 | 3 | 6 | 0 | 34 | 4 | 6 | 2 | 11 | 1 | 5.84 (3.06, 11.16) | 0 | 0.76 | <0.00001 |
| Nausea | 3 | 3 | 0 | 3 | 19 | 4 | 3 | 0 | 2 | 0 | 2.30 (1.16, 4.54) | 41 | 0.15 | 0.02 |
| Anorexia | 3 | 5 | 0 | 2 | 8 | 7 | – | – | 6 | 1 | 0.95 (0.47, 1.93) | 0 | 0.43 | 0.89 |
| Vomiting | 4 | 6 | 2 | 1 | 15 | 4 | 3 | 0 | 7 | 3 | 1.84 (1.00, 3.41) | 23 | 0.27 | 0.05 |
| Constipation | 3 | 3 | – | – | – | – | 0 | 0 | – | – | ||||
| Neurologic-sensory | ||||||||||||||
| Neuropathy | 2 | 3 | – | – | – | – | 0 | 0 | – | – | ||||
| Pain | ||||||||||||||
| Abdominal | 7 | 9 | 3 | 1 | 8 | 0 | 0 | 1 | 6 | 0 | 1.81 (0.91, 3.63) | 44 | 0.13 | 0.09 |
| Muscular | – | – | 1 | 0 | – | – | 0 | 0 | – | – | ||||
| Stomach | – | – | 0 | 0 | – | – | – | – | ||||||
| Headache | 1 | 2 | – | – | 4 | 0 | 0 | 0 | 0 | 0 | 1.84 (0.11, 31.90) | 56 | 0.13 | 0.67 |
| Joint | 7 | 1 | – | – | – | – | 1 | 2 | – | – | ||||
| Bone | 3 | 15 | – | – | – | – | 0 | 0 | – | – | ||||
| Tumor | 13 | 8 | – | – | – | – | – | – | – | – | ||||
| Pulmonary | ||||||||||||||
| Cough | 1 | 1 | 0 | 0 | – | – | 0 | 0 | – | – | ||||
| Dyspnea | 16 | 11 | 0 | 0 | 8 | 4 | 1 | 2 | – | – | 1.45 (0.79, 2.67) | 0 | 0.61 | 0.23 |
| Voice changes | – | – | 0 | 0 | – | – | – | – | – | – | ||||
| Dermatologic | ||||||||||||||
| Rash or desquamation | 4 | 1 | 6 | 0 | – | – | 0 | 0 | – | – | ||||
| Hand-foot skin reaction | 25 | 0 | 11 | 0 | 34 | 4 | 2 | 1 | – | – | 11.78 (5.16, 26.93) | 22 | 0.28 | <0.00001 |
| Alopecia | 1 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | ||||
| Pruritus | 1 | 0 | 0 | 0 | – | – | – | – | – | – | ||||
I, intervention; C, control; CI, confidence interval; RR, risk ratio; P-H, P-value of Q for heterogeneity test; I2, 0–25%, no heterogeneity; 25–50%, modest heterogeneity; ≥50%, high heterogeneity; Hb, hemoglobin.
Discussion
This meta-analysis aimed to identify the therapeutic effect and associated adverse events of the currently used multikinase inhibitors sorafenib, sunitinib, pazopanib and axitinib in advanced RCC. The results demonstrate that individual use of sorafenib, sunitinib, pazopanib and axitinib is more effective in terms of PDR and ORR. However, the results also show that multikinase inhibitors are associated with a higher risk of grade 3 or 4 adverse events for hypertension and gastrointestinal effects, including diarrhea, nausea and vomiting. Although much progress has been achieved in understanding the molecular mechanism of advanced RCC and in the development of targeting drugs, the overall efficiency of these therapies is far from satisfactory. Considering the poor physical condition of patients with advanced RCC, the appropriate selection of treatments and the proper management of adverse events are required to improve the quality of life of such patients.
Although the combination of bevacizumab and IFN may provide a higher ORR compared with either agent alone, the higher frequency or grade of adverse events may be unbearable for some patients (23). However, although the individual use of sorafenib, sunitinib or temsirolimus is less toxic, the efficiency of these drugs in improving the ORR is limited (23). In clinical practice, the balance between therapeutic effect and associated adverse events should be carefully evaluated prior to the final selection of a treatment. Previous studies observed that patients suffered hypertension as early as the first day of taking sorafenib (24) and axitinib (25). Rini et al (26) observed that the median time of all-grade hypertension onset was independent of baseline antihypertensive use. However, the onset of grade 3 or 4 hypertension occurred significantly earlier in patients that received antihypertensive medications than in those that did not at baseline. Thus, patients receiving antihypertensive medication might be more biologically susceptible to earlier axitinib-induced hypertension (26). Therefore, it is recommended that clinicians consider collaborating with patients and other practitioners to manage treatment-induced hypertension actively. Prior to treatment, it is necessary for practitioners to assess the blood pressure status, cardiovascular risk factors and previous antihypertensive medication use of the patients. Based on this assessment, patients may be divided into normotensive, uncontrolled hypertensive, sub-optimally medication-controlled hypertensive or medication-controlled hypertensive groups (25). For patients with uncontrolled hypertension, short-acting antihypertensive agents could be provided. Following the initiation of multikinase treatment, short-acting antihypertensive agents may be switched to long-acting agents. For patients with sub-optimally controlled hypertension, treatment-induced hypertension could be controlled through increasing the dose of current medication or adding new medication. Pretreatment evaluation of risk factors of cardiovascular diseases, such as peripheral vascular disease, diabetes mellitus, cardiac conditions and renal disease is essential since patients with these risk factors may require close monitoring and the aggressive management of cardiovascular events during treatment (27). In addition, clinicians should to check whether patients have received hypertension-inducing agents such as hormones or steroids, which may complicate the management of axitinib-induced hypertension (28). It is recommended that during treatment with multikinase inhibitors, blood pressure should be monitored regularly in-clinic and at home. In order to gain a higher level of compliance of the patient to treatment, blood pressure assessment prior to treatment, including an assessment of the likelihood of hypertension developing during therapy and home monitoring could be provided. The clinician could support patients by training them and their family members in the proper use of the blood pressure-monitoring device, calibration of the device and recording and the reporting of measurements.
Gastrointestinal adverse events, including diarrhea, nausea and vomiting were commonly reported for these multikinase inhibitors. Although these events might not lead to treatment discontinuation and can be properly managed by pharmacological intervention and dietary modifications, in elderly patients, these events might lead to serious dehydration if they are not well controlled (29). Previous studies also reported that treatment-related diarrhea can be persistent for the duration of multikinase therapy and mild-to-moderate diarrhea can reduce the mobility and independence of patients, impairing quality of life (30–32). Therefore, it is recommended that clinical guidelines for the management of cancer treatment-related gastrointestinal adverse events should be followed (33). The major limitation of this study is the small number of studies involved. This small selection of studies was not adequate to perform patient subgroup analyses.
In conclusion, multikinase inhibitors can significantly control disease progress and improve the ORR. However, they are also associated with a higher risk of grade 3 and 4 hypertension and gastrointestinal events. Proper management of these events is necessary to improve quality of life.
References
- 1.Decastro GJ, McKiernan JM. Epidemiology, clinical staging and presentation of renal cell carcinoma. Urol Clin North Am. 2008;35:581–592. doi: 10.1016/j.ucl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Garcia JA, Cowey CL, Godley PA. Renal cell carcinoma. Curr Opin Oncol. 2009;21:266–271. doi: 10.1097/CCO.0b013e32832a05c8. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 5.van Spronsen DJ, de Weijer KJ, Mulders PF, De Mulder PH. Novel treatment strategies in clear-cell metastatic renal cell carcinoma. Anticancer Drugs. 2005;16:709–717. doi: 10.1097/01.cad.0000167901.58877.a3. [DOI] [PubMed] [Google Scholar]
- 6.Van Veldhuizen PJ, Hussey M, Lara PN, Jr, et al. A phase II study of gemcitabine and capecitabine in patients with advanced renal cell cancer: Southwest Oncology Group Study S0312. Am J Clin Oncol. 2009 doi: 10.1097/COC.0b013e3181925176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178:1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Xu CF, Bing NX, Ball HA, et al. Pazopanib efficacy in renal cell carcinoma: evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol. 2011;29:2557–2564. doi: 10.1200/JCO.2010.32.9110. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Escudier B, Rini B, et al. Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial. Br J Cancer. 2013;108:1571–1578. doi: 10.1038/bjc.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escudier B, Pluzanska A, Koralewski P, et al. AVOREN Trial Investigators: Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim HL, Belldegrun AS, Freitas DG, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170:1742–1746. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley … Sons, Inc; Chichester, UK: 2008. [DOI] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 20.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol. 2013;14:1233–1242. doi: 10.1016/S1470-2045(13)70464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudier B, Albiges L, Sonpavde G. Optimal management of metastatic renal cell carcinoma: Current status. Drugs. 2013;73:427–438. doi: 10.1007/s40265-013-0043-1. [DOI] [PubMed] [Google Scholar]
- 24.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-bractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 26.Rini BI, Quinn DI, Baum M, et al. Hypertension among patients with renal cell carcinoma receiving axitinib or sorafenib: analysis from the randomized phase III AXIS trial. Target Oncol. 2014 doi: 10.1007/s11523-014-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmonds K, Hull D, Spencer-Shaw A, et al. Strategies for assessing and managing the adverse events of sorafenib and other targeted therapies in the treatment of renal cell and hepatocellular carcinoma: recommendations from a European nursing task group. Eur J Oncol Nurs. 2012;16:172–184. doi: 10.1016/j.ejon.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien BE, Kaklamani VG, Benson AB., III The assessment and management of cancer treatment-related diarrhea. Clin Colorectal Cancer. 2005;4:375–381. doi: 10.3816/CCC.2005.n.009. discussion 382–373. [DOI] [PubMed] [Google Scholar]
- 30.Bellmunt J, Eisen T, Fishman M, Quinn D. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2011;78:24–32. doi: 10.1016/j.critrevonc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 31.La Vine DB, Coleman TA, Davis CH, Carbonell CE, Davis WB. Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma. Am J Clin Oncol. 2010;33:217–220. doi: 10.1097/COC.0b013e3181a650a6. [DOI] [PubMed] [Google Scholar]
- 32.Dutcher JP, Tannir N, Bellmunt J, Escudier B. Experience with sorafenib and the elderly patient. Med Oncol. 2010;27:1359–1370. doi: 10.1007/s12032-009-9388-4. [DOI] [PubMed] [Google Scholar]
- 33.Benson AB, III, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]