Abstract
The aim of the present study was to investigate the effect of microRNA (miR)-92a on cystatin C expression in patients with type II diabetes and lower limb ischemia. A total of 199 patients diagnosed with type II diabetes were included in the study and divided into three experimental groups: Simple type II diabetes mellitus (T2DM; n=60) group; type II diabetes with light to moderate occlusion (LLI-LM; n=70) group; and the type II diabetes with severe occlusion (LLI-S; n=69) group according to the patient ankle-brachial index score. In addition, 60 healthy individuals were examined as a control population. The expression levels of various biochemical indices were detected, including cystatin C in the peripheral blood. The expression levels of miR-92a and cystatin C mRNA were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and the correlation between miR-92a, cystatin C and the pathological development of type II diabetic lower limb ischemia was analyzed. The protein expression levels of cystatin C were detected using western blot analysis. Bioinformatic analysis indicated that miR-92a was able to downregulate cystatin C expression, and this result was supported by endothelial cell transfection. In the transfection assay, an miR-92a mimic downregulated cystatin C expression, while an miR-92a inhibitor upregulated cystatin C expression. The results of the RT-qPCR indicated that the expression levels of miR-92a in the LLI-S group were reduced compared with those in the T2DM and LLI-LM groups, and significantly lower compared with those in the negative control group. Platelet-derived miR-92a appeared to downregulate cystatin C expression in patients with type II diabetes and lower limb ischemia. Therefore, the combined detection of miR-92a and cystatin C may be useful as a method for clinically screening patients with type II diabetes for lower limb ischemia.
Keywords: type II diabetic lower limb ischemia, platelet, miR-92a, cystatin C
Introduction
Lower limb ischemia typically occurs as a result of peripheral arterial disease (PAD) and manifests with foot ulcers and necrotic lesions that may require amputation, severely affecting patients' quality of life (1). Early-stage diabetes may be accompanied by vascular malformation, atherosclerosis and thrombotic plaque (2). Diabetic lower limb ischemia frequently occurs in diabetic patients with an extensive medical history. In the early stages of ischemia, the disease presents with no clear symptoms. However, following the emergence of clinical symptoms, irreversible pathological damage may occur in the blood vessels, which complicates treatment. Therefore, methods for the early diagnosis of diabetes-associated lower limb ischemia are required to reduce the risk for diabetic patients with PAD and ensure quality of life. A previous study indicated that the systemic inflammation factor C-reactive protein (CRP) may function as a diagnostic marker for increased risk of PAD (3). However, CRP is not a specific diagnostic marker for PAD and there are a number of limitations to its use (4).
The cysteine proteinase inhibitor cystatin C is composed of 120 amino acid residues and is expressed in various organisms, including plants, animals and protozoa. The gene encoding cystatin C is located on band 2, area 13 in the short arm of human chromosome 20. The gene is ∼4.3 kb in length, including 3 exons and 2 introns, and may be consistently transcribed and translated in all nucleated cells (5). Under physiological conditions, cystatin C is able to inhibit endogenous cysteine protease activity. Previous studies demonstrated that cystatin C is a sensitive indicator for evaluating early kidney damage; however, recent studies have indicated that the imbalanced expression of cystatin C is notably associated with the occurrence and development of cardiovascular diseases, including hypertension, coronary heart disease, atherosclerosis and diabetic lower limb ischemia (6–9). Furthermore, Liu et al observed a significant association between the expression of cystatin C and diabetic lower limb ischemia, with elevated expression of cystatin C indicating an increased risk of lower limb ischemia in diabetic patients (10).
MicroRNAs (miRNAs) are a group of small non-coding RNAs, typically 18–22 nucleotides in length, that are able to regulate gene expression. miRNAs regulate target genes by interfering with transcription or inhibiting translation, in addition to participating in numerous signaling pathways. miRNAs are widely expressed in various body fluids, including blood (plasma, blood platelet, red blood cells and nucleated blood cells) and urine, and are not degraded by endogenous RNA polymerases (11). In addition, numerous reports have indicated that blood miRNAs function as signaling molecules in intercellular signaling pathways (12,13). miRNAs serve crucial functions in multiple cardiovascular cell processes, including development, proliferation, migration, apoptosis, metabolism, damage, regeneration, repair and phenotype change. miRNAs also participate in the occurrence and development of numerous cardiovascular diseases, including coronary heart disease, myocardial infarction, heart failure, hypertension, arrhythmia, myocardial fibrosis, cardiac hypertrophy and heart failure (14–16). Furthermore, miRNAs possess marked tissue, pathological and normal-state specificity and sensitivity, which are criteria of ideal biomarkers. In the process of atherosclerosis, circulating platelets in the blood may directly adhere to vascular lesion sites and release various regulatory factors, including miRNAs, accelerating disease progression (17).
The present study aimed to investigate the association between miR-92a and cystatin C expression levels. In addition, the potential use of miR-92a as a new diagnostic marker for diabetic lower limb ischemia was further analyzed.
Materials and methods
Patient selection
Between May 2012 to December 2013, 199 patients diagnosed with diabetes were enrolled in this study. Patients were grouped according to their ankle-brachial index (ABI) score, as follows: Patients with an ABI score of 0.91–1.30 formed the simple type II diabetes mellitus group (T2DM group; n=60), patients with an ABI of 0.41–0.90 comprised the diabetes with light to moderate lower limb ischemia group (LLI-LM group; n=70) and patients with an ABI <0.40 comprised the diabetes with severe lower limb ischemia group (LLI-S group, n=69). The gender composition of each group was as follows: T2DM group, 30 males and 30 females; LLI-LM group, 40 males and 30 females; and the LLI-S group, 38 males and 31 females. Furthermore, 60 healthy outpatients were selected at random over the same period as a control population, which consisted of 32 males and 28 females. Patients with diabetes (screened using the oral glucose tolerance test), hypertension, other endocrine and metabolic diseases, or a family history of diabetes were excluded from the control population. In addition, subjects were excluded if they exhibited infection, diabetic ketoacidosis, blood system diseases, and any history of cancer, hormone drug use or surgical stress. Prior written informed consent was obtained from all patients and the study protocol was approved by the ethics committee of Shandong Provincial Hospital (Jinan, China).
Clinical data and biochemical indices
Height, weight, waist and hip circumference, and systolic and diastolic blood pressure were measured for each patient, and the body mass index (BMI) and waist-hip ratio were subsequently calculated. Cystatin C was detected using immune transmission nephelometry (Shanghai Beijia Medical Devices Co. Ltd., Shanghai, China). Total cholesterol (TC) and fasting plasma glucose (FPG) were detected using the Total Cholesterol and Blood Glucose Test kits (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) according to the manufacturers' instructions. Triglyceride (TG) levels were determined using the TG Assay kit (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.) according to the manufacturers' instructions. High density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were detected using a HDL-C Assay kit (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.). Hemoglobin A1c (HbA1c) was detected by high pressure liquid chromatography (HLC-723G7; Tosoh, Tokyo, Japan) and fasting insulin (FINS) using an Insulin Assay kit (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). Homeostasis model assessment of insulin resistance (HOMA-IR) was employed, using the formula: HOMA-IR = [FINS (mU/l) × FPG (mmol/l)]/22.5. Patient bilateral limb ABI data were obtained using a Philips iU22 Doppler Ultrasound system (Philips Healthcare, Best, The Netherlands). ABI was calculated using the formula: ABI = ankle artery systolic/brachial artery systolic blood pressure.
Leukocyte-depleted platelet (LDP) preparation
Peripheral blood samples were collected and citrate dextrose (85 mM trisodium citrate, 78 mM citrate and 111 mM glucose) was then added, followed by centrifugation at 80 × g for 10 min. EDTA (2 mM) was added to the platelet-rich plasma, and platelets were precipitated by centrifugation at 1,000 × g for a further 10 min. The pellet was resuspended in 3 ml bead buffer (0.8% NaCl, 0.02% KCl, 0.144% Na2HPO4, 0.024% KH2PO4, 0.5% bovine serum albumin and 2 mM EDTA) and 40 µl human CD45 MicroBeads reagent (Miltenyi Biotec, Bergisch Gladbach, Germany) was added, followed by incubation at room temperature for 45 min with gentle mixing. A MACS magnetic bead separation system (Miltenyi Biotec) was employed to separate LDPs and obtain platelets with >99.99% purity.
Bioinformatics
The online prediction software packages miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/), miRanda (http://www.microrna.org/) and Targetscan (http://www.targetscan.org) were used to predict genes potentially involved in the regulation of cystatin C expression, using the cystatin C gene sequence. The presence of any predicted gene in platelets, and its effect when transfected into endothelial cells were subsequently investigated.
Cell culture and transfection
Human pulmonary artery endothelial cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and maintained in a tissue-culture incubator at 37°C with an atmosphere of 95% O2 and 5% CO2.
For cell transfection, pGCMV/EGFP/miR-92a mimic and inhibitor plasmids were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA) and were transfected into the endothelial cells using Liposome 2000 (Invitrogen Life Technologies) according to the manufacturer's instructions. Briefly, cells at the logarithmic phase were seeded into 24-well plates at 2×105/well one day prior to transfection. The following day, at a cell confluence of 80%, the cells were transfected with pGCMV/EGFP/miR-92a mimic or inhibitor using Liposome 2000 and incubated with OptiMEM I (Invitrogen Life Technologies) for 6 h. Endothelial cells without transfection (endothelial cell control, EC) and with mock transfection (negative control, NC) were used as controls. At 4 h after transfection, the transfection medium was replaced with DMEM high glucose culture medium containing 10% FBS. Cells were collected at 48 h following transfection for further analysis.
SYBR-green fluorescence reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the patient's platelets or transfected endothelial cells using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. The extracted RNA was reverse transcribed into cDNA using an M-MLV reverse transcription kit (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's instructions. Following the reverse transcription, fluorescence qPCR was performed. Primer sequences were as follows: Cystatin C, forward 3′-AGA TCT ACG CTG TGC CTT GG-5′ and reverse 3′-CAG AGC CTG TGG GGT AAA CA; miR-92a, forward 3′-ACT ATT GCA CTT GTC CCG-5′. The reaction system was composed of 12.5 µl SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.), 1 µl PCR forward primer, 1 µl reverse primer or Uni-miR qPCR Primer (Takara Biotechnology Co., Ltd.), 2 µl cDNA template and 8.5 µl ddH2O, with a total volume of 25 µl. Each sample was evaluated in triplicate. The amplification program was set up as follows: Pre-denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. The 2−ΔΔT ± standard error of the mean was used to calculate the relative miRNA expression level. The U6 gene was used as an internal control.
Western blot analysis
At 48 h after transfection, the endothelial cells were collected, lysed and centrifuged at 12,000 × g for 5 min at 4°C, and the supernatant was retained. A 50-µg protein sample was subjected to SDS-PAGE (10%; Beyotime Institute of Biotechology, Haimen, China) and the proteins were subsequently transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membrane was blocked with non-fat milk for 1 h at room temperature. Next, monoclonal mouse anti-human anti-cystatin C primary antibodies (1:1,000; #ab24327; Abcam, Cambridge, MA, USA) were added and the membranes were incubated overnight at 4°C. After washing three times with Tris Buffered Saline with Tween® 20 (TBST; Bioscience & Biotechnology Co., Ltd., Shanghai, China), polyclonal goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies (1:2,000; #ab6789; Abcam) were added and the membranes were incubated for 2 h at room temperature. Image Lab imaging software (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to perform data analysis. β-actin was used as an internal control and the relative levels of cystatin C protein were calculated against the levels of β-actin.
Statistical analysis
All preliminary data were tested for normality and processed using the SPSS statistical software, version 16.0 (SPSS Inc., Chicago, IL, USA). Final data are expressed as the mean ± standard deviation. Analysis of variance was used for multi-group comparison, and the t-test was used for comparison between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of clinical data
To compare the differences in clinical features, clinical data of each group was compared. The BMI, waist circumference and waist-hip ratio of the T2DM, LLI-LM and LLI-S groups were significantly higher compared with those of the control group (P<0.05). The waist circumference of the LLI-S group was significantly higher compared with those of the T2DM and LLI-LM groups (P<0.05), while no statistically significant difference was observed between the T2DM and LLI-LM groups. The waist-hip ratio of the LLI-S group was significantly higher compared with that of the T2DM group (P<0.05), but not that of the LLI-LM group. The systolic blood pressure in the LLI-LM and LLI-S groups was significantly higher compared with those of the control and T2DM groups (P<0.05), and the value of the LLI-S group was the highest among the groups. Furthermore, the difference between the systolic blood pressure in the control and T2DM groups was not statistically significant. The diastolic blood pressure in the LLI-S group was the highest among the groups. The diastolic blood pressure in the T2DM, LLI-LM and LLI-S groups was significantly higher compared with that in the control group. In addition, the diastolic blood pressure in the LLI-LM and LLI-S groups was significantly higher compared with that in the T2DM group (P<0.05); however, the difference between the LLI-LM and LLI-S groups was not statistically significant (Table I). These results confirmed that patients in the LLI-S group were more serious cases of diabetic lower limb ischemia than the T2DM and LLI-LM groups were.
Table I.
Analysis of clinical and biochemical indices in each group.
| Variable | NC | T2DM | LLI-LM | LLI-S |
|---|---|---|---|---|
| Number (M/F) | 60 (32/28) | 60 (30/30) | 70 (40/30) | 69 (38/31) |
| Age (years) | 51.0±10.20 | 52.3±7.86 | 60.5±8.53a,b | 60.8±8.2a,b |
| BMI (kg/m2) | 22.32±1.73 | 23.68±2.03a | 24.75±3.13a | 26.42±3.51a,b |
| WC (cm) | 74.13±6.25 | 83.9±11.29a | 87.1±7.89a | 91.08±9.67a–c |
| WHR | 0.80±0.07 | 0.85±0.09a | 0.89±0.05a | 0.91±0.05a,b |
| SBP (mmHg) | 116±15 | 121±20 | 135±17a,b | 145±25a–c |
| DBP (mmHg) | 72±7 | 78±16a | 83±11a,b | 85±12a,b |
| FPG (mmol/l) | 5.18±1.59 | 8.13±2.23a | 8.26±2.81a | 8.34±2.63a |
| FINS (mU/l) | 6.85±2.58 | 9.12±4.32a | 10.35±4.12a,b | 10.40±4.43a,b |
| HOMA-IR | 1.57±1.86 | 3.16±2.03a | 3.80±2.12a,b | 3.85±1.96a,b |
| HbA1c (%) | 5.09±0.41 | 7.71±1.63a | 8.25±2.11a | 8.66±1.78a |
| TC (mmol/l) | 4.61±1.13 | 4.68±1.01 | 4.82±1.2 | 4.92±1.36 |
| TG | 1.39±1.40 | 1.43±1.23 | 1.51±1.19 | 1.59±1.53 |
| HDL-C (mmol/l) | 1.16±0.34 | 1.12±0.25a | 1.10±0.21a | 1.05±0.31a |
| LDL-C (mmol/l) | 2.28±0.67 | 2.43±0.81 | 2.85±0.75a,b | 2.65±0.82a,b |
| Cystatin C (mg/l) | 0.76±0.26 | 0.78±0.39 | 1.07±0.53a,b | 1.55±0.78a–c |
Data expressed as the mean ± standard deviation.
P<0.05 vs. the NC group
P<0.05 vs. the T2DM group
P<0.05 vs. the LLI-LM group. NC, negative control; T2DM, type II diabetes mellitus; LLI-LM, low to moderate lower limb ischemia; LLI-S, severe lower limb ischemia; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, hemoglobin Alc; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Comparison of biochemical indices in each group
Biochemical indices of each group were tested and compared among the groups. As presented in Table I, FPG, FINS, HOMA-IR and HbA1C values in the T2DM, LLI-LM and LLI-S groups were significantly higher compared with those in the control group (P<0.05). The FINS and HOMA-IR values in the LLI-LM and LLI-S groups were significantly higher compared with those in the T2DM group (P<0.05). HDL-C levels in the T2DM, LLI-LM and LLI-S groups were significantly lower compared with those in the control group (P<0.05). No significant differences were observed in the levels of FPG, HbA1C and HDL-C among the T2DM, LLI-LM and LLI-S groups. The LDL-C levels in the LLI-LM and LLI-S groups were significantly increased compared with those in the control and T2DM groups (P<0.05); however, the difference between the LLI-LM and LLI-S groups was not statistically significant. The expression levels of cystatin C in the LLI-LM and LLI-S groups were significantly higher compared with those in the control and T2DM groups, and were significantly higher in the LLI-S group compared with the LLI-LM group (P<0.05). The difference in cystatin C expression levels was not statistically significant between the T2DM and control groups. Furthermore, no statistically significant difference was observed in the TC and TG values among the groups (Table I). These results indicate that the expression levels of cystatin C are closely associated with the level of diabetic lower limb ischemia.
miR-92a suppresses the expression of cystatin C
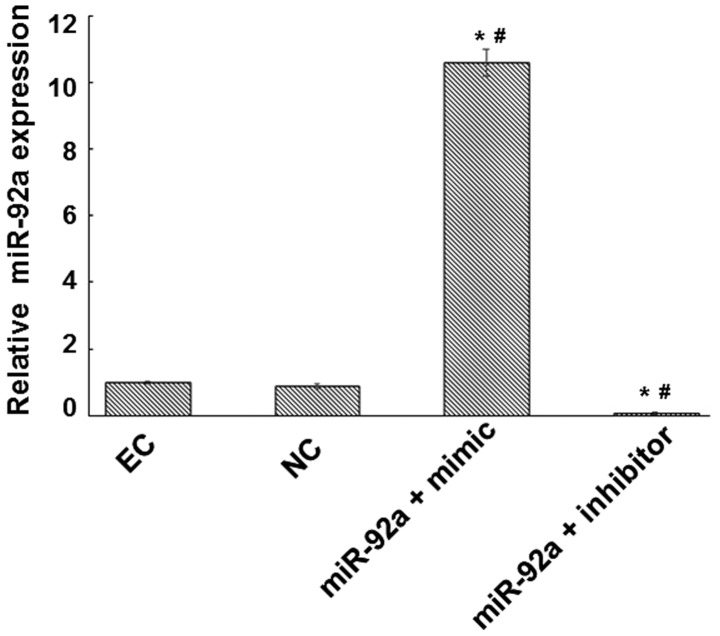
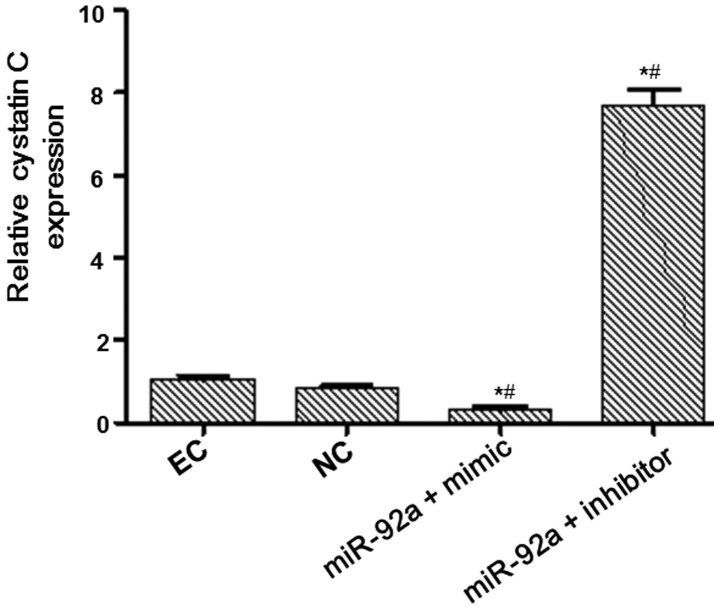
Bioinformatic analysis and associated confirmatory experiments were performed in order to identify an miRNA able to regulate cystatin C expression. miRWalk, miRanda and Targetscan suggested miR-92a as a potential candidate. Detection of miR-92a expression in platelets was subsequently performed, based on these bioinformatic results. Platelets are able to contact with and adhere to endothelial cells in the vascular surface during the atherosclerotic process. Therefore, an miR-92a mimic and an inhibitor were respectively transfected into endothelial cells to determine whether miR-92a was able to regulate cystatin C at the mRNA and protein levels. Fluorescence RT-qPCR was used to detect miR-92a expression and evaluate the transfection efficiency. The expression of miR-92a was increased by a factor of 10.57 in the miR-92a + mimic group and reduced by a factor of 14.29 in the miR-92a + inhibitor group compared with that in the NC group (Fig. 1). RT-qPCR and western blot analysis were performed to measure the mRNA and protein expression levels of cystatin C (Figs. 2 and 3). The mRNA and protein expression levels of cystatin C were significantly reduced in the miR-92a + mimic group compared with those in the NC group, and significantly increased in the miR-92a + inhibitor group. These results suggest that miR-92a is able to downregulate the expression of cystatin C at the mRNA and protein levels.
Figure 1.
Evaluation of transfection efficiency. Endothelial cells were transfected with miR-92a mimic or inhibitor. At 48 h after transfection, cells were collected and miR-92a expression was analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). U6 gene was used as an internal control. RT-qPCR results are presented. *P<0.05 vs. the control group. #P<0.05 vs. the NC group. EC, endothelial control; NC, negative control; miR, microRNA..
Figure 2.
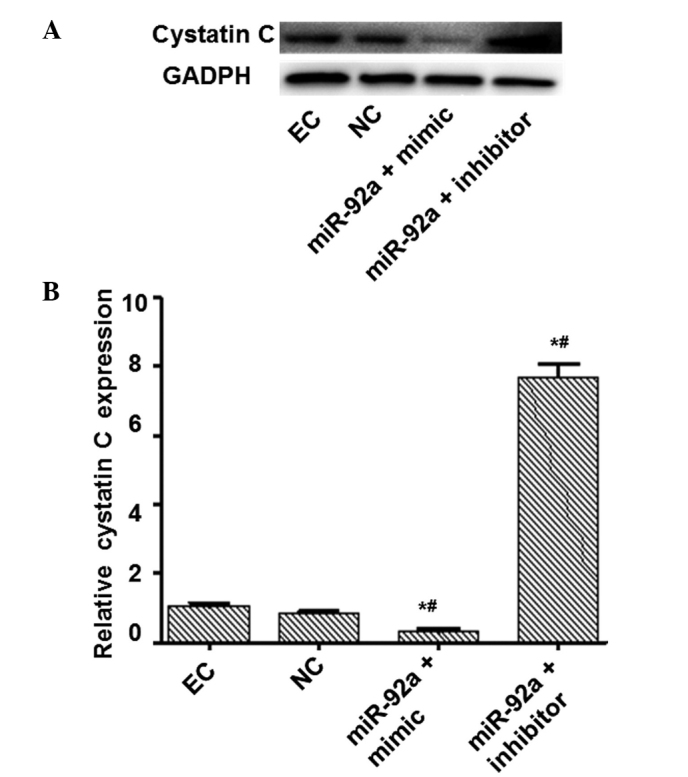
Analysis of cystatin C protein expression. Endothelial cells were transfected with miR-92a mimic and inhibitor. At 48 h after transfection, cells were collected and cystatin C protein expression levels determined by western blot analysis. GAPDH was used as an internal control. Representative and quantitative western blot analysis results are presented. *P<0.05 vs. the EC group. #P<0.05 vs. the NC group. EC, endothelial control; NC, negative control; miR, micro RNA.
Figure 3.
Analysis of cystatin C mRNA expression. Endothelial cells were transfected with miR-92a mimic or inhibitor. At 48 h after transfection, cells were collected and cystatin C expression at the mRNA level was analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). U6 gene was used as an internal control. RT-qPCR results are presented. *P<0.05 vs. the EC group. #P<0.05 vs. the NC group. EC, endothelial control; NC, negative control; miR, microRNA.
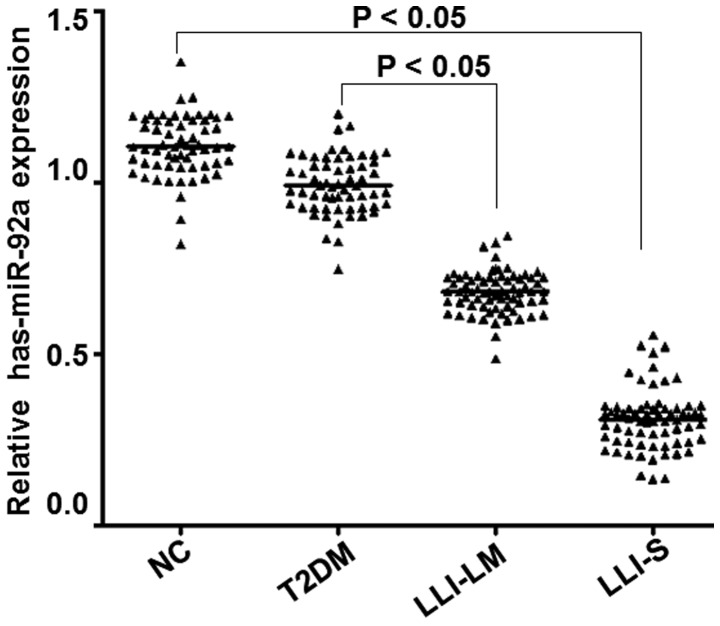
Expression of miR-92a in different groups
Fluorescence RT-qPCR was employed to detect the expression of the miR-92a gene in vivo, in order to determine whether miR-92a expression differed between groups. As presented in Fig. 4, miR-92a expression levels in the peripheral blood platelets of the LLI-LM and LLI-S groups were significantly reduced compared with those in the control and T2DM groups (P<0.05). Furthermore, miR-92a expression levels decreased with the severity of diabetic lower limb ischemia. In addition, miR-92a expression in the LLI-S group was significantly reduced compared with that in the LLI-LM group (P<0.05), whereas no significant difference was observed between the T2DM and control groups (P>0.05). These results indicate that miR-92a expression levels reduce in correlation with the severity of diabetic lower limb ischemia.
Figure 4.
Analysis of miR-92a expression in platelets of the control, T2DM, LLI-LM and LLI-S groups. Leukocyte-depleted platelets (LDPs) were isolated from peripheral blood of each group. miR-92a expression was determined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). U6 gene was used as an internal control. RT-qPCR results are presented. P<0.05 vs. the NC group. P<0.05 vs. the T2DM group. NC, normal control; T2DM, type II diabetes mellitus; LLI-LM, low to moderate lower limb ischemia; LLI-S, severe lower limb ischemia; miR, microRNA.
Discussion
Lower limb ischemia is a severe complication of diabetes. The condition results from atherosclerosis of the lower extremity arteries and the obstruction of blood circulation in the lower limbs. Numerous studies have suggested that a large number of platelets are involved in the atherosclerotic process and may promote plaque formation (18–21). Liu et al reported that the abnormal expression of cystatin C in serum may be used as a diagnostic indicator of diabetic lower limb ischemia (10). Furthermore, previous studies indicate that cystatin C is closely associated with the occurrence and development of cardiovascular diseases, and is abnormally expressed in diseases such as lower limb ischemia, hypertension, coronary heart disease and heart failure (22–24). Taglieri et al observed that high levels of serum cystatin C increased levels of CRP, induced inflammation, elevated concentrations of procoagulant factors and were closely associated with atherosclerosis (23).
In the present study, bioinformatic analysis was used to predict the genes that regulate cystatin C expression, and the results indicated that cystatin C may be a target of miR-92a. Transfection of miR-92a into endothelial cells demonstrated that miR-92a is able to regulate the expression of cystatin C. This observation suggests that in the pathological progression of atherosclerosis, platelets may release miR-92a directly into endothelial cells via cell-cell interactions and thereby regulate cystatin C expression. Furthermore, the expression levels of miR-92a were determined in the platelets of patients in the control, T2DM, LLI-LM and LLI-S groups. Data analyses demonstrated that the platelet-derived miR-92a was negatively correlated with serum cystatin C expression in patients. miR-92a expression was particularly low in the platelets of diabetic patients with severe lower limb ischemia, while serum cystatin C levels remained notably high. This result clearly indicates that with the detection of platelet-derived miR-92a and serum cystatin C expression, combined with the comprehensive evaluation of clinically relevant manifestations, the early diagnosis of diabetic lower limb ischemia may be accurately achieved. Early diagnosis of diabetic lower limb ischemia may aid the early detection and treatment of lower limb ischemic disease, thus reducing patient risk.
Acknowledgements
The authors thank Dr. Xing Jin for supervising this study.
References
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.CIR.97.5.425. [DOI] [PubMed] [Google Scholar]
- 4.Arain FA, Cooper Jr LT. Peripheral arterial disease: Diagnosis and management. Mayo Clin Proc. 2008;83:944–949. doi: 10.1016/S0025-6196(11)60771-4. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson M, Olafsson I, Palsdottir A, Ulvsbäck M, Lundwall A, Jensson O, Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: More than simply a marker of glomerular filtration rate. Clin Chem. 2005;5l:321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe S, Okura T, Liu J, Miyoshi K, et al. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895–899. doi: 10.1291/hypres.26.895. [DOI] [PubMed] [Google Scholar]
- 8.Arpegård J, Ostergren J, de Faire U, Hansson LO, Svensson P. Cystatin C - A marker of peripheral atherosclerotic disease? Atherosclerosis. 2008;199:397–401. doi: 10.1016/j.atherosclerosis.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 9.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Shen J, Zhao J, et al. Cystatin C: A strong marker for lower limb ischemia in Chinese type 2 diabetic patients? PloS One. 2013;8:e66907. doi: 10.1371/journal.pone.0066907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38:S2–S7. doi: 10.1038/ng1794. (Suppl) [DOI] [PubMed] [Google Scholar]
- 12.Stuwe E, Tóth KF, Aravin AA. Small but sturdy: Small RNAs in cellular memory and epigenetics. Genes Dev. 2014;28:423–431. doi: 10.1101/gad.236414.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Tao T, Xu B, et al. MiR-361-5p acts as a tumor suppressor in prostate cancer by targeting signal transducer and activator of transcription-6 (STAT6) Biochem Biophys Res Commun. 2014;445:151–156. doi: 10.1016/j.bbrc.2014.01.140. [DOI] [PubMed] [Google Scholar]
- 14.Loyer X, Potteaux S, Vion AC, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Belkin N, Feinberg MW. Endothelial microRNAs and atherosclerosis. Curr Atheroscler Rep. 2013;15:372. doi: 10.1007/s11883-013-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers KC, Rye KA, Tabet F. MicroRNAs in the onset and development of cardiovascular disease. Clin Sci (Lond) 2014;126:183–194. doi: 10.1042/CS20130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 18.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox JN, Smith KM, Williams LT, Schwartz SM, Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988;82:1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo Y, Ley KF. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004;14:18–22. doi: 10.1016/j.tcm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Nomura S, Suzuki M, Katsura K, et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 1995;116:235–240. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 22.Shi GP, Sukhova GK, Grubb A, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55:1932–1943. doi: 10.1373/clinchem.2009.128397. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe S, Okura T, Liu J, Miyoshi K, Fukuoka T, Hiwada K, Higaki J. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895–899. doi: 10.1291/hypres.26.895. [DOI] [PubMed] [Google Scholar]