Abstract
During behavioral quiescence the neocortex generates spontaneous slow oscillations that consist of Up and Down states. Up states are short epochs of persistent activity, but their underlying source is unclear. In neocortex slices of adult mice, we monitored several cellular and network variables during the transition between a traditional buffer, which does not cause Up states, and a lower-divalent cation buffer, which leads to the generation of Up states. We found that the resting membrane potential and input resistance of cortical cells did not change with the development of Up states. The synaptic efficacy of excitatory postsynaptic potentials mediated by non-NMDA receptors was slightly reduced, but this is unlikely to facilitate the generation of Up states. On the other hand, we identified two variables that are associated with the generation of Up states: an enhancement of the intrinsic firing excitability of cortical cells and an enhancement of NMDA-mediated responses evoked by electrical or optogenetic stimulation. The fact that blocking NMDA receptors abolishes Up states indicates that the enhancement in intrinsic firing excitability alone is insufficient to generate Up states. NMDA receptors have a crucial role in the generation of Up states in neocortex slices.
Keywords: slow oscillation, slice, cortex, Up state, Down state, arousal
neocortex slow oscillations, characteristic of slow-wave sleep and anesthesia in vivo (Steriade et al. 1993), can be generated in slices maintained in vitro (Castro-Alamancos 2009; Crunelli and Hughes 2010; McCormick et al. 2003). Slow oscillations are characterized by rhythmic cycles of synaptically mediated depolarization and action potentials (Up states) followed by decrease of synaptic inputs, leading to membrane hyperpolarization and cessation of firing (Down states). Slow oscillations consisting of Up and Down states occur spontaneously in isolated cortical slices of adult ferrets (Sanchez-Vives and McCormick 2000; Shu et al. 2003b), developing [postnatal day (P)14 to P21] (Wester and Contreras 2013) or adult (Cunningham et al. 2006) rats, and adult mice (Castro-Alamancos and Rigas 2002; Rigas and Castro-Alamancos 2007) bathed in an artificial cerebrospinal fluid (ACSF) that contains a concentration of divalent cations that resembles the “natural” CSF (i.e., ∼1 mM [Ca2+]o and [Mg2+]o). This concentration is lower than that in the traditional ACSF (i.e., 2 mM or higher) typically used in slices.
Slice studies have used this method to determine the balance of excitatory and inhibitory conductances during slow oscillations (Shu et al. 2003b), the impact of slow oscillations on afferent inputs (Rigas and Castro-Alamancos 2009; Shu et al. 2003a), the role of thalamocortical and intracortical afferents in controlling slow oscillations (Favero and Castro-Alamancos 2013; Rigas and Castro-Alamancos 2007), and the effects of neuromodulators on slow oscillations (Favero et al. 2012; Wester and Contreras 2013), among other studies.
For the most part, previous studies have not focused on the cellular and synaptic variables that change in cortical networks, as the ACSF is adjusted, to allow the generation of slow oscillations. This requires holding intracellular and extracellular recordings during the transition between before and after generation of slow oscillations. A previous study mentioned that the amplitude of postsynaptic potentials (PSPs) evoked by electrical stimulation did not change significantly in five cells held during this transition (see Methods in Reig et al. 2006). Unpaired comparisons between different cells in different ACSF conditions found that short-term depression was reduced by the ACSF that produces slow oscillations (Reig et al. 2006), which is expected in light of the known effects of reducing [Ca2+]o on short-term depression in neocortex (Castro-Alamancos and Connors 1997). Here we monitored cellular and synaptic properties of cortical networks during this transition in the same cells (paired comparisons). The results show that a main change in cortical networks associated with the development of slow oscillations in slices is the enhancement of NMDA synaptic responses. Together with the fact that slow oscillations are abolished by NMDA receptor antagonists, this highlights a critical role of these receptors in the generation of neocortex slow oscillations in vitro.
METHODS
All procedures were reviewed and approved by the Animal Care Committee of Drexel University. Slices were prepared as previously described (Favero et al. 2012; Favero and Castro-Alamancos 2013; Rigas and Castro-Alamancos 2007, 2009) from adult (>8 wk) CD-1 or Thy1-COP4/EYFP (line18) mice. Some CD-1 mice were anesthetized with ketamine-xylazine (100 and 5 mg/kg) and injected with AAV2/9-hSyn-ChR2(H134R)-eYFP (Univ. of Pennsylvania Vector Core) in the somatosensory thalamus (0.2–0.4 μl) with the following coordinates (from bregma): posterior 1.5, lateral 2.0, and ventral 3.0 mm. Slices were prepared from these animals 1–2 wk after the injection.
For slice preparation, mice were deeply anesthetized with an overdose of ketamine. Upon losing all responsiveness to a strong tail pinch, the animal was decapitated and the brain was rapidly extracted. Slices (400 μm thick) were cut in the thalamocortical plane (Agmon and Connors 1991) with a vibratome. Slices were transferred to an interface chamber, where they were bathed constantly (1–1.5 ml/min) with ACSF at 32.5°C. We used two ACSFs: control and low buffer. The control ACSF contained (in mM) 126 NaCl, 3.5 KCl, 1.25 NaH2Po4, 26 NaHCO3, 10 dextrose, 2 MgSO4·7H2O, and 2 CaCl2·2H2O. The low buffer ACSF was identical to the control ACSF except for MgSO4·7H2O and CaCl2·2H2O, which were lowered to 1 mM. Field potential (FP) recordings were made with low-impedance (∼0.5 MΩ) glass pipettes filled with ACSF. Blind whole cell recordings were obtained from layer V, IV, and III cells of somatosensory cortex with patch electrodes of 4–12 MΩ impedance. For current-clamp recordings, the electrodes were filled with internal solution containing (in mM) 135 K-gluconate, 4 KCl, 2 NaCl, 0.2 EGTA, 10 Tris-phosphocreatine, 0.3 TrisGTP, 10 HEPES, and 4 MgATP (290 mosM). Under our conditions, the Nernst equilibrium potential is −81 mV for Cl− and −96.7 mV for K+. In most cases, the internal solution contained neurobiotin (0.2%) to label the recorded cells. The results from cells located in different layers (V–III) were pooled together because we found no significant differences in the effects of the experimental manipulations.
After each experiment, the slices were fixed in 4% paraformaldehyde with 1% glutaraldehyde and later cryoprotected with sucrose (30%) and resectioned on a cryostat (80 μm). Nonfluorescent sections were incubated in 3% hydrogen peroxide, followed by 0.2% Triton X-100 and by incubation in 2% goat serum. Incubation with ABC reagent (Vector Labs) occurred overnight. The following day, diaminobenzidine was applied to the sections. After color development, sections were mounted and cleared in xylene. Fluorescent sections (from animals injected with AAV or line18) were mounted, coverslipped with DAPI mounting medium, and photographed with a fluorescent microscope. Fluorescent sections that contained neurobiotin-filled cells were incubated in 0.2% Triton X-100 and 2% goat serum followed by DyLight 594-Streptavidin. Sections were then mounted, coverslipped with DAPI mounting medium, and photographed with a fluorescent microscope. The enhanced yellow fluorescent protein (eYFP) from Channelrhodopsin-2 (ChR2) expression appears greenish, the neurobiotin-filled cell appears reddish, and DAPI appears blueish. Examples of these fluorescent sections and of reconstructed cells recorded in similar experiments can be found in previous publications (Favero et al. 2012; Favero and Castro-Alamancos 2013; Rigas and Castro-Alamancos 2007).
A concentric bipolar stimulating electrode (125-μm diameter) was used to electrically stimulate the cortex (intracortical). The intracortical electrical stimulating electrode was placed lateral to the recording electrodes (∼400 μm) in layers III and II. To derive an input-output curve, five stimulation intensities, typically between 10 and 50 μA, were used. A 200-μm-core diameter multimode optic fiber was used to apply pulses of blue light in the cortex (around the recording site). The light source was an LED (∼473 nm) driven by pulses of 0.2- to 5-ms duration (or longer to test whether the cell responded directly to the light). The blue light intensity was controlled by adjusting the output range of the light source or the duration of the pulse and could be easily monitored by recording the evoked short-latency FP response. In addition, the intensity (irradiance) of the light beam exiting the optic fiber was measured by flashing a photodiode power sensor placed in the location of the slice. The light intensity range was 0–8 mW/mm2, which corresponds to relative values of 0–5. The FP electrode was first used to identify the cortical region with a strong and short-latency response evoked by electrical stimulation and light. Intracellular recordings were then obtained adjacent to the FP electrode. Drugs were dissolved in ACSF at the indicated concentrations.
Electrical or blue light stimulations were applied at a minimum of 5 s apart and recurred at an interval of 5 s or higher. During each stimulus the membrane potential (Vm) could be set at different potentials by applying negative and positive current pulses (before the synaptic stimulus onset), up to the level that produced spontaneous firing. This allowed derivation of the reversal potential for each point of the synaptic response and estimation of the excitatory and inhibitory synaptic conductance (Gsynexc and Gsyninh, 0-mV and −75-mV reversal potential, respectively) before and during a drug (see Favero et al. 2012; Favero and Castro-Alamancos 2013 for details).
Up states were detected off-line by using a threshold detector in the FP recording (see Rigas and Castro-Alamancos 2007, 2009). Relatively rare transient FP events that are not Up states are easily rejected by setting the detection algorithm to reject short-duration events (<50 ms). In addition, all detected events are sent to a sorting algorithm (similar to those used to sort spikes), and this allows classification of all detected Up states based on several projections (e.g., principal components, etc.). Finally, all detected events (selected or unselected as Up states) are inspected by eye to ensure that the procedure was adequate.
For statistical analyses, data were first tested for normality with the Shapiro-Wilk test. If the data were considered normally distributed, parametric statistics were applied (repeated-measures ANOVA or paired t-test). Otherwise, we applied nonparametric statistics (Wilcoxon signed ranks for paired comparisons, Mann-Whitney for nonpaired comparisons, Kruskal-Wallis for multiple groups). To compare input-output curves or firing excitability between two conditions we used a two-factor repeated-measures ANOVA. The buffer factor had two levels (control and low buffer), and the intensity factor had as many levels as the intensities tested.
RESULTS
Low buffer leads to generation of Up states that depend on NMDA receptors.
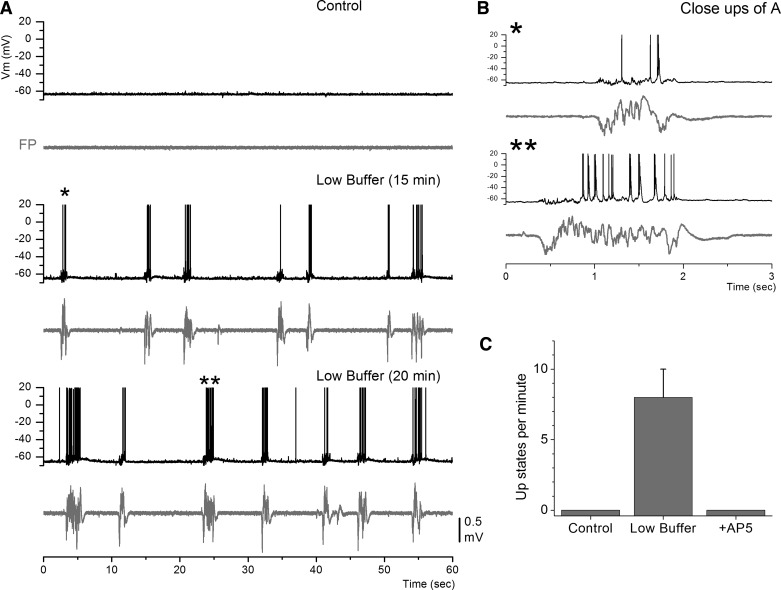
Traditionally, cortical slices of adult mice maintained in vitro are studied with ACSF buffer in which the concentration of divalent cations (Ca2+ and Mg2+) is 2 mM or higher (termed here “control buffer”). Under these conditions, adult cortical slices show nil spontaneous network activity. As described above, lowering the divalent cation concentration to ∼1 mM (termed here “low buffer”) in cortical slices of adult mice leads to the generation of spontaneous and evoked network activity that resembles cortical slow oscillations observed in vivo. Figure 1 shows the appearance of slow oscillations consisting of Up and Down states upon changing between control buffer and low buffer. During control buffer there was little or nil spontaneous network activity reflected in both the Vm of the recorded cell and the population FP. In contrast, after switch to low buffer, network activity consisting of Up and Down states readily emerged. This activity was rapidly and completely abolished by blocking NMDA receptors (Fig. 1C; d-AP5, 50 μM; n = 12), as previously described (Favero and Castro-Alamancos 2013). Note that Up states are not abolished by blocking AMPA receptors; instead, Up states become less frequent and shorter in duration but are readily evoked by electrical or optogenetic stimulation of cortical pathways (Favero and Castro-Alamancos 2013).
Fig. 1.
Low buffer generates spontaneous slow oscillations consisting of Up and Down states in neocortex slices. A: whole cell intracellular [membrane potential (Vm)] and field potential (FP) recordings from a cell in somatosensory cortex before and during low buffer. B: close-ups of Up states marked by asterisks in A. C: population data showing the abolishment of spontaneous Up states by application of d-AP5 (50 μM).
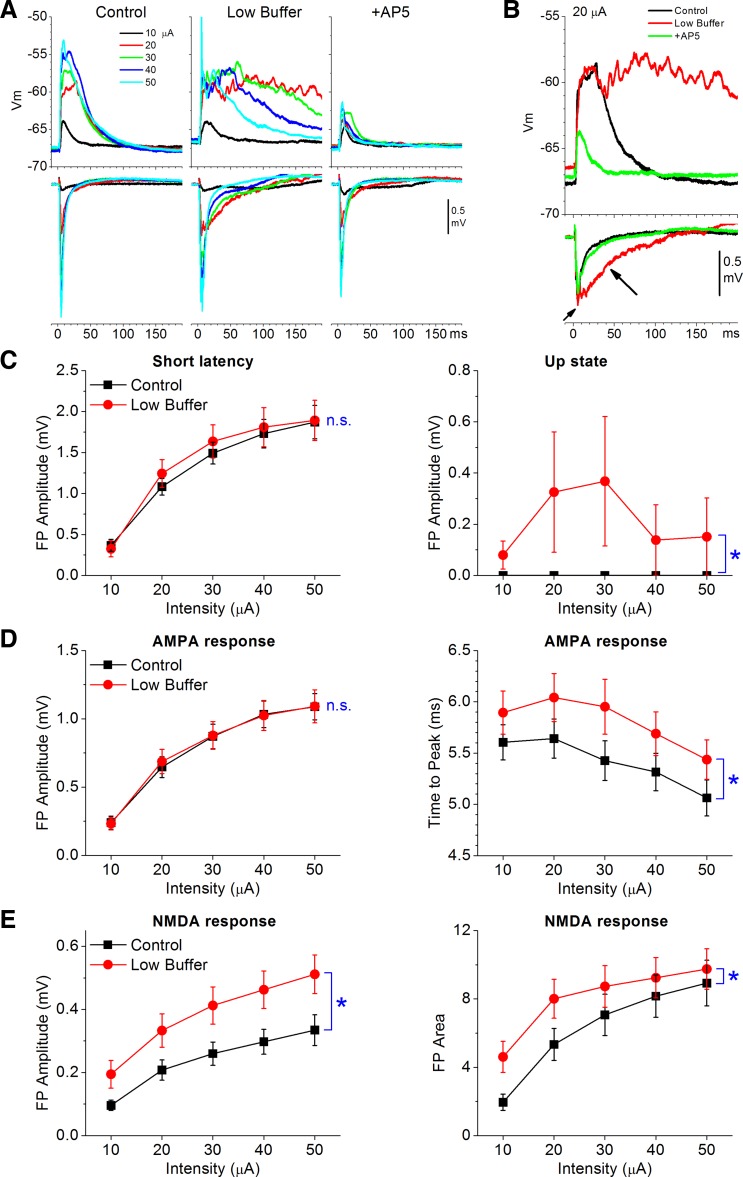
Similar to the effects on spontaneous Up states, low buffer also unmasks evoked Up states that depend on NMDA receptors. FP and intracellular responses were evoked by a stimulating electrode placed in layers III and II during control and low buffers. Figure 2, A and B, show a typical experiment in which the buffer was changed from control to low buffer. Each trace is an average of 5–10 FP and intracellular responses evoked by 5 different intensities (10–50 μA) to produce an input-output curve during each condition. Subsequent addition of AP5 (50 μM) completely abolished the evoked Up states, leaving only the non-NMDA-mediated response (termed AMPA response, for simplicity; note that GABA receptors are not blocked). As previously, we measured the amplitude of short-latency evoked FP responses (<15 ms) and the amplitude of the longer-latency evoked Up states (Favero and Castro-Alamancos 2013; Rigas and Castro-Alamancos 2007). Low buffer had negligible effects on the amplitude of the short-latency responses (see below) but led to the development of evoked Up states (Fig. 2C; n = 6, P < 0.001). Note also that evoked Up states are suppressed by higher intensities (because of stronger feedforward inhibition driven by cooperativity) (Favero and Castro-Alamancos 2013). This is reflected in the population data (Fig. 2C) because different slices have different thresholds for Up state induction and suppression.
Fig. 2.
Effect of low buffer on input-output curves of electrically evoked synaptic responses. A: example showing averaged synaptic responses (Vm and FP) evoked at different intensities by a stimulating electrode placed in layers III and II during control buffer, low buffer, and subsequent addition of AP5. B: overlaid responses evoked by 1 intensity (20 μA) for the data shown in A. Arrows indicate short- and long-latency responses. C: population data showing the effects of low buffer on the amplitude of short-latency (2–15 ms) and long-latency (15–50 ms; reflecting the Up states) FP responses under normal conditions (no glutamate receptor antagonists). D: population data showing the effects of low buffer on AMPA FP responses (in the presence of AP5): peak amplitude (left) and time to peak (right). E: population data showing the effects of low buffer on NMDA FP responses (in presence of CNQX): peak amplitude (left) and area (right) of FP response (3–50 ms). Note that low buffer enhances NMDA responses. *P < 0.01. n.s., Not significant.
Low buffer enhances NMDA-mediated responses.
The dependence of Up states on NMDA receptors led us to test the hypothesis that low buffer causes Up states by enhancing NMDA synaptic responses as a result of lowering the divalent cation concentration in the ACSF. At resting Vm, the NMDA receptor channel is blocked by Mg2+ depending on the [Mg2+]o in the ACSF; at lower [Mg2+]o, NMDA responses are potentiated (Mayer et al. 1984; Nowak et al. 1984). Cortical slices bathed in 1–2 mM [Mg2+]o are known to show evoked NMDA responses at resting Vm (Castro-Alamancos and Connors 1996; Espinosa and Kavalali 2009; Kanter et al. 1996). In contrast to the potentiating effects of lowering [Mg2+]o on NMDA synaptic responses, lowering [Ca2+]o is well known to suppress synaptic responses by reducing release probability (Katz 1969), including in neocortex (Castro-Alamancos and Connors 1997; Rozov et al. 2001). To decipher these contrasting effects on synaptic responses, we tested the effect of low buffer on isolated AMPA- and NMDA-mediated responses. We focused on evoked responses because spontaneous events (such as mini frequencies) are difficult to compare between control and low buffer conditions because of the absence of any spontaneous network activity during the former and the development of Up states during the latter.
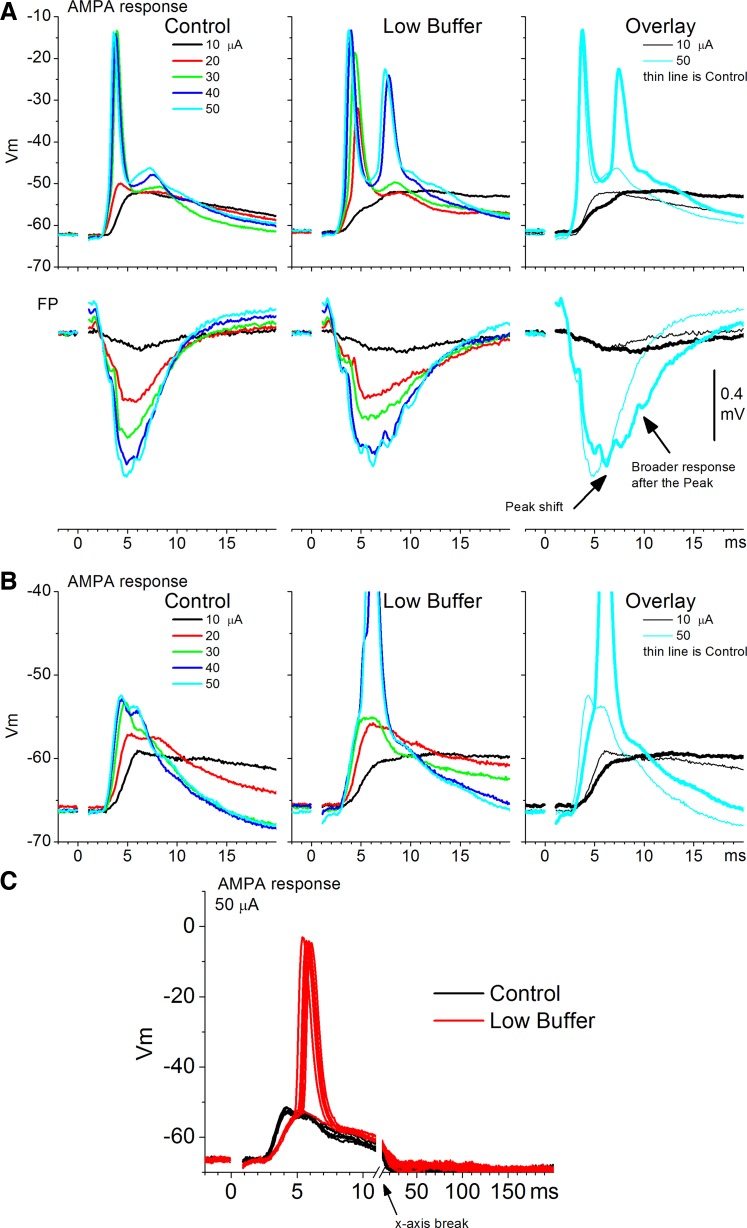
AMPA responses, measured in the presence of AP5 (50 μM), consist of sharp short-latency FP responses (<20 ms) coincident with intracellular PSPs. Low buffer had no significant effect on the peak amplitude of short-latency FP AMPA responses (n = 19 slices,; P = 0.8; Fig. 2D, left). Also, low buffer did not affect FP AMPA responses >20 ms, and thus did not generate evoked Up states (n = 19 slices, P = 0.4). However, low buffer had subtle but consistent effects on the short-latency AMPA response evoked by electrical stimulation. Figure 3 shows these effects in two example cells. First, low buffer slightly but significantly (n = 19 slices, P = 0.003) delayed the time to peak of the evoked FP response (Fig. 2D, right, and Fig. 3A). This change in the FP response was associated with a significant reduction in the rising slope of the evoked excitatory PSPs (EPSPs) in simultaneously recorded cells (n = 5 cells, P = 0.01; Fig. 3). Second, the short-latency FP response was slightly broadened so that the falling phase of this response occurred slightly later. The FP broadening was associated with an increase in spike probability in the evoked intracellular response on top of the slower rising EPSP slope (Fig. 3C). We return to this increase in firing excitability below.
Fig. 3.
Examples depicting the effect of low buffer on AMPA responses. A: an experiment showing simultaneous FP and intracellular recordings of AMPA responses evoked by electrical stimulation at different intensities during control and low buffer. Right: overlay of responses evoked by 2 intensities for comparison. Bottom: arrows indicate a shift in the peak and a broadening of the FP response. B: another experiment showing intracellular responses as described in A. C: individual traces used to derive the average traces shown in B for the 50-μA stimulus intensity. Note the reduction in the slope of the excitatory postsynaptic potential (PSP) and the enhanced spiking during low buffer.
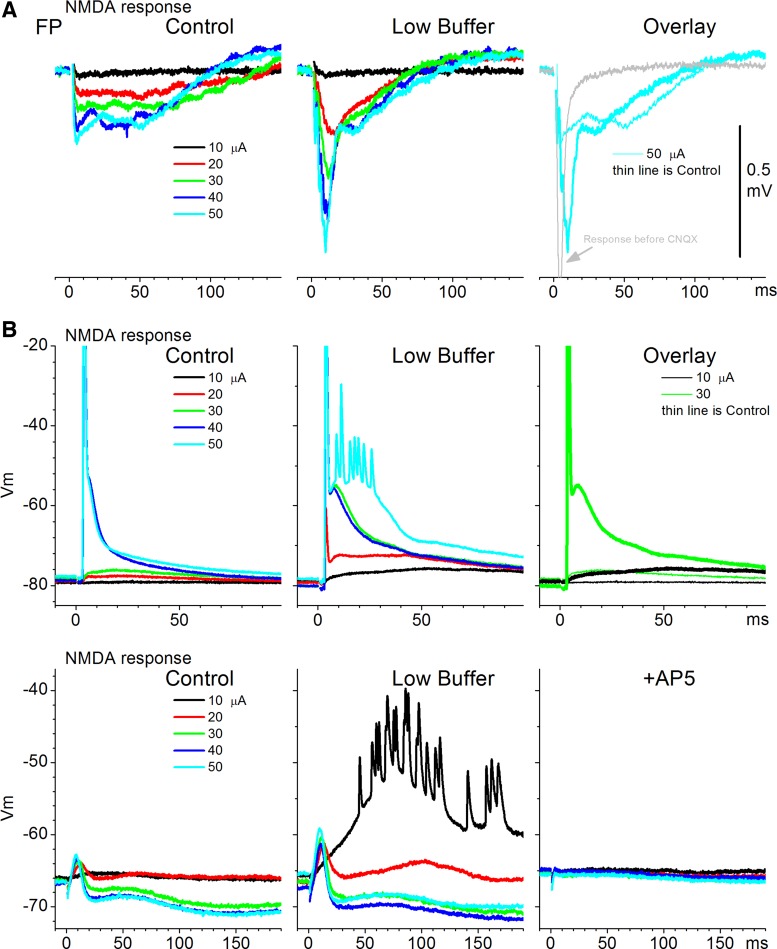
NMDA responses, measured in the presence of CNQX (10 μM), consist of slower-rising and longer-lasting FP and PSPs than AMPA responses (Fig. 2E and Fig. 4). Low buffer had a robust enhancing effect on both FP and intracellular NMDA responses, which were abolished by subsequent AP5. Figure 4 shows these effects in three example experiments: one FP (Fig. 4A) and two intracellular (Fig. 4B) recordings. The second cell example in Fig. 4B, bottom, showed an evoked EPSP followed by an inhibitory PSP (IPSP) at higher intensities; both were abolished by subsequent application of AP5, confirming that they were driven by NMDA receptors. Population measurements revealed that low buffer significantly increased both the peak amplitude (n = 21 slices, P < 0.001; Fig. 2E, left) and the area of the FP NMDA response (P < 0.001; Fig. 2E, right) measured between 3 and 50 ms after stimulus. The peak amplitude of intracellular EPSPs, excluding spikes, was also significantly enhanced by low buffer (n = 8 cells, P = 0.001).
Fig. 4.
Examples depicting the effect of low buffer on NMDA responses. A: 1 FP experiment is shown. NMDA FP responses are evoked by electrical stimulation at different intensities during control and low buffer. Right: overlay of the responses evoked by 1 intensity for comparison and the normal response evoked before addition of CNQX to isolate NMDA responses. B, top and bottom: 2 additional experiments. Intracellular NMDA responses are evoked by electrical stimulation at different intensities during control and low buffer. Top right: overlay of responses evoked by 2 of the intensities for comparison. Bottom right: responses evoked after AP5.
These results indicate that low buffer enhances NMDA-mediated responses and slightly suppresses the synaptic efficacy of AMPA responses. In addition, low buffer seems to enhance the firing excitability of cortical cells, which is addressed below.
Low buffer also enhances optogenetically evoked NMDA responses.
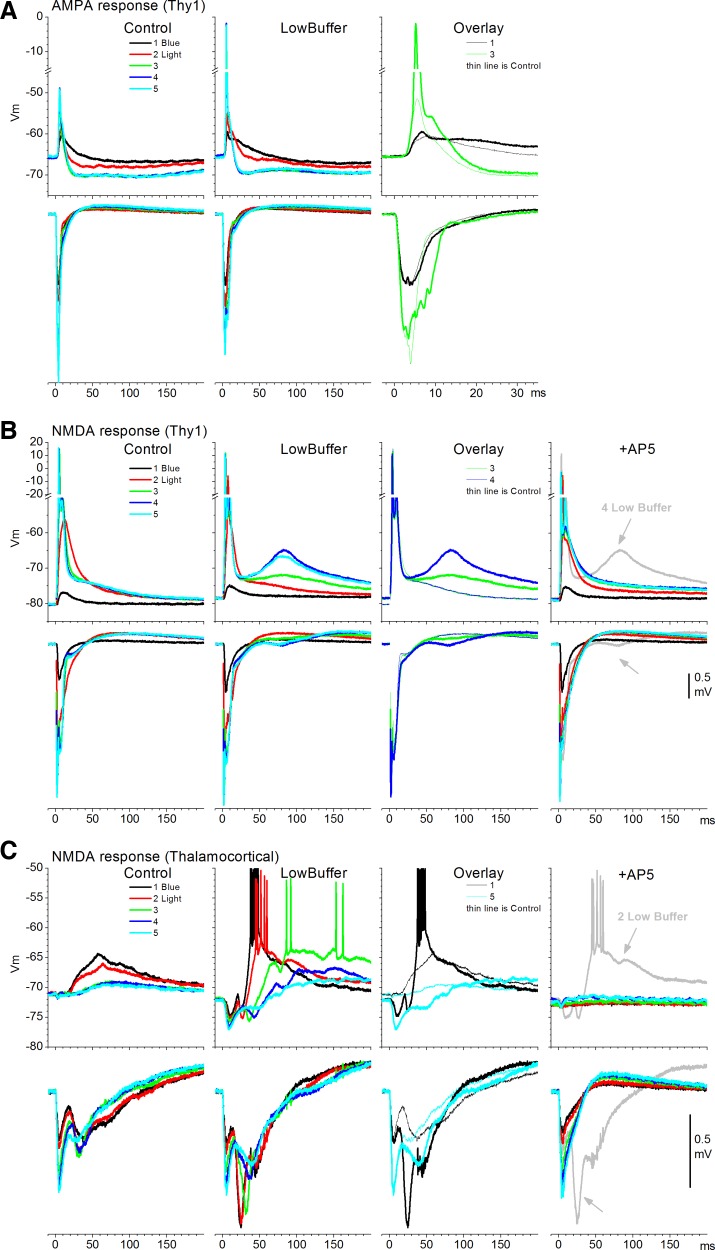
Electrical stimulation within neocortex is fairly unselective; it directly excites the membranes of all excitatory and inhibitory cells surrounding the stimulating electrode. To address this confound, we used slices from Thy1(line18) mice, which primarily express ChR2 in excitatory cortical cells (Wang et al. 2007). In these mice, layer V pyramidal cells of the somatosensory cortex robustly express ChR2 (Favero and Castro-Alamancos 2013). We also used a few CD-1 (n = 4) mice that express ChR2 in thalamocortical fibers. This was accomplished by infusing an AAV into the somatosensory thalamus of CD-1 mice to express ChR2 in thalamocortical fibers (Cruikshank et al. 2010; Favero and Castro-Alamancos 2013; Zhang et al. 2006, 2007). Note that FP and intracellular responses evoked by optogenetic stimulation (blue light) have been characterized previously in these mice (Favero and Castro-Alamancos 2013). In Thy1 slices, many cortical cells respond directly to blue light, and FP responses have a short-latency response component driven by direct activation of responding cells, which is not abolished by glutamate receptor antagonists or TTX. In CD-1 slices that express ChR2 in thalamocortical fibers, cortical cells do not respond directly to blue light, but the FP has a short-latency component that reflects the direct activation of thalamocortical fibers, which is also not abolished by the above-mentioned blockers. Here the effect of low buffer was tested on isolated AMPA and NMDA responses evoked by a spot of blue light delivered by an optic fiber centered in layer IV.
Figure 5 shows examples obtained from three experiments. In the first case (Fig. 5A), AMPA responses (during AP5) were evoked by five different intensities of blue light in a Thy1 slice. As per electrical stimulation, the spike probability increased during low buffer compared with control, and there was also a corresponding broadening of the evoked FP response. Similar effects were obtained in four other cells. Population measurements of FP responses revealed that low buffer did not significantly affect the peak amplitude of short-latency AMPA responses in Thy1 mice (n = 18, P = 0.4). Also, low buffer did not unmask long-latency responses (>20 ms). Measurement of the rising slope of EPSPs evoked in intracellular recordings by blue light did not reveal a significant change (n = 5 cells, P = 0.3). This was different compared with electrically evoked responses, which decreased, and may be due to the fact that ChR2 channels provide an additional Ca2+ source (Zhang et al. 2006), which may counteract the effect of lowering [Ca2+]o on synaptic transmission.
Fig. 5.
Effect of low buffer on optogenetically evoked responses. A: an experiment showing simultaneous FP and intracellular AMPA responses evoked by blue light pulses at different intensities during control and low buffer in a Thy1 slice. Right: overlay of responses evoked by 2 intensities for comparison. B: another experiment showing simultaneous FP and intracellular NMDA responses evoked by blue light pulses at different intensities during control and low buffer in a Thy1 slice. Third panel from left: overlay of responses evoked by 2 intensities for comparison. Fourth panel: effect of AP5 on the responses evoked by the 5 intensities and 1 of the traces before AP5 (low buffer) for comparison. This cell responded directly to blue light (in the presence of glutamate receptor antagonists), indicating that it expressed Channelrhodopsin-2 (ChR2). C: a third experiment showing simultaneous FP and intracellular NMDA responses evoked by blue light pulses at different intensities during control and low buffer in a slice expressing ChR2 in thalamocortical fibers. Third panel from left: overlay of responses evoked by 2 intensities for comparison. Fourth panel: effect of AP5 and 1 of the traces before AP5 (low buffer) for comparison. AP5 abolished the intracellular responses and most of the FP response except for the short-latency fiber volley reflecting the direct activation of thalamocortical fibers.
Figure 5B shows NMDA responses (during CNQX) evoked by five different intensities of blue light in a Thy1 slice in a cell that expresses ChR2. As per electrical stimulation, low buffer caused a significant enhancement of the NMDA response evoked by blue light, which appeared as a long-latency depolarization. In this cell, the unmasked NMDA response was of fairly long latency and subsequent application of AP5 completely abolished it. AP5 left intact the short-latency response, indicating that this cell expressed ChR2, responding directly to blue light in the presence of glutamate receptor antagonists. This was confirmed by using long pulses of blue light that directly depolarize the cell for the duration of the pulse, as previously shown (Favero and Castro-Alamancos 2013). Similar effects in cells expressing or not expressing ChR2 (see Fig. 5C) were obtained in six other cells. Population measurements of FP responses revealed that low buffer significantly increased the peak amplitude and the area of the evoked NMDA response (3–50 ms) in Thy1 mice (n = 26, P < 0.001).
Figure 5C shows NMDA responses (during CNQX) evoked by five different intensities of blue light in a slice from an animal expressing ChR2 in thalamocortical fibers. This cell showed NMDA responses at the lowest intensities, but these were inhibited at higher intensities (see Favero and Castro-Alamancos 2013). Low buffer caused the NMDA-evoked responses to become much larger and also revealed IPSPs driven by the thalamocortical stimulation, indicating that the enhancement of the NMDA response produced a stronger thalamocortical response that more effectively drove inhibitory interneurons (Cruikshank et al. 2010; Sun et al. 2006). As expected, the intracellular NMDA-evoked response was completely abolished by AP5. The postsynaptic FP response was also abolished, except for the thalamocortical fiber volley that is driven by ChR2 ion channels (Favero and Castro-Alamancos 2013). Similar results were obtained in three cells. Population measurements of FP responses revealed that low buffer significantly increased the area of the evoked NMDA FP response (3–50 ms) in slices expressing ChR2 in thalamocortical fibers (n = 5, P < 0.001). Thus low buffer appears to also potentiate NMDA responses driven by thalamocortical fibers, which may explain the high effectiveness of thalamocortical stimulation in triggering Up states (Favero and Castro-Alamancos 2013; Rigas and Castro-Alamancos 2007).
Effect of low buffer on isolated IPSPs.
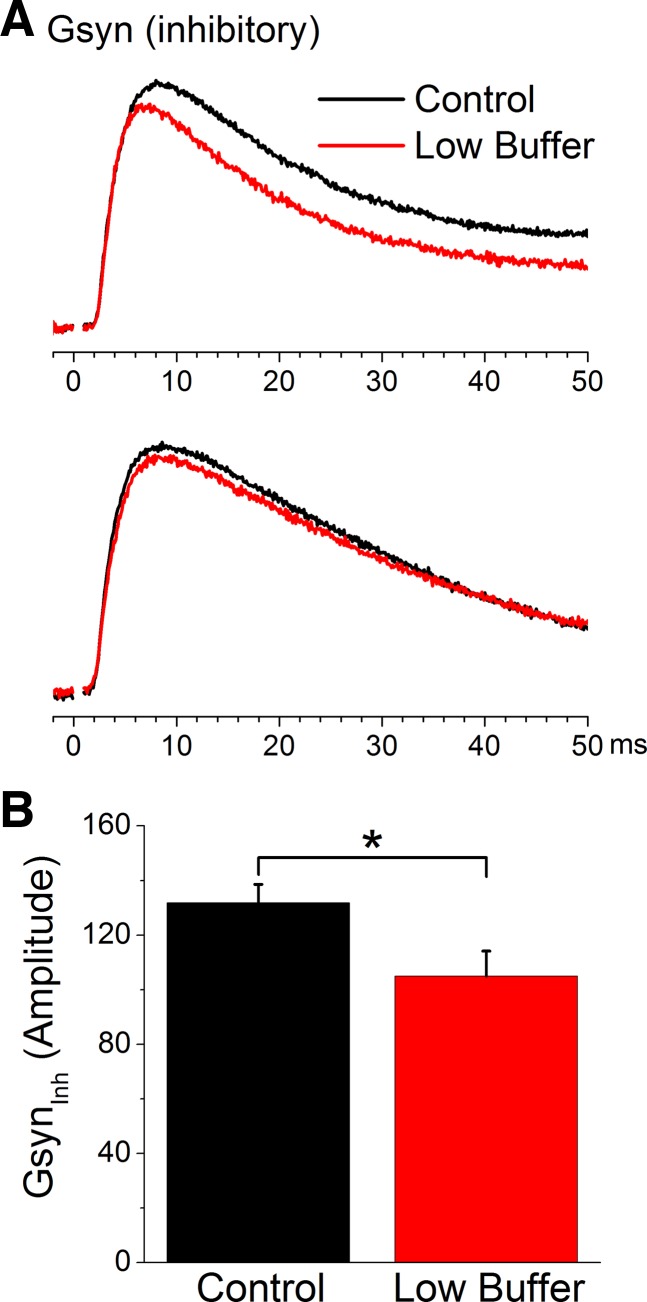
We next determined the effect of low buffer on isolated IPSPs evoked in the presence of glutamate receptor antagonists (CNQX and AP5, 10 and 50 μM, respectively) by a stimulating electrode located adjacent (200–400 μm lateral) to the recorded cells within layers V–III. We estimated the inhibitory synaptic conductance from synaptic potentials evoked at different Vm set by variable current pulses (see Favero et al. 2012; Favero and Castro-Alamancos 2013). Figure 6A shows the effect of low buffer on two different cells. Both of these cells show a relatively small suppression of the evoked inhibitory conductance. However, this effect was statistically significant when considering the population of cells (n = 8, P = 0.002; Fig. 6B). Thus low buffer produces a very small but significant suppression of the isolated inhibitory conductance.
Fig. 6.
Low buffer slightly suppresses the isolated inhibitory synaptic conductance (Gsyn) evoked by intracortical electrical stimulation. A: 2 examples showing the effect of low buffer on the inhibitory synaptic conductance derived from isolated inhibitory PSPs evoked at different Vm. B: population data showing the effects of low buffer on the peak amplitude of the isolated inhibitory synaptic conductance evoked by electrical stimulation. *P < 0.05.
Low buffer increases firing excitability.
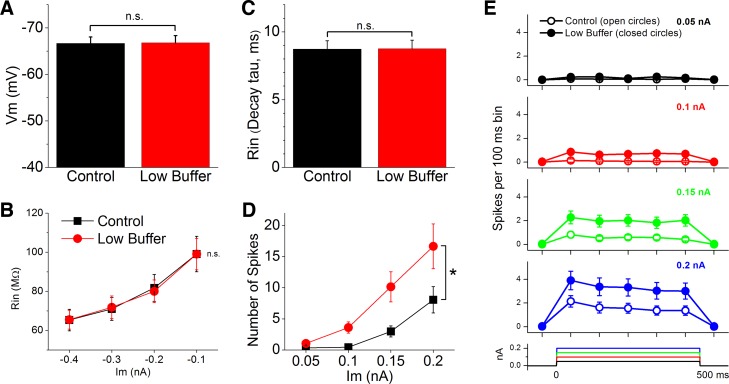
From the population of cells that were recorded during control and low buffer, we measured resting Vm, input resistance, and intrinsic firing. Vm did not change significantly as a consequence of low buffer for the whole population of cells measured (Fig. 7A; n = 39 cells, P = 0.87). To measure input resistance we applied negative current pulses of different amplitudes (−0.1 to −0.4 nA). Input resistance was estimated in two different ways. First, we measured the voltage drop caused by each of the negative current pulses (−0.4 to −0.1 nA; Fig. 7B). Second, we measured the decay time constant of the voltage after the offset of the −0.2-nA current pulse (Fig. 7C), which is less sensitive to changes in access resistance that may confound changes in input resistance. We found that low buffer did not cause a significant change in input resistance measured with either of these two methods (n = 32 cells, P > 0.5).
Fig. 7.
Low buffer increases firing excitability. A: effect of low buffer on resting Vm. B: effect of low buffer on input resistance (Rin) estimated by measuring the voltage drop caused by negative current pulses (Im) of different amplitudes. C: effect of low buffer on input resistance estimated by measuring the decay time constant (τ) after pulse offset. D: effect of low buffer on firing excitability estimated by measuring the number of spikes evoked by 500-ms positive current pulses of different amplitudes. *P < 0.05. E: same data shown in D but plotted as perievent time histograms with 100-ms bins.
The preceding results that tested evoked excitatory responses suggest that low buffer increases firing excitability. To measure intrinsic firing we applied positive current pulses (500 ms) of different amplitudes. The amplitude of the current pulses was the same for each cell before and after low buffer but varied slightly between cells as a function of input resistance (the mean currents applied ranged between 0.05 and 0.2 nA). We found that low buffer significantly increased the number of spikes evoked by the same current pulse (n = 32 cells, P < 0.001; Fig. 7, D and E). Thus low buffer increases intrinsic firing excitability.
DISCUSSION
Effects of low buffer.
The low buffer that leads to the generation of Up states in neocortex slices consists of [Mg2+]o and [Ca2+]o lowered from the traditional 2 mM to 1 mM. Here we show that low buffer is associated with a potentiation of NMDA-mediated responses and NMDA receptors are required for Up states to occur. Thus NMDA receptors seem to be at the basis of Up states in neocortex. Apart from enhancing NMDA receptor responses, we also found that low buffer produces two other major effects.
First, synaptic efficacy is slightly reduced. This is apparent in both the rising slope of the non-NMDA EPSPs and the amplitude of isolated IPSPs evoked by electrical stimulation and is a direct consequence of lowering [Ca2+]o on synaptic transmission (Katz and Miledi 1968). Importantly, even though lowering [Ca2+]o alone reduces synaptic efficacy in neocortex slices, it does not lead to the development of Up states (Castro-Alamancos and Connors 1997). Thus a reduction in synaptic efficacy caused by lower [Ca2+]o does not underlie Up state generation. It is also worth mentioning that a reduction in release probability in neocortex caused by lowering [Ca2+]o causes a significant increase in synaptic facilitation of subsequent inputs (Castro-Alamancos and Connors 1997; Katz and Miledi 1968), which could be somewhat supportive of ongoing Up states. Another important consideration is that low buffer involves lowering both divalent cations, so the suppressive effect of lowering [Ca2+]o on synaptic transmission is countered by the simultaneous lowering of [Mg2+]o, which is known to enhance synaptic transmission (Katz 1969). The reduction in synaptic efficacy caused by low buffer will be less than if only [Ca2+]o was lowered. In conclusion, the relatively small reduction in synaptic efficacy caused by low buffer is unlikely to be a major factor in the generation of Up states. Interestingly, the reduction in synaptic efficacy produced by low buffer was observed for synaptic responses evoked by electrical stimulation but not for responses evoked by blue light. This difference can be explained by the fact that ChR2 channels allow Ca2+ to flow through them, providing an additional Ca2+ source (Zhang et al. 2006) that can support synaptic transmission in ways different from electrical stimulation.
Second, low buffer consistently enhanced cell firing excitability. This is caused by the well-known actions of lowering the overall divalent cation concentration on ion channel gating. Lowering the cation concentration lowers the activation threshold of many channels, including Na+ channels (Frankenhaeuser and Hodgkin 1957; Hille 2001; Wang et al. 2004). It is also worth noting that lowering [Ca2+]o can lead to less efficient activation of calcium-activated potassium channels, which can facilitate excitability during sustained firing (Storm 1990). The enhanced firing excitability associated with low buffer can boost the network activity associated with Up states.
NMDA receptors and Up states.
NMDA receptors have been proposed to contribute to the ability of prefrontal cortical neurons to exhibit persistent activity related to short-term memory (Lisman et al. 1998). Several models have emphasized a critical role for NMDA receptor dendritic spikes (Schiller et al. 2000) in the generation of Up states (Antic et al. 2010; Kepecs and Raghavachari 2007; Milojkovic et al. 2005). NMDA receptors are thought to facilitate sustained recurrent excitation because of their slow kinetics and voltage dependence, and they can ease the propagation of bursting in cortical networks (Polsky et al. 2009).
Although block of non-NMDA and NMDA receptors has been reported to suppress spontaneous Up states (McCormick et al. 2003; Sanchez-Vives and McCormick 2000), the relative importance of NMDA compared with AMPA receptors in the generation of Up states was recognized recently (Favero and Castro-Alamancos 2013). In adult mice, we found that blocking NMDA receptors completely abolishes Up states while blocking AMPA receptors does not; the frequency and duration of spontaneous Up states are reduced by AMPA antagonists, but these are still evoked reliably by afferent stimulation. The fact that Up states are abolished by blocking NMDA receptors, together with the present findings showing that NMDA responses are robustly enhanced by the low buffer that triggers Up states, points toward a prominent role of NMDA receptors in the generation of Up states in slices.
Interestingly, spontaneous recurrent network activity, consisting of Up and Down states, has also been shown to arise in slices of developing mice (P14–P18) bathed in a “traditional” slice buffer (i.e., ∼2 mM [Ca2+]o and [Mg2+]o) (MacLean et al. 2005; Mao et al. 2001), although other studies using rats of the same age appear to have required low buffer (Wester and Contreras 2013). In any case, NMDA receptor responses of developing neocortex slices around this age have kinetics very different (i.e., much longer decay time constants) from adult slices (Carmignoto and Vicini 1992; Crair and Malenka 1995), which is also the case in other brain regions (Hestrin 1992; Lester et al. 1990). The longer decays of NMDA responses could facilitate the generation of Up states in developing slices bathed in 2 mM buffer.
NMDA receptors are well known to be located postsynaptically, where their properties have been extensively characterized (Dingledine et al. 1999). However, there is also growing evidence that NMDA receptors are located presynaptically at some synaptic connections in various cortical regions, including entorhinal cortex (Berretta and Jones 1996), visual cortex (Buchanan et al. 2012), and somatosensory cortex (Brasier and Feldman 2008), where they enhance neurotransmitter release (Corlew et al. 2008; Kunz et al. 2013). Thus it is possible that presynaptic NMDA receptors facilitate the generation of Up states by potentiating neurotransmitter release at specific synapses that are involved in the generation of Up states. Alas, testing the pre- versus postsynaptic contribution of NMDA receptors to the generation of Up states will be tricky because each cell serves both pre- and postsynaptic roles in highly interconnected and recurrent neocortex circuits. In addition, Up states are network events, and so blocking NMDA receptors in single or a few cells (e.g., through the patch internal solution) is unlikely to have a major impact on the network event.
An important question relates to the role of NMDA receptors in the generation of slow oscillations in vivo. NMDA receptors have been shown to mediate persistent visual-evoked responses in neocortex (Miller et al. 1989), and application of AP5 locally into the barrel cortex or thalamus attenuates spontaneous activity (Hirata and Castro-Alamancos 2006). In principle, it is possible that NMDA receptors have a critical role in the generation of Up states in vivo, but future work needs to address this question directly in vivo. Alas, this is not a trivial experiment to conduct in vivo because of various technical difficulties, such as the difficulty in blocking NMDA receptors in all cortical regions to avoid slow oscillations propagating from them to the test region or the confounds from the impact of cortical activity changes on thalamic activity that will feed back to the cortex.
Conclusions.
Here we monitored several cellular and network variables during the transition between a traditional buffer and the low buffer that leads to the generation of Up states in slices of adult rodents. We found variables that did not change during this transition, variables that changed but are unlikely to cause the development of Up states, and variables that changed and are associated with the development of Up states. Among the variables that did not change significantly were the resting Vm and input resistance of cortical cells. Among the variables that changed but are unlikely to facilitate the development of Up states was a reduction in synaptic efficacy associated with lowering [Ca2+]o. We identified two other variables that can be associated with the development of Up states: first, an enhancement of the firing excitability of cortical cells caused by lowering the overall concentration of divalent cations and second, an enhancement of NMDA-mediated responses caused by lowering [Mg2+]o. The fact that blocking NMDA receptors alone is sufficient to completely abolish Up states implies that NMDA receptors are critically important for the generation of Up states in cortical slices and that the change in firing excitability has only a supportive role.
GRANTS
This work was supported by the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.C.-A. conception and design of research; M.A.C.-A. and M.F. performed experiments; M.A.C.-A. and M.F. analyzed data; M.A.C.-A. interpreted results of experiments; M.A.C.-A. prepared figures; M.A.C.-A. drafted manuscript; M.A.C.-A. edited and revised manuscript; M.A.C.-A. and M.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alon Poleg-Polsky for comments on this article.
REFERENCES
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991. [DOI] [PubMed] [Google Scholar]
- Antic SD, Zhou WL, Moore AR, Short SM, Ikonomu KD. The decade of the dendritic NMDA spike. J Neurosci Res 88: 2991–3001, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75: 339–344, 1996. [DOI] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci 28: 2199–2211, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, Tudor Jones AA, Oyrer J, Sjöström PJ. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron 75: 451–466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258: 1007–1011, 1992. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Cortical up and activated states: implications for sensory information processing. Neuroscientist 15: 625–634, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci USA 93: 1335–1339, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Distinct forms of short-term plasticity at excitatory synapses of hippocampus and neocortex. Proc Natl Acad Sci USA 94: 4161–4166, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Rigas P. Synchronized oscillations caused by disinhibition in rodent neocortex are generated by recurrent synaptic activity mediated by AMPA receptors. J Physiol 542: 567–581, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist 14: 609–625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature 375: 325–328, 1995. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 13: 9–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci USA 103: 5597–5601, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999. [PubMed] [Google Scholar]
- Espinosa F, Kavalali ET. NMDA receptor activation by spontaneous glutamatergic neurotransmission. J Neurophysiol 101: 2290–2296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M, Castro-Alamancos MA. Synaptic cooperativity regulates persistent network activity in neocortex. J Neurosci 33: 3151–3163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M, Varghese G, Castro-Alamancos MA. The state of somatosensory cortex during neuromodulation. J Neurophysiol 108: 1010–1024, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol 137: 218–244, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357: 686–689, 1992. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- Hirata A, Castro-Alamancos MA. Relief of synaptic depression produces long-term enhancement in thalamocortical networks. J Neurophysiol 95: 2479–2491, 2006. [DOI] [PubMed] [Google Scholar]
- Kanter ED, Kapur A, Haberly LB. A dendritic GABAA-mediated IPSP regulates facilitation of NMDA-mediated responses to burst stimulation of afferent fibers in piriform cortex. J Neurosci 16: 307–312, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool, UK: Liverpool Univ. Press, 1969. [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol 195: 481–492, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Raghavachari S. Gating information by two-state membrane potential fluctuations. J Neurophysiol 97: 3015–3023, 2007. [DOI] [PubMed] [Google Scholar]
- Kunz PA, Roberts AC, Philpot BD. Presynaptic NMDA receptor mechanisms for enhancing spontaneous neurotransmitter release. J Neurosci 33: 7762–7769, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature 346: 565–567, 1990. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci 1: 273–275, 1998. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48: 811–823, 2005. [DOI] [PubMed] [Google Scholar]
- Mao BQ, Hamzei-Sichani F, Aronov D, Froemke RC, Yuste R. Dynamics of spontaneous activity in neocortical slices. Neuron 32: 883–898, 2001. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261–263, 1984. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex 13: 1219–1231, 2003. [DOI] [PubMed] [Google Scholar]
- Miller KD, Chapman B, Stryker MP. Visual responses in adult cat visual cortex depend on N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 86: 5183–5187, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J Neurosci 25: 3940–3951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465, 1984. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel B, Schiller J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J Neurosci 29: 11891–11903, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R, Gallego R, Nowak LG, Sanchez-Vives MV. Impact of cortical network activity on short-term synaptic depression. Cereb Cortex 16: 688–695, 2006. [DOI] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci 27: 4261–4272, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Impact of persistent cortical activity (up states) on intracortical and thalamocortical synaptic inputs. J Neurophysiol 102: 119–131, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol 531: 807–826, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature 404: 285–289, 2000. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci 23: 10388–10401, 2003a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003b. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26: 1219–1230, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, Wang D, Zhang F, Boyden E, Deisseroth K, Kasai H, Hall WC, Feng G, Augustine GJ. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci USA 104: 8143–8148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang J, Cottrell JE, Kass IS. Small physiologic changes in calcium and magnesium alter excitability and burst firing of CA1 pyramidal cells in rat hippocampal slices. J Neurosurg Anesthesiol 16: 201–209, 2004. [DOI] [PubMed] [Google Scholar]
- Wester JC, Contreras D. Differential modulation of spontaneous and evoked thalamocortical network activity by acetylcholine level in vitro. J Neurosci 33: 17951–17966, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature 446: 633–639, 2007. [DOI] [PubMed] [Google Scholar]