Abstract
There is mounting evidence for the idea that performance in a visuomotor rotation task can be supported by both implicit and explicit forms of learning. The implicit component of learning has been well characterized in previous experiments and is thought to arise from the adaptation of an internal model driven by sensorimotor prediction errors. However, the role of explicit learning is less clear, and previous investigations aimed at characterizing the explicit component have relied on indirect measures such as dual-task manipulations, posttests, and descriptive computational models. To address this problem, we developed a new method for directly assaying explicit learning by having participants verbally report their intended aiming direction on each trial. While our previous research employing this method has demonstrated the possibility of measuring explicit learning over the course of training, it was only tested over a limited scope of manipulations common to visuomotor rotation tasks. In the present study, we sought to better characterize explicit and implicit learning over a wider range of task conditions. We tested how explicit and implicit learning change as a function of the specific visual landmarks used to probe explicit learning, the number of training targets, and the size of the rotation. We found that explicit learning was remarkably flexible, responding appropriately to task demands. In contrast, implicit learning was strikingly rigid, with each task condition producing a similar degree of implicit learning. These results suggest that explicit learning is a fundamental component of motor learning and has been overlooked or conflated in previous visuomotor tasks.
Keywords: explicit learning, implicit learning, visuomotor rotation, motor adaptation, motor learning
visuomotor rotation tasks have proven to be a rich experimental paradigm for elucidating principles of sensorimotor learning (Cunningham 1989; for review, see Krakauer 2009). Generally, the goal of the task is to terminate a virtual cursor, controlled by motion of the hand, within a designated target region. During the learning phase, an angular rotation is imposed on the cursor and participants can learn to counter the rotation by moving their hand in an equal and opposite direction. When the rotation is removed, participants exhibit persistent aftereffects considered to be a hallmark of motor adaptation (for review, see Taylor and Ivry 2014).
Traditionally, learning was thought to arise predominantly from a single process—through the updating of an internal forward model based on sensory-prediction errors or the difference between predicted and actual cursor feedback (Synofzik et al. 2008; Tseng et al. 2007; Wolpert et al. 1998). Recent work, however, has shown that a number of other processes can operate during learning, such as reinforcement learning (Huang et al. 2011; Izawa and Shadmehr 2011), use-dependent plasticity (Verstynen and Sabes 2011; White and Diedrichsen 2010), and explicit strategies (Benson et al. 2011; Mazzoni and Krakauer 2006; Taylor and Ivry 2011). In particular, explicit aiming strategies are capable of fully compensating for a rotation in a single trial (Mazzoni and Krakauer 2006). Interestingly, however, as participants continue to use an explicit strategy, their reaches begin to drift in the direction of the strategy, increasing directional error and deteriorating performance (Mazzoni and Krakauer 2006). If training is continued further, this drift eventually reverses and task performance is restored (Taylor and Ivry 2011). This complex, nonmonotonic behavior can be explained by a dynamic interplay between explicit learning, related to fluctuations in aiming strategies, and implicit learning, related to the training of a forward model (Mazzoni and Krakauer 2006; Taylor and Ivry 2011).
It is worth noting that the operation of these distinct processes was only exposed when participants were provided with an explicit strategy at the beginning of the learning phase (Mazzoni and Krakauer 2006; Taylor and Ivry 2011). The degree to which explicit and implicit learning are present in standard visuomotor learning tasks has been debated, and a number of attempts have been used to dissociate their relative contributions through posttests (Hegele and Heuer 2010, 2013; Heuer and Hegele 2008), dual-task manipulations (Taylor and Thoroughman 2007, 2008), and modeling (Taylor and Ivry 2011). Recently, we developed a simple method to directly assess explicit strategies by having participants verbally report their aiming direction on each trial, which was made possible by including numbered visual landmarks in the workspace for participants to select (Taylor et al. 2014). We found that explicit and implicit learning processes were dissociable and followed unique time courses, with explicit learning exhibiting large fluctuations early in training before stabilizing late in training and implicit learning proceeding in a slow and gradual manner throughout training. Notably, participants who were asked to report their aiming strategies only learned at a slightly faster rate than participants who did not report aiming strategies—the standard procedure in visuomotor tasks—suggesting that aiming strategies may be present in standard visuomotor rotation tasks and that they may be a common compensation mechanism.
While this new approach revealed how the time course of explicit aiming strategies unfolds during training, it is uncertain how the shape of this time course responds to different task configurations and demands. In the previous study, we only tested participants with one configuration of visual landmarks used for reporting aiming strategies. It is possible that our previously characterized time course of explicit learning was a function of the configuration of visual landmarks and that, with a different configuration of landmarks, explicit learning would follow a different time course. For example, if the visual landmarks always aligned with the target location, it could potentially provide a strong cue to the participants and allow them to quickly determine the appropriate landmark to counter the rotation, leading to a steplike explicit learning function considering that they would simply have to report the same landmark for each trial. However, if the visual landmarks were unrelated to the target location, then it might be more difficult for participants to quickly figure out an appropriate aiming strategy, leading to a different time course of explicit learning. Thus the purpose of experiment 1 was to characterize the time course of explicitly learning an aiming strategy under different landmark conditions and to determine whether the shape of the previously reported time course of explicit learning was more general in nature. Similarly, in our previous study explicit learning was characterized by using only one rotation size (i.e., 45°) and eight target locations, and while the extent of explicit learning has been examined with different rotation sizes in prior studies, it was only assessed with posttests at the end of the experiment (Heuer and Hegele 2008). Therefore, in the present set of experiments, we sought to better characterize how explicit aiming strategies and implicit learning may change as a function of visuomotor rotation task demands. Specifically, we manipulated the orientation of the visual landmarks used for reporting aiming strategies, the number of training targets, and the size of the rotation. We found that aiming strategies were successfully employed under all task conditions, suggesting that explicit learning is highly flexible and rapidly accommodates different task demands. While aiming strategies increased in magnitude with the number of targets, implicit learning decreased. Moreover, aiming strategies scaled with the size of the rotation, while implicit learning did not. Altogether, we find that aiming strategies are more responsive to task demands than implicit learning and that overall learning in a visuomotor rotation task appears to be more reflective of explicit processes than implicit processes.
METHODS
Participants and Experimental Apparatus
Ninety young adults [52 women, 38 men; mean age 20 (SD 2.2) yr] participated in one of three visuomotor rotation experiments. Participants were recruited from the research participation pool maintained by the Department of Psychology at Princeton University and received either class credit or $12.00 for participation. All participants had normal or corrected-to-normal vision and were right-handed, as verified by the Edinburgh Handedness Inventory (Oldfield 1971). All participants provided informed consent, and the experimental protocol was approved by the Princeton University Institutional Review Board.
Participants performed a center-out reaching task, which required sliding their right hand across a digitizing tablet while holding a digitizing pen (Intuos 3, Wacom, Vancouver, WA). Reaching movements were directed to virtual targets located 7 cm from the start position, and the tablet sampled the movement trajectory at 100 Hz. All stimuli were displayed by a 17-in., 1,024 × 768-pixel-resolution LCD computer monitor with a refresh rate of 60 Hz (Dell, Dallas, TX). The monitor was mounted 25.4 cm above the tablet, which occluded visual feedback of the hand. Visual feedback for the movement trajectory, when provided, was in the form of a small circular cursor (0.31-cm radius). The game was controlled by custom software (Pygame, http://python.org) from a laptop computer (Apple, Cupertino, CA).
General Procedure
At the beginning of each trial, participants were required to position their hand at the center of the workspace (start position), which was indicated by a small empty circle (0.41-cm radius) at the center of a horizontally positioned visual display located ∼11.4 cm from the participants' chair. Participants were guided to the center by a larger empty circle that indicated the radial distance from their current position to the start position. Once the hand was within 0.5 cm of the start position, a small white cursor appeared indicating the hand position underneath the display. After participants maintained the start position for 1 s, a circular green target (0.60-cm radius) appeared. The number of target locations varied between experiments (see specific methodology for each experiment below).
Participants were instructed to make a ballistic-style reaching movement by “shooting” their right hand through the target, with additional instruction to refrain from stopping at the target. Movement onset was defined as exceeding a radial distance of 0.5 cm from the start position. Movement duration was quantified as the time from movement onset to cursor termination on the virtual ring. Only end-point feedback, presented as the static position of the cursor on the virtual ring (7 cm), was provided for each reach (except for the first 16 familiarization trials in the Baseline block, which provided continuous online feedback). For all other trials in the Baseline block, and all trials in the Baseline-Report block and the Rotation block, the white cursor was removed as soon as the participant's hand exceeded a radial distance of 0.5 cm from the start position and reappeared in the form of a red cursor when the participant's movement exceeded a radial distance of 7 cm. If the reach exceeded 400 ms in duration, participants received an auditory warning that the movement was “too slow.” End-point feedback showing where the cursor landed on the virtual ring was provided for 1.5 s before the display was cleared; then, participants were required to return to the start position using the guiding circle mentioned above. In all experiments, participants were instructed that the goal of the task was to land the red cursor on the green target. For each trial, if any part of the red cursor overlapped with the target participants were awarded with 1 point and a pleasant “ding” sound was played; otherwise, they failed to earn any points and an unpleasant “buzz” sounded. Scores were tallied throughout the experiment and displayed every 40 trials.
The visual workspace also included numbered landmarks presented on a virtual ring, whose orientation varied between conditions in experiment 1 (see below and Fig. 1). Participants were instructed to report their aiming location before initiating a movement. Specifically, they were instructed, “You may have noticed that there were little numbers flanking the target. I would like you to tell me, before moving, the number that you think you should aim to in order to get your cursor on the target. So if you think that you should aim directly at the target, then please say ‘green.’ But if you think that you should aim somewhere else, please tell me what that number is.” Occasionally, when a participant forgot to report the number of a landmark before moving, the experimenter gave him or her the following verbal reminder: “Please continue to report the number to which you are aiming before you start moving.” Moreover, every 40 trials, the game software reminded participants that “the goal of the task is to get the red cursor on the green target. Always aiming directly at the target may not be effective.” These instructions were provided automatically by the game software and read from a script by the experimenter to ensure that all participants were delivered the same instructions.
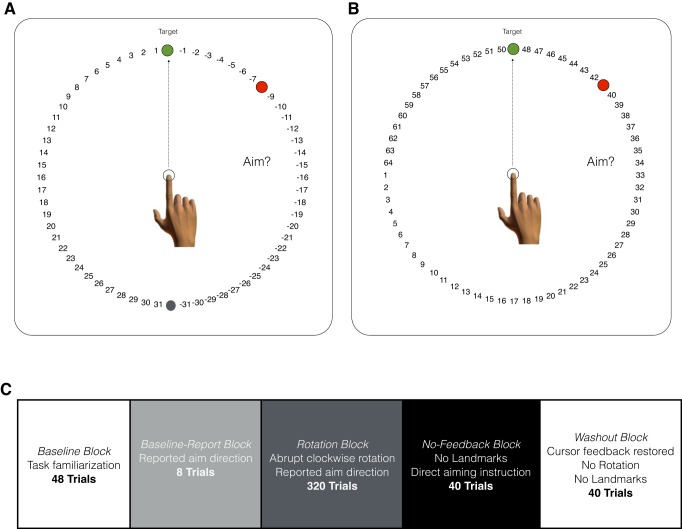
Fig. 1.
Task workspace and structure. A: Rotating condition: a circular array of numbered landmarks flanked each side of the target and rotated along with the target such that 1 and −1 were always adjacent to the target. This configuration was also used in experiments 2 and 3. B: Fixed condition: the numbered landmarks remained fixed relative to the workspace regardless of target location. Before each movement, participants were instructed to verbally report where they needed to aim to get their cursor on the target. C: task block structure.
All experiments followed the same five-block structure (Fig. 1C). In the first block, participants were provided with veridical feedback for 48 trials (Baseline block) to familiarize them with the task using continuous online feedback for the first 16 trials. At the start of the second block, participants were provided with the landmark reporting instructions as outlined above and continued to receive veridical feedback for the next eight trials (Baseline-Report block). Occasionally, these instructions prompted a few participants on some trials to aim to locations other than the target during this Baseline-Report block for reasons that are not entirely known. A clockwise rotation was abruptly introduced in the third block of 320 trials (Rotation block). In the fourth block of 40 trials, visual feedback of the cursor, the rotation, and the landmarks were all removed (No-Feedback block). Additionally, participants were provided with specific aiming instructions: “I want you to aim directly for the green target—so stop aiming to the numbers. You no longer need to report where you are aiming on each trial. In fact, the numbers will not even be present on the screen. We will also not show you feedback for a while. You will just hear a ‘knocking’ sound that will inform you that you reached far enough. Remember: just aim to the green target on every trial.” In the final block of 40 trials (Washout block), veridical cursor feedback was restored.
Experiment 1: Visual Landmark Orientation
Twenty participants were equally divided into two groups, which differed in the orientation of the landmarks. In the Rotating condition, the numbers rotated along with the direction of the target, such that they increased and decreased in the counterclockwise and clockwise directions, respectively. For example, if the target appeared at 90°, then the number 1 was in the counterclockwise direction from the target and the number −1 was in the clockwise direction (Fig. 1A). In the Fixed condition, the numbers did not rotate with the direction of the target but remained in a fixed orientation regardless of the target direction. For example, if the target appeared at 90° then the number 50 was in the counterclockwise direction from the target and the number 48 was in the clockwise direction (Fig. 1B). The target could appear at one of eight locations, which were separated by 45° along a virtual ring with a radius of 7 cm (0°, 45°, 90°, 135°, 180°, 225°, 270°, 315°). The sequence of target locations was pseudorandomly presented, such that each target location was experienced before a particular target location was repeated, and each participant received a different randomized sequence of target locations. In each condition, 63 landmarks were displayed at any given trial (including the gray target shown in Fig. 1A). For the Rotating condition, numbered landmarks ranged from 1 to 31 and −1 to −31, separated by gray and green targets. For the Fixed condition, numbered landmarks ranged from 1 to 64, and the green target was presented on top of one of the numbered landmarks as it followed its pseudorandomized location sequence. In experiment 1, the rotation was 45° in the clockwise direction.
Experiment 2: Number of Training Targets
Thirty participants were equally divided into three groups, which differed in the number of the targets. In the One-Target condition, there was one target in the 0° direction. In the Two-Target condition, there were targets in the 0° and 180° directions. Finally, in the Four-Target condition, there were targets in the cardinal directions of 0°, 90°, 180°, and 270°. Target locations for the Four-Target condition were pseudorandomized identically to experiment 1. For all conditions, the aiming landmarks were oriented such that they rotated along with the direction of the target (identical to the Rotating condition in experiment 1; Fig. 1A). The imposed rotation was 45° in the clockwise direction.
Experiment 3: Rotation Size
Forty participants were equally divided into four groups, which differed in the size of the rotation. In the Fifteen, Thirty, Sixty, and Ninety conditions, the rotation sizes were 15°, 30°, 60°, and 90°, respectively. There were eight possible target directions, which were pseudorandomized identically to experiment 1. The aiming landmarks rotated along with the direction of the target (identical to the Rotating condition of experiment 1; Fig. 1A).
Movement Analysis
Kinematic and statistical analyses were performed with MATLAB (MathWorks, Natick, MA). To assess task performance, we focused on the end-point hand angle measured when movements passed a radial distance of 7 cm. Each movement trajectory, regardless of the actual target location, was rotated to a common reference axis with the target location set at 0°. The end-point hand angle was computed by drawing a straight line between referent points positioned at the start position and 7 cm along the trajectory and computing the angle of this line. Positive angles indicate a counterclockwise deviation from the target, and negative angles indicate a clockwise deviation from the target. The end-point hand angles were then averaged in eight-trial bins (epicycles) for each participant for further analyses. We opted to average eight trials because our task included up to eight target directions that were pseudorandomized (when applicable; see above). Moreover, since the sequence of target locations (when applicable) was randomized across participants, this procedure also removes biases associated with specific target locations. Note that all data in figures are reported in end-point hand angles, not target errors.
Our planned analyses focused on four phases of the experiment. To verify that that there were no differences between groups prior to our experiment manipulations, we submitted the averaged eight-trial epicycle of the Baseline-Report block to a one-way ANOVA with Group as the single factor. Note that two-sample t-tests were used for all analyses of experiment 1, because they are equivalent to an ANOVA for two groups. The epicycle for the Baseline-Report block was then subtracted from all subsequent phases of end-point hand angles to remove any potential systematic biases in reaching.
To quantify learning, we focused our analyses on the first and last eight-trial epicycles of the Rotation block. The first epicycle was used as a proxy for the rate of learning, while the last epicycle was used to quantify the amount of learning. We performed two stages of analysis on each measure. First, for each group we submitted the rate and amount of learning to separate one-sample t-tests to determine whether there were significant changes with respect to the Baseline-Report block. Second, we examined whether there were differences in the rate and amount of learning between groups by submitting these values to separate one-way ANOVAs with Group as the single factor. Since these measures were subjected to two statistical analyses, we adjusted the α value to 0.025 for significance according to the Bonferroni correction.
To quantify the size of the aftereffect, we focused on the first epicycle of eight trials in the No-Feedback block. Similar to the analyses in the Rotation block, we first verified the presence of an aftereffect by submitting the difference between the No-Feedback block and the Baseline-Report block epicycles to a one-sample t-test. Subsequently, we tested for differences between groups by submitting these values to a one-way ANOVA with Group as the single factor. Note that since the epicycle is the average of eight trials, it reduces the apparent size of the aftereffect since significant unlearning can occur when feedback is present or absent (Kitago et al. 2013; Taylor et al. 2013, 2014; Taylor and Ivry 2013), but averaging over eight trials of an epicycle reduces any target direction bias. The α value was also adjusted to 0.025 because these measures were subjected to two statistical tests.
To quantify the contributions of explicit and implicit learning, we performed a procedure similar to that described above. Explicit learning was converted into a measure of the participants' aiming angle by multiplying the verbally reported landmark by the spacing of the numbered landmarks (5.625°) for each trial. Implicit learning was computed by subtracting the aiming angles from the end-point hand angle on each trial. Then, both explicit and implicit learning measures were averaged in eight-trial bins. Our analyses of explicit and implicit learning focused only on the first and last epicycles of the Rotation block. These values were subjected to a two-stage analysis, first verifying their change within group before comparing the differences between groups, so the α value was set to 0.025.
The purpose of experiment 1 was to examine whether explicit learning varied as a function of the visual landmarks; therefore, we also quantified the variability of aiming—the probability that participants changed their aim from one trial to the next, the magnitude of this change, and how likely participants were to switch aiming behaviors after a successful “hit” or an unsuccessful “miss” trial. To compute the probability of aim change, the verbally reported landmark on the current trial was compared to the previous trial. If these values were different, then that trial was marked with the value “1”; otherwise it was marked with the value “0.” These values were then averaged into bins of eight trials for each participant. The magnitude of aim was also computed as the difference between the verbally reported landmark on the current trial and the previous trial, but the magnitude of the difference was preserved and converted into an angular measure by multiplying by the landmark spacing constant of 5.625°. Since these values are discrete in nature, we focused on the average of the entire block rather than on any particular epicycle of trials. The likelihood of switching aiming strategies after a hit or miss trial was submitted to a two-way ANOVA with factors of Group and Outcome, with Outcome as a repeated measure.
For all measures subjected to statistical analyses, we report the mean and the 95% confidence interval of the mean. For all statistical tests, we report the uncorrected P value but note when it fails to pass correction for multiple comparisons according to the Bonferroni correction.
RESULTS
Experiment 1: Visual Landmark Orientation
All participants practiced reaching to the targets with veridical feedback during the Baseline block to become familiarized with the task. In the Baseline-Report block, participants continued to reach to the targets with veridical feedback while reporting the number of the landmark to which they were aiming. For participants in the Rotating condition, the landmarks rotated along with the target (Fig. 1A) and all participants reported aiming directly to the green target. In contrast, for participants in the Fixed condition, the landmarks remained fixed relative to the workspace (Fig. 1B); thus these participants needed to report a different landmark number on each trial if they continued aiming directly to the green target. Only 2 of 10 participants reported a landmark number that was not directly on top of the target, and this was generally directed toward an adjacent landmark for only a few trials. Moreover, participants did not substantially differ in their end-point hand angles during the epicycle of the Baseline-Report block (t18 = 1.92, P = 0.07), measuring 1.54 ± 0.78° and 0.38 ± 0.90° in the Rotating and Fixed conditions, respectively (see Fig. 3A).
Fig. 3.
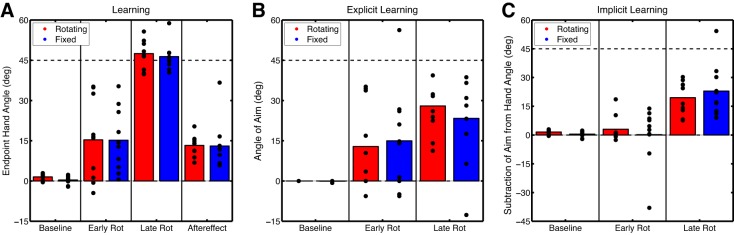
Experiment 1 phases of interest for each block. A: average end-point hand angles for the Baseline-Report block epicycle (Baseline) and first (Early Rot) and last (Late Rot) epicycles of the Rotation block and the aftereffect for the first epicycle of the No-Feedback block (Aftereffect). B: explicit learning: average angle of aiming location for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. C: implicit learning: subtraction of aiming location from end-point hand angle for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. Bars represent the group mean of each 8-trial bin (epicycle), and circles represent the individual participants for the Rotating and Fixed conditions.
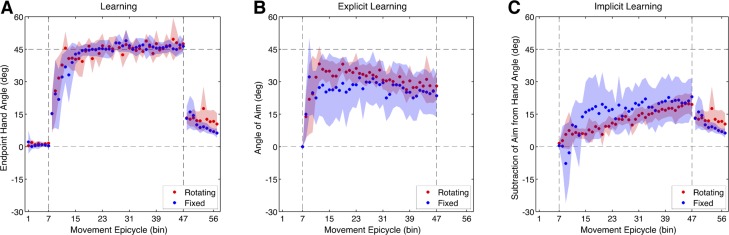
After the Baseline-Report block, a 45° clockwise rotation was introduced for 320 trials and participants in both groups quickly learned to counter the rotation (Fig. 2A). Participants in both the Rotating and Fixed conditions rapidly adjusted their end-point hand angles within the first epicycle of the Rotation block, measuring 15.3 ± 9.32° (t9 = 2.98, P = 0.015) and 15.2 ± 7.26° (t9 = 3.86, P = 0.004), but there were no differences between groups (t18 = 0.17, P = 0.87). By the last epicycle of the Rotation block, participants in both the Rotating and Fixed conditions had fully compensated for the rotation, measuring 47.5 ± 3.43° (t9 = 26.3, P < 0.001) and 46.4 ± 3.14° (t9 = 27.9, P < 0.001), respectively. There were no differences between groups in the amount of learning (t18 = 0.02, P = 0.99). Thus the difference in landmark orientation did not have a significant effect on the rate or amount of overall learning (Fig. 3A).
Fig. 2.
Experiment 1 learning time courses. A: end-point hand angle for Rotating and Fixed landmarks. B: explicit learning: angle of aiming location (verbally reported landmark). C: implicit learning: subtraction of aiming direction from end-point hand angle. Vertical dashed lines denote when the rotation was introduced and removed. Movement epicycles represent the average of an 8-trial bin, and shading represents the 95% confidence interval of the mean.
During the No-Feedback block the rotation was removed, cursor feedback was absent, and landmarks were erased from the screen. In addition, participants were instructed to aim directly to the target to measure the presence of aftereffects (Taylor et al. 2014). Aftereffects were present for both the Rotating and Fixed conditions, measuring 13.3 ± 2.39° (t9 = 9.26, P < 0.001) and 13.0 ± 5.57° (t9 = 4.48, P = 0.002), respectively, although aftereffect size was not significantly different between groups (t18 = 0.30, P = 0.76; Fig. 3A).
Explicit and implicit learning.
Participants in both groups reported the number of the landmark to which they were aiming for each trial in the Rotation block. The time series of these aiming locations provides a measure of explicit learning during rotation training (Fig. 2B). To measure the rate and amount of explicit learning, we focused on the first and last epicycles of the Rotation block, respectively. On average, participants in both the Rotating and Fixed conditions rapidly adjusted their aiming angle in the first epicycle of the Rotation block, measuring 12.9 ± 10.0° and 15.0 ± 11.7°, respectively, but, accounting for multiple comparisons, these adjustments were only marginal (Rotating: t9 = 2.52, P = 0.03; Fixed: t9 = 2.50, P = 0.03) and there were no significant differences between groups (t18 = 0.27, P = 0.79). By the last epicycle of the Rotation block, participants in both the Rotating and Fixed conditions were significantly aiming to a large degree, measuring 28.0 ± 6.35° (t9 = 0.8.63, P < 0.001) and 23.4 ± 9.92° (t9 = 4.62, P = 0.001), respectively; between-group differences were not significant (t18 = 0.77, P = 0.45). Therefore, selecting an aiming location by the end of training in the Fixed condition was not significantly more difficult than selecting an aiming location in the Rotating condition (Fig. 3B). Indeed, the time courses of explicit learning for the Rotating and Fixed conditions were similar (r = 0.52, P = 0.005).
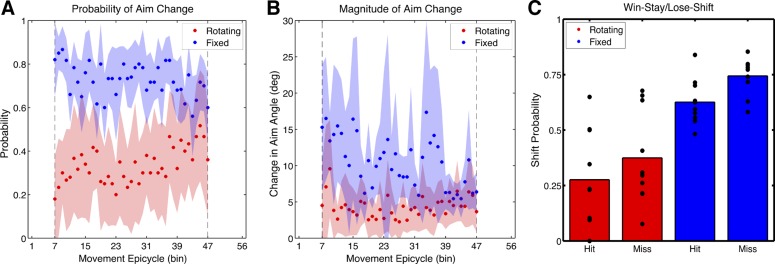
From Fig. 2, the Fixed group appears to aim to a lesser degree throughout the Rotation block—though not significantly less— even when the analysis focused on the average aiming angle throughout the entire Rotation block (t18 = 0.79, P = 0.44). However, between participants, the aim was more variable in the Fixed condition compared with the Rotating condition (t18 = 3.98, P < 0.001). Upon closer inspection, the probability of aim change throughout the Rotation block was also, on average, larger for the Fixed condition (Fig. 4A). The degree to which the aim changed from trial to trial was also larger for the Fixed condition (t18 = 5.27, P < 0.001; Fig. 4B). For these analyses, the angles of reported aiming landmarks were converted to angles relative to the target direction; therefore, the aiming variance observed in the Fixed condition was not simply attributable to needing to report different landmarks on each trial. Finally, we computed how likely participants were to change their aiming behavior after a hit or miss trial during the Rotation block and submitted these data to a mixed factorial ANOVA with factors of Group and Outcome (repeated measure). We found that there was a main effect of Group [F(1,18) = 47.5, P < 0.0001] and a main effect of Outcome [F(1,38) = 4.3, P = 0.04] but no interaction [F(1,9) = 0.03, P = 0.85]. Accordingly, all participants exhibited a win-stay, lose-shift behavior such that they were more likely to change their aiming behavior after a miss trial compared with a hit trial, although participants in the Fixed condition were more likely to change their aim regardless of task outcome (Fig. 4C). Differences in landmark orientation appear to change the consistency of aiming strategies, but explicit learning appears to follow a similar overall time course in both conditions.
Fig. 4.
Changes in explicit aiming during the Rotation block. A: probability of aim direction change during the verbal reporting phase for the Rotating and Fixed landmark conditions. B: average change in aiming direction from trial n and trial n − 1 across subjects. C: win-stay/lose-shift: probability of aim change after a successful or unsuccessful trial. Subjects in the Fixed condition are more likely to switch aiming strategies whether hitting or missing the target. Data were averaged into 8-trial bins (epicycles), and shading represents the 95% confidence interval of the mean.
To estimate implicit learning, we subtracted the aiming angle from the hand angle on each trial (Fig. 2C). In contrast with overall learning, implicit learning appears quite slow. It was not significant for participants in either the Rotating or the Fixed condition over the first epicycle of the Rotation block, measuring 3.05 ± 4.00° (t9 = 1.49, P = 0.17) and 0.22 ± 9.28° (t9 = 0.05, P = 0.96), respectively (Fig. 3C). Furthermore, there were no differences between groups (t18 = 0.55, P = 0.59). By the end of the last epicycle of the rotation block, implicit learning was much greater for both the Rotating and Fixed groups, measuring 19.5 ± 5.53° (t9 = 7.17, P < 0.001) and 22.9 ± 8.40° (t18 = 5.35, P < 0.001), respectively. The amount of implicit learning at the end of the Rotation block was not different between groups (t18 = 0.68, P = 0.51; Fig. 3C). Thus implicit learning appears to progress quite slowly, and the final amount of learning did not differ as a function of landmark orientation. This lack of a difference in implicit learning also supports the lack of a difference in the aftereffect (above) between groups, which was verified by comparing the size of the final amount of implicit learning with the size of the aftereffect (t18 = 0.66, P = 0.52).
In summary, the orientation of the landmarks appeared to have only a minor effect on explicit learning and no significant effect on implicit learning. Landmark orientation also appeared to decrease the consistency of the reported aiming location when the number of the landmark was fixed relative to the workspace. This led to an increased probability of aim change, and the magnitude of aim change was greater for participants in the Fixed condition. Nonetheless, the explicit learning time courses were very similar on average. Implicit learning was relatively unaffected by landmark orientation. In the next experiment, we investigated how explicit and implicit learning vary as a function of the number of target locations.
Experiment 2: Number of Training Targets
All participants practiced reaching to the target(s) with veridical feedback during the Baseline block to become familiarized with the task. For the eight trials preceding the introduction of the rotation (Baseline-Report block), participants were also instructed to report where they planned to aim using the numbered landmarks. In the One-Target group, 4 of 10 participants aimed to a location other than the green target. Three of 10 and 1 of 10 participants aimed to a location other than the green target in the Two-Target and Four-Target groups, respectively. In all cases, this aiming strategy lasted only for a few trials, and while most reported aiming locations were generally directed toward landmarks neighboring the target, some participants aimed to numbered landmarks that were far from the target. We suspect that this was most likely due to a failure of the participant to understand the instruction to report an intended aim location for the first few trials. Nonetheless, despite having a different number of targets between groups, there were no differences in the mean end-point hand angles in the Baseline-Report block [F(2,27) = 1.03, P = 0.37], measuring −4.91 ± 11.2°, 4.46 ± 9.55°, and 2.90 ± 8.10° for the One-, Two-, and Four-Target groups, respectively (see Fig. 6A).
Fig. 6.
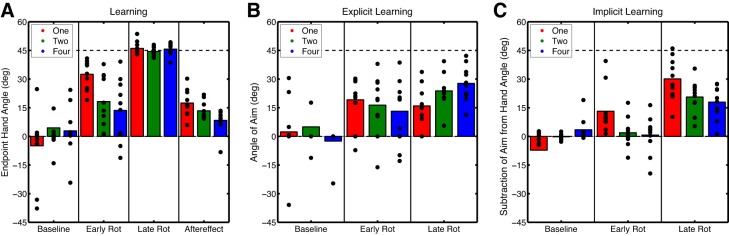
Experiment 2 phases of interest for each block. A: average end-point hand angles for the Baseline-Report block epicycle and first and last epicycles of the Rotation block and the aftereffect for the first epicycle of the No-Feedback block. B: explicit learning: average angle of aiming location for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. C: implicit learning: subtraction of aiming location from end-point hand angle for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. Bars represent the group mean of each 8-trial bin (epicycle), and circles represent the individual participants for the One-Target, Two-Target, and Four-Target conditions.
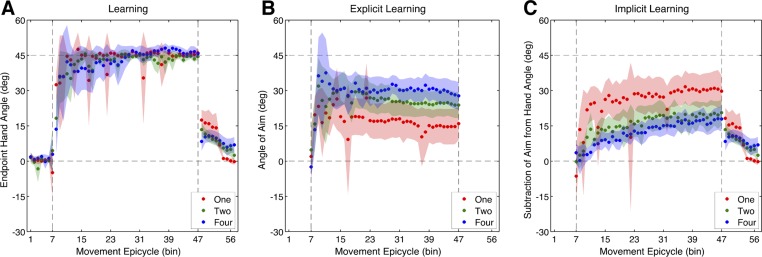
After the Baseline-Report block, a 45° clockwise rotation was introduced for 320 trials and all groups learned to counter the rotation (Fig. 5A). Participants in the One-Target condition rapidly began to counter the rotation. Within the first epicycle of the Rotation block they changed their hand angle to 32.5 ± 4.68° (t9 = 6.93, P < 0.001). While on average, participants in the Two- and Four-Target conditions changed their hand angles by 18.2 ± 8.51° and 13.6 ± 10.2°, respectively, these changes were not significantly deviant from the Baseline-Report block (Two-Target: t9 = 1.75, P = 0.11; Four-Target: t9 = 1.51, P = 0.17). This lack of significant change can be partially attributed to variance in the Baseline-Report block and the first epicycle of the Rotation block. Nonetheless, despite this variance, there was a near significant difference in learning rates (when corrected) between groups during the first epicycle [F(2,27) = 4.54, P < 0.02; Fig. 6A]. Post hoc t-tests revealed significant differences between the One- and Four-Target conditions (t18 = 3.00, P = 0.008) and marginal differences (when corrected) between the One- and Two-Target conditions (t18 = 2.50, P = 0.024). There was no difference between the Two- and Four-Target conditions (t18 = 0.29, P = 0.77). However, by the last epicycle of the Rotation block the One-, Two-, and Four-Target groups had fully compensated for the rotation, measuring 46.1 ± 2.12° (t9 = 8.61, P < 0.001), 44.4 ± 1.41° (t9 = 7.88, P < 0.001), and 45.7 ± 1.96° (t9 = 9.72, P < 0.001), respectively (Fig. 6A). There were no significant differences in the amount of learning between groups by the last epicycle [F(2,27) = 1.22, P = 0.31]. Thus participants in the One-Target condition displayed a faster rate of learning, but all groups learned an equivalent amount by the end of the Rotation block.
Fig. 5.
Experiment 2 learning time courses. A: end-point hand angle for One-Target, Two-Target, and Four-Target conditions. B: explicit learning: angle of aiming location (verbally reported landmark). C: implicit learning: subtraction of aiming direction from end-point hand angle. Vertical dashed lines denote when the rotation was introduced and removed. Movement epicycles represent the average of an 8-trial bin, and shading represents the 95% confidence interval of the mean.
After the Rotation block, cursor feedback was absent and the rotation was removed. In addition, the landmarks were erased and participants were instructed to aim directly to the green target. The One- and Two-Target groups presented significant aftereffects, measuring 17.5 ± 4.37° (t9 = 3.82, P = 0.004) and 13.4 ± 2.90° (t9 = 3.91, P = 0.004), respectively, while the Four-Target group showed a marginal aftereffect of 8.37 ± 3.91° (t9 = 2.18, P = 0.06). While aftereffect size appears to decrease as the number of targets increase, this remains only a trend [F(2,27) = 2.81, P = 0.08; Fig. 6A].
Explicit and implicit learning.
The time series depicting participants' reported aiming locations appeared to fluctuate nonmonotonically during the Rotation block, displaying an initial increase followed by a gentle decline with increased training (Fig. 5B). To quantify this shape, we focused on the first and last epicycles of the Rotation block, respectively. Participants in the One- and Two-Target conditions reported aiming to locations in a direction to counter the rotation within the first epicycle, measuring 19.2 ± 8.00° [t9 = 4.70, P = 0.001] and 16.4 ± 9.76° [t9 = 3.29, P = 0.009], respectively (Fig. 6B). Participants in the Four-Target condition displayed marginal changes (when corrected) in aiming direction, measuring 13.2 ± 10.5° (t9 = 2.45, P = 0.04). Comparison of these aiming directions between groups reveals no difference in the rate of explicit learning [F(2,27) = 0.39, P = 0.68]. By the last epicycle of the Rotation block, all groups continued to report aiming to locations in a direction to counter the rotation. One-, Two-, and Four-Target groups displayed aiming directions of 16.0 ± 7.00° (t9 = 4.47, P = 0.002), 23.8 ± 5.50° (t9 = 8.50, P < 0.001), and 27.8 ± 5.60° (t9 = 9.30, P < 0.001), respectively. Overall, there was a trend (when corrected) for the final amount of explicit learning to increase with an increasing number of target directions [F(2,27) = 3.67, P = 0.04; Fig. 6B]. It is worth noting that the One-Target group shows a larger average aiming angle over the first epicycle compared with the last epicycle, while the Two- and Four-Target groups show the opposite trend. Thus participants appear to rapidly adjust their aim and change it over the course of training irrespective of the number of targets.
To estimate implicit learning, we subtracted the aiming angle from the hand angle on each trial (Fig. 5C). In contrast with overall learning, implicit learning appears quite slow and monotonic. Only participants in the One-Target condition displayed rapid implicit learning during the first epicycle, measuring 13.2 ± 7.53° (t9 = 3.42, P = 0.008). Comparatively, implicit learning was lesser for participants in the Two- and Four-Target conditions [F(2,27) = 4.6, P = 0.02], measuring only 1.86 ± 4.5° (t9 = 0.75, P = 0.47) and 0.68 ± 6.22° (t9 = 0.21, P = 0.83), respectively. Post hoc t-tests revealed only marginal differences (when corrected) between the One- and Two-Target groups (t18 = 2.47, P = 0.02) and the One- and Four-Target groups (t18 = 2.50, P = 0.02), with no difference between the Two- and Four-Target groups (t18 = 0.29, P = 0.77; Fig. 6C). By the last epicycle, implicit learning was robust in the One-, Two-, and Four-Target groups, measuring 30.1 ± 6.88° (t9 = 8.58, P < 0.001), 20.6 ± 5.33° (t9 = 7.58, P < 0.001), and 17.9 ± 5.39° (t9 = 6.51, P < 0.001), respectively. Between-group differences in the final amount of implicit learning were significant [F(2,27) = 4.52, 0.02; Fig. 6C]. Post hoc t-tests revealed that this difference was marginal (when corrected) between the One-Target group and the Two-Target (t18 = 2.14, P = 0.04) and Four-Target (t18 = 2.74, P = 0.014) groups. There was no difference between the Two- and Four-Target groups (t18 = 0.70, P = 0.49). These data suggest that implicit learning is more robust and accumulates at a rapid rate when training at fewer targets. While the size of the final amount of implicit learning appears to be greater than the size of the aftereffect, it was not significant [F(2,27) = 0.68, P = 0.51].
In summary, overall learning was faster with fewer targets, which was expected. Explicit and implicit learning varied to some degree as a function of the number of targets. Increasing the number of targets led to a slightly slower rate of explicit learning and a slightly larger amount of explicit learning by the end of the Rotation block. In contrast, increasing the number of targets led to a slower rate and a smaller final amount of implicit learning. We deduce that an apparent inverse relationship between explicit and implicit learning yields the overall learning curve. In the next experiment, we examined how explicit and implicit learning change as a function of rotation size.
Experiment 3: Rotation Size
All participants practiced reaching to eight targets with veridical feedback during the Baseline block to become familiarized with the task. Similar to experiments 1 and 2, participants were instructed to report their desired aiming location before each movement for eight trials while continuing to reach with veridical feedback (Baseline-Report block). The majority of participants always reported aiming directly toward the green target, but in each group several participants reported aiming to other locations. Only 1 of 10 participants in the Fifteen group reported aiming to a location other than the green target, while 4 of 10 participants reported aiming to locations other than the green target for some of the trials in each of the Thirty, Sixty, and Ninety groups. Most of these aiming locations neighbored the green target and, overall, averaged <5° away from it. For the Fifteen, Thirty, Sixty, and Ninety groups end-point hand angles measured 2.69 ± 3.00°, −3.13 ± 8.27°, −4.09 ± 9.57°, and 2.63 ± 4.69°, respectively, and these values were not significantly different between groups [F(3,36) = 1.07, P = 0.38; see Fig. 8A]. We did not expect group-level differences because different rotation sizes had yet to be introduced.
Fig. 8.
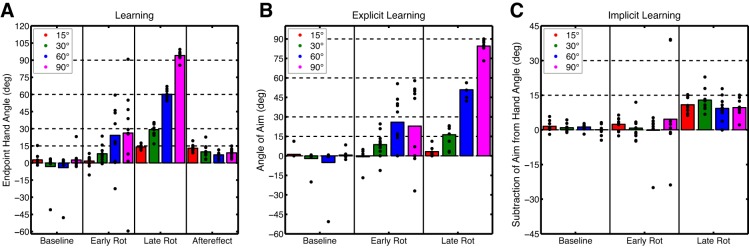
Experiment 3 phases of interest for each block. A: average end-point hand angles for the Baseline-Report block epicycle and first and last epicycles of the Rotation block and the aftereffect for the first epicycle of the No-Feedback block. B: explicit learning: average angle of aiming location for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. C: implicit learning: subtraction of aiming location from end-point hand angle for the Baseline-Report block epicycle and first and last epicycles of the Rotation block. Bars represent the group mean of each 8-trial bin (epicycle), and circles represent the individual participants for the Fifteen, Thirty, Sixty, and Ninety degree rotation conditions.
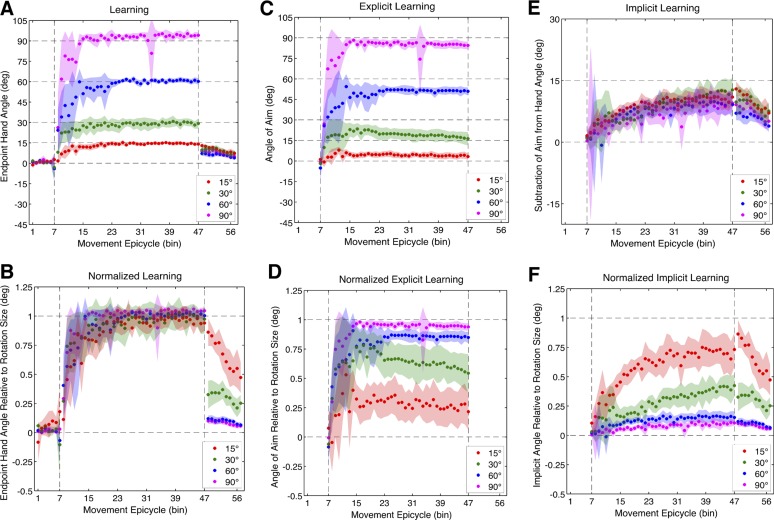
After the Baseline-Report block, a rotation was introduced for 320 trials with the magnitude differing by group. All participants learned to counter the rotation during the Rotation block (Fig. 7A), but the rate of learning appeared to be greater as rotation magnitude increased. Over the first epicycle of the Rotation block, participants in the Fifteen group did not significantly adjust their hand angle (t9 = 0.35, P = 0.73), measuring only 1.68 ± 3.23°. In contrast, participants in the Thirty and Sixty groups significantly adjusted their hand angle during the first epicycle, measuring 8.10 ± 4.48° (t9 = 2.38, P = 0.04) and 24.3 ± 15.9° (t9 = 4.63, P = 0.001), respectively. While the adjustment over the first epicycle was large for the Ninety group (26.2 ± 24.3°), it only trended toward significance (t9 = 1.97, P = 0.08). These differences in learning rates were nearly significant (when corrected) between groups [F(3,36) = 3.30, P = 0.03; Fig. 8A]. Post hoc comparisons failed to achieve significance when corrected for multiple comparisons (P > 0.04). By the last epicycle of the Rotation block, all groups learned to counter the rotation completely. The final amount of learning for the Fifteen, Thirty, Sixty, and Ninety groups measured 14.1 ± 1.02° (t9 = 6.13, P < 0.001), 29.2 ± 3.76° (t9 = 6.35, P < 0.001), 60.2 ± 2.39° (t9 = 12.7, P < 0.001), and 94.1 ± 2.29° (t9 = 50.3, P < 0.001), respectively (Fig. 8A). As expected, given that participants learned to counter differentially sized rotations and that we focused our analyses on end-point hand angle, the final amount of learning was different between groups [F(3,36) = 85.2, P < 0.001]. It is clear that each condition performed similarly by the end of the Rotation block when the end-point hand angle is normalized by the size of the rotation for each group (Fig. 7B).
Fig. 7.
Experiment 3 learning time courses. A: end-point hand angle for Fifteen, Thirty, Sixty, and Ninety degree rotation conditions. B: normalized learning: end-point hand angle divided by the size of the rotation for each group. C: explicit learning: angle of aiming location (verbally reported landmark). D: normalized explicit learning: average angle of aiming location divided by the size of the rotation for each group. E: implicit learning: subtraction of aiming direction from end-point hand angle. F: normalized implicit learning: subtraction of aiming direction from end-point hand angle divided by the size of the rotation for each group. Vertical dashed lines denote when the rotation was introduced and removed. Movement epicycles represent the average of an 8-trial bin, and shading represents the 95% confidence interval of the mean.
Immediately after the Rotation block, cursor feedback was absent, the rotation was removed, and the numbered landmarks were erased. In addition, participants were instructed to aim directly toward the green target. All groups displayed significant aftereffects during the first epicycle of the No-Feedback block, measuring 12.9 ± 1.77° (t9 = 8.67, P < 0.001), 9.76 ± 3.61° (t9 = 3.02, P = 0.01), 7.13 ± 1.61° (t9 = 3.39, P = 0.008), and 8.91 ± 2.29° (t9 = 3.04, P = 0.01). Despite between-group differences in rotation magnitude, aftereffect sizes were not significantly different between groups [F(3,36) = 0.47, P = 0.70; Fig. 8A].
Explicit and implicit learning.
The time series of verbally reported aiming locations provided an estimate of explicit learning. Surprisingly, explicit learning appeared to scale to the size of the rotation for most groups during the Rotation block (Fig. 7C). Similar to experiments 1 and 2, we focused our analyses on the first and last epicycles of the Rotation block to provide measures of the rate and final amount of explicit learning, respectively. Participants in the Fifteen group failed to display large aiming angle shifts over the first epicycle of the Rotation block, measuring −0.70 ± 3.69° (t9 = 0.37, P = 0.72). In contrast, participants in the Thirty and Sixty groups showed significant shifts in their aiming direction, measuring 8.66 ± 6.32° (t9 = 2.68, P = 0.025) and 25.9 ± 11.8° (t9 = 4.29, P = 0.002), respectively. While the Ninety group also displayed a large shift in aiming direction, measuring 22.9 ± 18.8°, the shift was marginal (when corrected, t9 = 2.39, P = 0.04). Between groups, the rate of explicit learning was significantly different [F(3,36) = 4.36, P = 0.01; Fig. 8B]. Post hoc t-tests revealed that explicit learning in the Sixty group was significantly greater than in the Fifteen group (t18 = 4.2, P < 0.001), and comparisons between the other groups were only marginally significant when corrected for multiple comparisons (P > 0.02). By the last epicycle of the Rotation block, participants continued to report aiming to locations other than the target location. For the Fifteen, Thirty, Sixty, and Ninety groups, the mean aiming directions were 3.23 ± 2.33° (t9 = 2.73, P = 0.02), 12.3 ± 4.88° (t9 = 6.55, P < 0.001), 50.9 ± 2.90° (t9 = 34.4, P < 0.001), and 84.4 ± 2.82° (t9 = 58.7, P < 0.001), respectively. These differences were significant when compared between groups [F(3,36) = 449, P < 0.001; Fig. 8B]. It is worth noting that participants in the Fifteen group showed a small yet significant amount of explicit learning by the end of the Rotation block. From Fig. 7C, it appears that explicit learning for these participants peaked early in the Rotation block and then decreased slowly over training. Indeed, we searched for the epicycle with the maximum aiming direction and found that there was a maximum of 10.3 ± 1.78°, which was significant (t9 = 11.3, P < 0.001). This peak was highly variable between participants but on average occurred around 54 ± 42 trials into the Rotation block. All groups engaged in aiming to locations other than the target throughout the majority of the Rotation block, and the degree of aiming appeared to scale with the size of the rotation, with more explicit learning increasing relative to the size of the rotation (see normalized results in Fig. 7D).
Similar to experiments 1 and 2, the implicit learning for all groups gradually and monotonically increased during the Rotation block. Interestingly, implicit learning over the first epicycle was only marginally significant (when corrected) for the Fifteen group (t9 = 2.65, P = 0.027), measuring 2.39 ± 1.77°. The Thirty, Sixty and Ninety groups did not show significant implicit learning over the first epicycle (P > 0.47), measuring 0.75 ± 3.08°, −0.19 ± 5.48°, and 4.62 ± 26.5°, respectively. There were no between-group differences in the rate of implicit learning [F(3,36) = 0.09, P = 0.96; Fig. 8C]. The lack of significant implicit learning in the first epicycle appears to be partially attributable to a large degree of variance during the early phase of the Rotation block, perhaps due to an initial failure to properly report aiming location. By the last epicycle of the Rotation block, all groups displayed implicit learning. The final amount of implicit learning measured 10.9 ± 2.09° (t9 = 10.2, P < 0.001), 12.9 ± 2.86° (t9 = 8.84, P < 0.001), 9.30 ± 3.22° (t9 = 5.66, P < 0.001), and 9.66 ± 2.26° (t9 = 8.36, P < 0.001) for the Fifteen, Thirty, Sixty, and Ninety groups, respectively. The final amount of learning did not differ between groups [F(3,36) = 1.44, P = 0.25; Fig. 8C] despite gross differences in rotation size. Finally, similar to experiments 1 and 2, the final amount of implicit learning was slightly larger than the size of the aftereffect, but this difference was insignificant [F(3,36) = 1.18, P = 0.218]. When implicit learning was normalized by the size of the rotation, implicit learning appeared to increase with smaller rotation sizes (Fig. 7F).
In summary, we found that explicit learning scaled with respect to the size of the rotation. Critically, however, implicit learning did not—while aftereffects were slightly different between conditions, their size was disproportional to the size of the rotation. According to the size of the rotation, explicit learning appears to change with reference to a fixed amount of implicit learning to achieve an appropriately scaled overall learning function.
DISCUSSION
It has become increasingly clear that multiple processes can operate during sensorimotor learning (Smith et al. 2006), such as reinforcement learning (Huang et al. 2011; Izawa and Shadmehr 2011), use-dependent plasticity (Verstynen and Sabes 2011; White and Diedrichsen 2010), hand path priming (Jax and Rosenbaum 2009; van der Wel et al. 2007), and explicit aiming strategies (Benson et al. 2011; Hegele and Heuer 2010, 2013; Mazzoni and Krakauer 2006; Sulzenbruck and Heuer 2009; Taylor and Ivry 2011). Explicit forms of learning appear to make a greater contribution to sensorimotor learning than previously thought (Keisler and Shadmehr 2010), but the contribution of these processes is often measured with posttests (Hegele and Heuer 2010, 2013) or inferred through computational modeling (Taylor and Ivry 2011). Recently, our lab developed a simple but novel methodology to assess the evolution of explicit learning during training by having participants verbally report an intended aiming direction on each trial (Taylor et al. 2014). In addition, measuring a separate time course of explicit learning allowed us to infer the time course of underlying implicit learning (Taylor et al. 2014). This demonstration showed that learning was the combined result of a dynamic interplay between explicit and implicit forms of learning and, importantly, that explicit learning played a major role in the overall learning function.
In the present work, we sought to extend our previous research by systematically manipulating dimensions of a visuomotor rotation task to observe how explicit and implicit learning were affected. We found that explicit learning was only mildly dependent on the orientation of visual landmarks used for verbal reporting, that it increased with the number of training targets, and that it scaled with the size of an imposed rotation. The flexibility of explicit learning was in contrast to the relative rigidity of implicit learning, which was consistent regardless of landmark orientation and rotation size and only modestly decreased with an increasing number of targets. The strong contribution of explicit learning and relatively weak contribution of implicit learning challenge prior interpretations and principles regarding implicit learning drawn from visuomotor rotation tasks.
Explicit and Implicit Learning Independent of Visual Cues
To assess participants' explicit aiming strategies in our prior and present work, we included visual landmarks in the workspace display to allow participants to verbally report their aiming direction on each trial. We settled on this particular methodology as opposed to having participants respond by using a visually displayed radial line connecting the start position to the target, which has been used in other studies (Hegele and Heuer 2010, 2013), in order to decrease the total trial time. However, it remains possible that visual landmark orientation provides a contextual cue or prime that could bias reported aiming directions. Indeed, prior work from our lab concerning generalization suggests that visual cues can strongly bias the degree of generalization (Taylor et al. 2013).
In experiment 1, we set out to test whether the orientation of numbered aiming landmarks biases explicit aiming strategies. We compared two conditions in which the numbers either rotated with the target on each trial or remained fixed with respect to the workspace. The time series of verbally reported aiming landmarks were not radically different between these conditions. On average, reported aiming directions in the Fixed condition converted to a slightly smaller angular deviation from the target compared with the Rotating condition, but this was not significant. Additionally, participants' aiming directions in the Fixed condition were less consistent across trials and more apt to change from one trial to the next. These results make intuitive sense since the task itself forced participants to change the number marking a particular aiming direction from one trial to the next. It is unlikely that participants could accurately recall the landmark that was appropriate or a previously reported landmark for a given target location since they would not revisit that target until they completed reaching to all other target locations. This raises the question of how participants were choosing to aim to a particular numbered landmark on each trial. One possibility is that participants were learning to do a mental addition or subtraction from the number that was beneath the target to obtain an appropriate number. In a postexperiment questionnaire, we asked participants whether they were using any kind of mental addition or subtraction to determine their aiming location and only three participants reported using mental addition in the Fixed condition. An alternative possibility is that participants ignored the numbers entirely and simply counted over by a particular amount; however, participants did not report using this metastrategy, either. More likely, participants were learning an abstracted angular deviation and selecting the number that approximated that angular deviation. Indeed, this is what other experiments have found when testing for explicit strategies, in addition to their generalizability across the workspace, suggesting that they are highly flexible (Hegele and Heuer 2010, 2013; Heuer and Hegele 2008, 2011).
It appears that the time course of explicit learning is not just a function of visual landmarks used to report the aiming strategy. More generally, it is likely that participants use aiming strategies even when visual landmarks are not present, by forming and updating a spatial representation of a given environmental perturbation that may dynamically interact with implicit processes over time to maximize compensation and minimize cognitive load. In our previous work, we found very similar rates of learning during the Rotation block between conditions with landmarks and without landmarks (Taylor et al. 2014). Moreover, aftereffects were similar between conditions with and without landmarks and were approximately half the size of the rotation, suggesting that implicit learning proceeded in a similar fashion regardless of whether participants were verbally reporting an aiming location on each trial (Taylor et al. 2014). In the present experiment, we see similar implicit learning curves during the Rotation block and similarly sized aftereffects in the No-Feedback block, providing further evidence that implicit learning proceeds independently from explicit learning.
Number of Training Targets
In experiment 2, we varied the number of training targets between groups and found that as the number of targets increased the amount of explicit learning increased while implicit learning decreased. It could be expected that formulating an explicit aiming strategy is more difficult with multiple targets, particularly if participants were attempting to implement a strategy in Cartesian coordinates, considering that the perturbation is in polar coordinates. This is especially true for cases of end-point feedback, where there is little information to distinguish between a translational shift of the cursor and a rotation of the cursor. In fact, with end-point feedback, participants show generalization patterns that are more consistent with a translational shift than a rotation (Taylor et al. 2013; Taylor and Ivry 2013). For example, the appropriate strategy for the 0° target direction with a clockwise rotation requires choosing an aiming landmark that is upward, but when switching to the 180° target direction the appropriate landmark would need to be downward to counter the rotation. However, participants showed similar rates of explicit learning regardless of the number of target locations, suggesting that they quickly figured out that the appropriate aiming strategy was in polar coordinates. Moreover, previous experiments examining strategy generalization have found that strategies are appropriate for a rotation when provided with online feedback (Heuer and Hegele 2008, 2011).
In contrast, implicit learning decreased as the number of targets increased, which may be expected since increasing the number of targets reduces the number of repeated training trials at any single target location. Furthermore, given the limited breadth of generalization observed in visuomotor rotation tasks (Krakauer et al. 2000; Taylor and Ivry 2013), implicit learning at each target benefits little from training at other targets when their angular separation is large, as in our study. Considering that with an increased number of targets there is increased time between each movement to the same target location, it is possible that learning decays between repetitions at a particular target location. To add to this point, it has recently been shown that there are at least two timescales of forgetting in implicit learning: a temporally labile and a temporally stable component (Brennan et al. 2012; Hadjiosif and Smith 2013). The time constant for the temporally labile component is ∼20 s (Brennan et al. 2012; Hadjiosif and Smith 2013). Therefore, as the time between repetitions in a particular direction increases, only the stable component is observed, which makes a significantly smaller contribution to overall implicit learning. Indeed, we analyzed the intertrial time between repetitions at the same target direction and found that the intertrial time steadily increased from ∼11 s in the One-Target condition to 20.5 s in the Two-Target condition and to 32 s in the Four-Target condition. Consequently, not only did the number of trials involving a single target location decrease as the number of targets increased, but the time between repetitions also increased, which could explain the corresponding decrease in implicit learning.
It is worth noting that implicit learning in the One-Target condition was incomplete by the end of the Rotation block. Implicit learning quickly rose to ∼25° within the first quarter of training in the Rotation block but slowed over the last three quarters of the Rotation block, reaching only a semiasymptote of ∼30°. We computed the average slope of increase over the last 100 trials of the Rotation block, and it was only 0.003° per trial, which would require ∼5,000 trials for implicit learning to fully compensate for a 45° rotation, assuming that this rate is time invariant. This result is remarkable given the rapid rate of implicit learning during the early phase of learning a rotation, suggesting that there may be a deceleration in the rate of implicit learning over continued training in the Rotation block. Hence, it remains an open question as to whether it is possible for implicit learning to fully compensate for a 45° rotation.
Flexibility of Explicit Learning but Not Implicit Learning
A commonly held assumption is that explicit learning only contributes to learning when visual errors are large. Most visuomotor rotation or prism adaptation studies introduce a relatively large rotation (30° or greater) abruptly, and participants are generally aware of a decrease in performance—although they may not be aware of the rotation per se (Bedford 1999; Kagerer et al. 1997; Klassen et al. 2005; Mazzoni and Krakauer 2006; Michel et al. 2007). When visuomotor rotations are introduced gradually, participants generally do not report being aware of the perturbation (Kagerer et al. 1997; Klassen et al. 2005; Saijo and Gomi 2010; Taylor and Ivry 2011). These studies suggest that implicit learning proceeds independent of articulable awareness and that, for small rotations, explicit learning would make little contribution.
In experiment 3, we sought to quantify the relative contribution of explicit and implicit learning as a function of visual error size by manipulating the size of the rotation between groups. We found that explicit learning scaled with the size of the rotation and that participants reported aiming directions throughout learning even when the rotation was relatively small (15°). Curiously, implicit learning did not proportionally scale with the size of the rotation; the rate and final degree of implicit learning were statistically indistinguishable despite the groups having dramatically different sizes of rotations. In addition, aftereffects were quite similar between groups, ranging between 8° and 12°, confirming that implicit learning was disproportional to error size. These results depart from previous work that showed relative scaling (Thomas and Bock 2012), but in prior studies the rotation was simply removed at the onset of a washout block and participants were not instructed to stop aiming. Under these conditions, participants would be expected to continue to reach in the same manner as on the last few trials of the Rotation block, making it inevitable that the reach angles in the Washout block would be closer to the size of the rotation. In our present and previous work (Taylor et al. 2014), we demonstrate that the true size of the aftereffect can only be observed by instructing participants to aim directly to the target without cursor feedback. Moreover, the size of the aftereffects closely matches the extent of implicit learning. This provides two measures that reveal that implicit learning is disproportionate to rotation size.
The insensitivity of implicit learning is surprising given the number of studies that have shown proportional learning when perturbations were consistent (Fine and Thoroughman 2007; Scheidt et al. 2001; Semrau et al. 2012); insensitivity to error size is only observed when perturbations were infrequent or inconsistent (Fine and Thoroughman 2006; Semrau et al. 2012; Wei and Kording 2009). Additionally, this error proportionality has been elegantly described with recursive learning models (Fine and Thoroughman 2007; Scheidt et al. 2001; Thoroughman and Shadmehr 2000). Recently, however, studies that provide participants with an explicit strategy have observed that implicit learning (or angular drift) appears to reach a maximum or asymptotic value that falls short of the full size of the rotation (Mazzoni and Krakauer 2006; Taylor and Ivry 2011). Another recent study has shown that when visual error is clamped such that the angular deviation of the cursor is constant regardless of angular hand position, implicit learning proceeds similarly regardless of error size and participants are relatively unaware of its operation (Morehead et al. 2014). Given these conditions, implicit learning appears to be sensitive to error direction but not error magnitude (Morehead et al. 2014).
These studies, in conjunction with our present study, suggest that previous reports of error proportionality have been observed only when explicit and implicit learning are conflated. Therefore, pure implicit learning may be less sophisticated than previously thought; sensitivity to error size may be an exclusive feature of explicit learning, or it may be that the joint operation of implicit learning and explicit learning is necessary to reduce error in a proportional manner. However, it seems unlikely that explicit learning itself is entirely responsible for error proportional learning, considering that the early phase of explicit learning is highly variable and often goes in the wrong direction to counter the rotation. Only during the later phase of the learning time course does explicit learning appear stable and responsive to an increase in implicit learning. Explicit learning in the absence of implicit learning has yet to be systematically examined in a visuomotor rotation task. Future experiments are necessary to determine the source of error-proportional learning, whether it arises from a single process operation or the combination of a number of processes. It is possible that only the temporally labile component of implicit learning is sensitive to error magnitude, while the temporally stable component of implicit learning is only sensitive to direction.
Finally, the similar extent of implicit learning despite large changes in explicit learning suggests that implicit learning operates with a notable degree of independence from explicit learning. Previous studies that have provided participants with an explicit aiming strategy to counter a rotation have observed that participants initially have minimal target error but, over time, their hand drifts in the direction of the strategy such that directional error increases (Mazzoni and Krakauer 2006; Taylor et al. 2010; Taylor and Ivry 2011). This has been ascribed to the continued operation of a forward model based upon the difference between aiming direction and cursor feedback, not target error (Mazzoni and Krakauer 2006; Taylor and Ivry 2011). With continued training, the drift in hand direction eventually reverses, and this reversal has been attributed to changes in participants' aiming strategies, not implicit learning (Taylor and Ivry 2011). These studies, in conjunction with our present findings, suggest that implicit learning may indeed operate independently from explicit learning (i.e., aiming strategies). However, it does not appear that explicit and implicit learning are strictly independent, since explicit aiming strategies appear to change in response to continued implicit learning. It is possible that explicit learning responds appropriately to implicit learning, but not vice versa, suggesting a unidirectional dependency. Additionally, it is unknown whether this interaction is immediate, with implicit learning directly informing explicit learning, or indirect, with explicit learning responding to changes in target error solely as an outcome of implicit learning.
Conclusions
In our task, explicit learning is manifested as selecting aiming strategies to restore task performance. Utilizing aiming strategies to counter visuomotor errors is not a newly described phenomenon but has been described in previous prism adaptation studies (Martin et al. 1996; Redding and Wallace 1996; Weiner et al. 1983) and colloquially in the practice of “Kentucky windage,” which refers to the strategy of aiming to the side of a target to compensate for wind during rifle shooting. Only recently, however, has their contribution been quantified through postexperimental tests (Hegele and Heuer 2010, 2013) or on a trial-by-trial basis (Taylor et al. 2014). In this study, we find that explicit learning appears to be a fundamental process contributing to overall learning in a visuomotor rotation task, working in tandem with implicit learning to counter visuomotor rotations under a variety of task conditions. We propose that future experiments will need to include tests of explicit learning to thoroughly understand learning in a visuomotor rotation task.
GRANTS
K. M. Bond and J. A. Taylor were supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-084948 and the Princeton Neuroscience Institute's Innovation Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.B. and J.A.T. conception and design of research; K.M.B. performed experiments; K.M.B. and J.A.T. analyzed data; K.M.B. and J.A.T. interpreted results of experiments; K.M.B. and J.A.T. prepared figures; K.M.B. and J.A.T. drafted manuscript; K.M.B. and J.A.T. edited and revised manuscript; K.M.B. and J.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sam McDougle for helpful comments on the manuscript and Alyssa Bangel, Jon Berliner, Peter Butcher, Judy Fan, John Krakauer, Richard Ivry, and Ryan Morehead for insightful discussions. We also thank John Lindsay McCulloch for introducing us to the concept of Kentucky windage and how it might relate to aiming strategies.
REFERENCES
- Bedford FL. Keeping perception accurate. Trends Cogn Sci 3: 4–11, 1999. [DOI] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol 105: 2843–2851, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AA, Bakdash JZ, Proffitt DR. Treadmill experience mediates the perceptual-motor aftereffect of treadmill walking. Exp Brain Res 216: 527–534, 2012. [DOI] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15: 493–506, 1989. [DOI] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Motor adaptation to single force pulses: sensitive to direction but insensitive to within-movement pulse placement and magnitude. J Neurophysiol 96: 710–720, 2006. [DOI] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Trial-by-trial transformation of error into sensorimotor adaptation changes with environmental dynamics. J Neurophysiol 98: 1392–1404, 2007. [DOI] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiosif A, Smith M. Savings is restricted to the temporally labile component of motor adaptation (Abstract). In: Translational and Computational Motor Control. San Diego, CA: Am. Soc. Neurorehabilitation, 2013. [Google Scholar]
- Hegele M, Heuer H. Implicit and explicit components of dual adaptation to visuomotor rotations. Conscious Cogn 19: 906–917, 2010. [DOI] [PubMed] [Google Scholar]
- Hegele M, Heuer H. Age-related variations of visuomotor adaptation result from both the acquisition and the application of explicit knowledge. Psychol Aging 28: 333–339, 2013. [DOI] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging 23: 190–202, 2008. [DOI] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Generalization of implicit and explicit adjustments to visuomotor rotations across the workspace in younger and older adults. J Neurophysiol 106: 2078–2085, 2011. [DOI] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7: e1002012, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: rapid decay of dorsal stream information. Neuropsychologia 47: 1573–1577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997. [DOI] [PubMed] [Google Scholar]
- Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci 30: 14817–14823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitago T, Ryan SL, Mazzoni P, Krakauer JW, Haith AM. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci 7: 307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: 405–421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y. Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. J Cogn Neurosci 19: 341–350, 2007. [DOI] [PubMed] [Google Scholar]
- Morehead RJ, Taylor JA, Parvin D, Marrone E, Ivry RB. Implicit adaptation via visual error clamp (Abstract). In: Translational and Computational Motor Control. Washington, DC: Am. Soc. Neurorehabilitation, 2014. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform 22: 379–394, 1996. [DOI] [PubMed] [Google Scholar]
- Saijo N, Gomi H. Multiple motor learning strategies in visuomotor rotation. PLoS One 5: e9399, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Dingwell JB, Mussa-Ivaldi FA. Learning to move amid uncertainty. J Neurophysiol 86: 971–985, 2001. [DOI] [PubMed] [Google Scholar]
- Semrau JA, Daitch AL, Thoroughman KA. Environmental experience within and across testing days determines the strength of human visuomotor adaptation. Exp Brain Res 216: 409–418, 2012. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzenbruck S, Heuer H. Functional independence of explicit and implicit motor adjustments. Conscious Cogn 18: 145–159, 2009. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 18: 814–818, 2008. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hieber LL, Ivry RB. Feedback-dependent generalization. J Neurophysiol 109: 202–215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Comput Biol 7: e1001096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Context-dependent generalization. Front Hum Neurosci 7: 171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res 210: 217–253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum 9: 580–586, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J Neurophysiol 98: 317–326, 2007. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS One 3: e2485, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Bock O. Concurrent adaptation to four different visual rotations. Exp Brain Res 221: 85–91, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature 407: 742–747, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. [DOI] [PubMed] [Google Scholar]
- van der Wel RP, Fleckenstein RM, Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: evidence for abstract spatiotemporal forms in human motor control. J Exp Psychol Hum Percept Perform 33: 1117–1126, 2007. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Sabes PN. How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci 31: 10050–10059, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Kording K. Relevance of error: what drives motor adaptation? J Neurophysiol 101: 655–664, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33: 766–772, 1983. [DOI] [PubMed] [Google Scholar]
- White O, Diedrichsen J. Responsibility assignment in redundant systems. Curr Biol 20: 1290–1295, 2010. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]