Abstract
Spontaneous and stimulus-evoked excitatory postsynaptic currents (EPSCs) were recorded in calyx nerve terminals from the turtle vestibular lagena to quantify key attributes of quantal transmission at this synapse. On average, EPSC events had a magnitude of ∼42 pA, a rise time constant of τ0 ∼229 μs, decayed to baseline with a time constant of τR ∼690 μs, and carried ∼46 fC of charge. Individual EPSCs varied in magnitude and decay time constant. Variability in the EPSC decay time constant was hair cell dependent and due in part to a slow protraction of the EPSC in some cases. Variability in EPSC size was well described by an integer summation of unitary quanta, with each quanta of glutamate gating a unitary postsynaptic current of ∼23 pA. The unitary charge was ∼26 fC for EPSCs with a simple exponential decay and increased to ∼48 fC for EPSCs exhibiting a slow protraction. The EPSC magnitude and the number of simultaneous unitary quanta within each event increased with presynaptic stimulus intensity. During tonic hair cell depolarization, both the EPSC magnitude and event rate exhibited adaptive run down over time. Present data from a reptilian calyx are remarkably similar to noncalyceal vestibular synaptic terminals in diverse species, indicating that the skewed EPSC size distribution and multiquantal release might be an ancestral property of inner ear ribbon synapses.
Keywords: auditory, vestibular, synapse, unitary quanta
inner ear vestibular otolith organs including the saccule, utricle, and in some species the lagena are broad-band sensors that encode low-frequency changes in gravito-inertial acceleration or static tilt relative to gravity (Goldberg 2012) and encode high-frequency sound or vibration (Curthoys et al. 2006; Curthoys and Vulovic 2011; Edds-Walton and Fay 2003; Locke et al. 1999; Lu et al. 2003, 2004; McCue and Guinan 1994; Murofushi et al. 1995; Weeg et al. 2002; Young et al. 1977). This broad bandwidth necessitates specialized hair cell afferent synapses capable of transmitting both tonic stimuli and precise phasic stimuli. In amniotes, all vestibular organs contain chalice-shaped calyx afferent synaptic endings that receive excitatory signals from one or more mechanosensitive receptors called type 1 hair cells (Favre and Sans 1979; Gulley and Bagger-Sjoback 1979; Lorente de No 1926; Retzius 1881, 1884). At these hair cell-calyx contacts, information is transmitted through quantal release of glutamate packaged in synaptic vesicles (Matsubara et al. 1999; Rennie and Streeter 2006; Schessel et al. 1991) and through nonquantal (Yamashita and Ohmori 1990) presynaptic release of unconventional chemical transmitter(s) including protons (Highstein et al. 2014) and potassium ions (Contini et al. 2012; Lim et al. 2011). These two signaling modes are evident as quantal and nonquantal excitatory postsynaptic currents (EPSCs and nqEPSCs, respectively) in the calyx terminal (Holt et al. 2007). The nonquantal component persists after blocking quantal glutamatergic EPSCs (Highstein et al. 2014; Holt et al. 2007), suggesting that the two components are at least partially independent. Both quantal and nonquantal transmission can be evoked by depolarization of a single presynaptic hair cell (Highstein et al. 2014; Songer and Eatock 2013), with relative contributions to the total postsynaptic charge varying widely between individual hair cells. Since the temporal properties of quantal and nonquantal transmission differ, each contributes uniquely to encoding of the stimulus.
In the lagena, the indefatigable nature of the nqEPSC favors transmission of tonic signals such as head orientation relative to gravity and slowly varying signals such as low-frequency head movements (Highstein et al. 2014). As such, one of the roles of the postsynaptic vestibular calyx appears to be opposite to that of the presynaptic calyx of Held, which is specialized for transmission of spike timing and temporal information in the auditory brainstem (Held 1893; Ryugo and Fekete 1982; Taschenberger and von Gersdorff 2000; Wu and Kelly 1993). This pre- vs. postsynaptic distinction could explain why inner ear postsynaptic calyces and nqEPSCs are abundant in vestibular end organ synapses, which require transmission of tonic stimuli, but absent in mammalian cochlear auditory synapses specialized to transmit high-frequency phasic stimuli. Transmission of tonic signals from type 1 vestibular hair cell synapses to the calyx is further enhanced by the protracted time course of quantal EPSCs, which, for high vesicular release rates, can give rise to overlapping events summating to a tonic current (Sadeghi et al. 2014). Evidence suggests that this prolonged EPSC time course may be due to the kinetics of transmitter accumulation and clearance in the elongated chalice-shaped synaptic cleft (Sadeghi et al. 2014; Yamashita and Ohmori 1990). These prolonged EPSCs generate maintained depolarizations of the afferent neuron that lead to tonic increases in action potential rate. In addition to encoding tonic and low-frequency stimuli, calyx bearing otolith afferents also respond to sound and vibration, with action potentials phase locking to the stimulus with high temporal fidelity. This cannot be achieved by nonquantal transmission alone and suggests a fundamental role for quanta in transmitting phasic signals at the type 1 hair cell calyx synapse.
Transmission of acoustic signals from vestibular hair cells to noncalyceal bouton terminals is quantal, as perhaps first demonstrated in the goldfish saccule (Furukawa 1986; Starr and Sewell 1991). The size of individual EPSC events varies widely, well beyond what would be expected on the basis of diversity in synaptic vesicle size or loading. Several lines of evidence suggest that the diversity in EPSC size has advantages for precisely controlling action potential timing relative to the stimulus and might arise from the stimulus-dependent simultaneous release of a varying number of vesicles (Glowatzki and Fuchs 2002; Keen and Hudspeth 2006; Li et al. 2009; Schnee et al. 2011; Siegel 1992; Singer et al. 2004) or, alternatively, from dynamic control of a unitary vesicle fusion pore (Chapochnikov et al. 2014). Analogous results are found in the central nervous system (Jonas et al. 1993; Silver et al. 1996) and in the vestibular calyx (Yamashita and Ohmori 1990). In the goldfish saccule, the size of synaptic events also depends on stimulus history and, during maintained acoustic stimulation, afferent EPSPs and action potentials show adaptive run down (Furukawa and Matsuura 1978). Run down occurs in the absence of a change in the microphonic. This suggests that the probability of large EPSC events decreases over time even without adaptive rundown of hair cell mechanoelectrical transduction currents (Furukawa and Matsuura 1978). In dual hair cell-afferent terminal recordings, hair cell hyperpolarization reduces the fraction of large EPSC events (Li et al. 2009), demonstrating the dependence of large EPSC events on presynaptic potentials. Paired recordings in the bullfrog amphibian papilla clearly demonstrate the importance of large EPSC events in locking the timing of postsynaptic afferent nerve action potentials to a specific temporal phase of sinusoidal presynaptic stimuli (Li et al. 2014). Hence, the presence of large EPSC events in afferent terminals arising from unitary or multivesicular release is essential to sound localization and auditory signal processing (Carr and Macleod 2010; Koppl 1997). Although there is strong evidence for phase locking of afferent action potentials in type 1 calyx-bearing afferents (Rowe and Neiman 2012; Songer and Eatock 2013; Zhu et al. 2014), it is not known to what extent this might depend on kinetics and the size of stimulus evoked quantal EPSCs.
The present report addresses quantal transmission at the type 1 hair cell calyx synapse in the turtle lagena, with attention to kinetic diversity of EPSCs, EPSC size, and temporal properties of stimulus-evoked quantal release. The lagena is a mixed auditory/vestibular organ that responds to low frequency stimuli as well as sound and vibration. Results demonstrate adaptive run down of quantal release for tonic stimuli, provide evidence for multiquantal release, and quantify synapse-specific diversity of spontaneous and stimulus evoked EPSCs.
MATERIALS AND METHODS
The procedures were approved by the Institutional Animal Care and Use Committee at the Marine Biological Laboratory. Electrophysiological and imaging data were obtained from lagenae isolated from adult red-eared slider turtles, Trachemys scripta elegans. Data from 39 turtles are included in the present report. The otolithic membrane and otoconia were removed by incubation for ∼5 min in type XXIV proteinase (P8038; Sigma-Aldrich; 0.06 mg/ml in 0.1 mM Ca). Epithelia were perfused at 3 ml/min with an oxygenated media with the following (in mM): 125 NaCl, 4 KCl, 2.2 MgCl2, 2.8 CaCl2, 8 d-glucose, and 10 Na-HEPES, pH 7.53. Patch electrodes contained the following (in mM): 120 KCl, 2.8 MgCl2, 0.45 CaCl2, 5 EGTA, 2.5 Na2ATP, and 10 K-HEPES, pH 7.2. Experiments were performed using a ×63, 1.0 NA objective on a fixed-stage Zeiss Axioskop. Type 1 hair cells and calyx terminals were identified visually in the central striolar region of the lagena. Experimental protocols were computer controlled (PatchMaster; HEKA). An Axon 700b amplifier (Molecular Devices) was used for whole cell recording. Analog signals were recorded at 16 bits, filtered at 16 kHz (npi), and also sampled at 96 kHz (Apogee AD16x modified for DC coupling; IGOR Pro; Wavemetrics). Hair bundles of type 1 hair cells were stimulated with a fluid jet directly attached to a picospritzer (1A, WPI, pv 800) to deflect the bundle toward the kinocilium (Highstein et al. 2014). Whole cell voltage clamp of calyces was performed at room temperature. In 17 of 39 turtles, Lucifer yellow was added to the pipette to visualize filled calyces. Capacitance was compensated online (35.3 pF, SE 3.7), and series resistance (24.0 MΩ, SE 1.58) was compensated offline in the time domain. The total corrected membrane current Im, consisting of the synaptic currents plus membrane conduction currents, was estimated from the capacitance compensated electrode (amplifier) current IEC and electrode voltage VE using
| (1) |
where Gm and Cm are the whole cell membrane conductance and capacitance, RE is the uncompensated electrode access resistance, and CE is the electrode capacitance. Under voltage clamp conditions, the amplifier voltage VE is constant and dVE/dt can be neglected. In this correction we assumed voltage-dependent changes in Gm were small. The corrected membrane voltage was estimated using Vm ≈ VE − REIE. These equations were applied in the time domain. Individual EPSC events were identified automatically by high-pass filtering Im and finding fast transient events surpassing ∼18 pA in amplitude. Probability histograms show that the 18-pA threshold value was in the extreme tail of the EPSC amplitude distribution and below the unitary quantal size (see results).
Kinetics of individual EPSC events were quantified using
| (2) |
where t is time, tk is the time of the kth EPSC event, τO is the onset rise time constant, and H is the unit Heaviside step function. Recovery of the EPSC to baseline was described as a fast component decaying with time constant τR (μs) plus a slow component of relative amplitude ϕ decaying with time constant τS (μs). Parameters in Eq. 2 were found independently for each individual EPSC event using Levenberg-Marquardt optimization. The magnitude of each event (Mk = max|Ik|, pA), and for the case when τS >> τR, the magnitude is well approximated by Mk ≈ AkτR[(1 + τR/τO)−τO/τR + ϕ(1 + τR/τO)−τO/τS]/(τO + τR). The charge was computed as the time integral of Eq. 2, namely qk = Ak/[τR2/(τO + τR) + ϕτS2/(τO + τs)]. To determine the dominant recovery time constant, we set ϕ = 0 and optimized parameters in Eq. 2 for each individual EPSCs (e.g., see Figs. 1–3). The procedure first identified EPSC peaks using a threshold followed by a local search and then applied iterative Levenberg-Marquardt optimization in a window around the EPSC to estimate the start time, rise time constant, and decay time constant. A subset of events exhibited a slow tail, ϕ > 0. To fit events exhibiting the slow tail, we optimized all parameters in Eq. 2 subject to the regularizing constraints τR ≤ 1,000 μs and 0 ≤ ϕ ≤ 2.
Fig. 1.
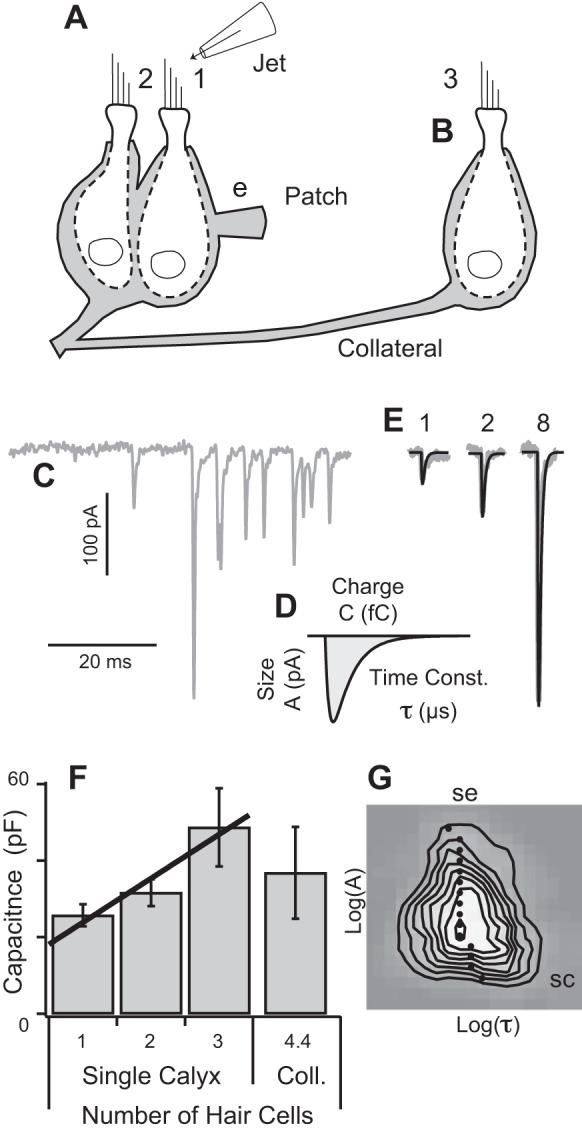
Voltage-clamp recordings from vestibular calyx terminals. A: asynchronous and evoked excitatory postsynaptic currents (EPSCs) were recorded using ∼6 MΩ patch electrodes (e) attached to the lateral membrane of calyx terminals (gray). Type 1 hair bundles were identified visually and a fluid jet was used to deflect the bundle in the direction of the kinocilium. B: subset of afferents branched to form synaptic contacts with remote hair cells. C: EPSC events exhibited a wide range of amplitudes (asynchronous example shown). D: individual EPSCs were fit with an exponential rise-fall function to determine the amplitude (pA), decay time constant (μs), and total charge (fC). E: EPSCs evoked by deflection of a single hair bundle decayed with the same time constant and could be described a superposition of simultaneous unitary quantal events, e.g., 1, 2, and 8 simultaneous quantal events (black). F: capacitance measured by the patch pipette correlated with number (N) of hair cells enveloped by the patched calyx (C = 12.4 + 11.5 × N pF ± 3.2; n = 15 calyces). Presence of a collateral calyx did not consistently increase the measured capacitance. G: 2-dimensional (2D) bin histogram showing there was very little correlation between asynchronous mEPSC size and time constant (n = 8 calyces; k = 770 events; black dots show the mean time constant at for all EPSCs within each size bin). SC, space clamp.
Fig. 3.
Diversity of EPSC kinetics. A and B: probability histograms showing the amplitudes (pA) and decay time constants (ms) of spontaneous mEPSCs (black), EPSCs evoked by deflection of hair bundle 1 (red), and EPSCs evoked by hair bundle 2 (green) recorded in a single calyx. Inset: average EPSCs. In this calyx, there were significant differences in size and speed depending upon the specific hair bundle stimulated. The bimodal distribution arises because the population of events includes EPSCs from hair cells 1 and 2. C and D: raw records comparing evoked EPSCs (C) to spontaneous mEPSCs (D). Red curves overlaying the raw data (black) illustrate a slow protracted tail in spontaneous mEPSCs that was absent (or overwhelmed) in bundle 1 evoked EPSCs. The presence or absence of this tail is the primary difference between EPSCs with slow vs. fast kinetics.
We estimated the unitary quantal size u and number of unitary quanta in each EPSC event ||Mk/u|| by minimizing the difference between the summed unitary quanta and the data, while simultaneously minimizing the number of unitary quanta per event. Specifically, in the time domain, we numerically optimized
| (3) |
Current reconstructions shown in Figs. 3–5 (red traces) are time domain summations of individual EPSCs, I(t) = Ik(t,tk), where the amplitude of the kth EPSC was determined from the summation of nk unitary quantal events by setting Mk = nku.
Fig. 5.
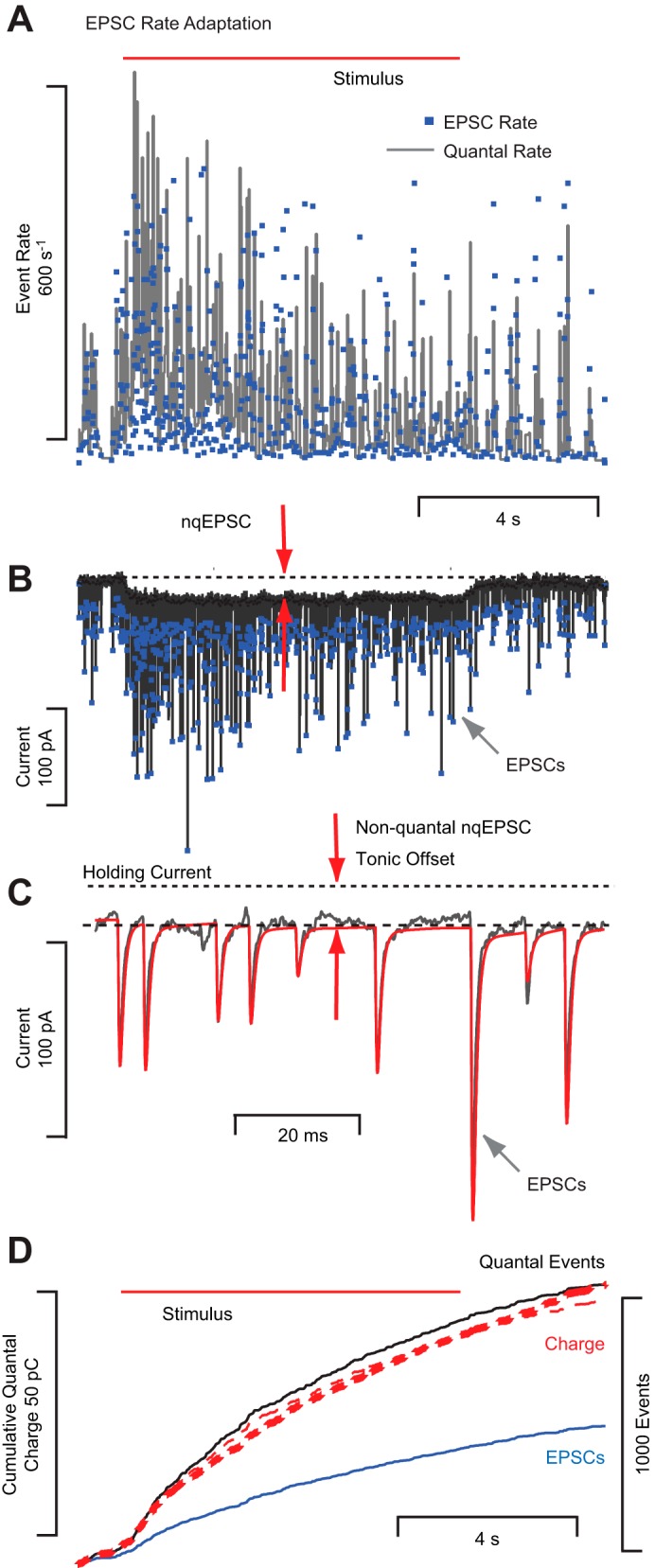
Quantal EPSCs and nonquantal (nq)EPSCs can be evoked simultaneously. Same notation as Fig. 4 but with longer time scale and recording from a different calyx. A: adaptation of EPSC instantaneous rate and unitary quantal rate. B: raw current trace showing superposition of EPSCs and nqEPSC. C: expanded section showing a modest EPSC tail and tonic nqEPSC offset. D: cumulative quantal charge (subtracting nqEPSC) provides an estimate of ∼44 fC per unitary event in this cell.
RESULTS
Voltage-clamp recordings from visually identified calyces in the striolar region of the vestibular lagena contained both spontaneous miniature excitatory postsynaptic currents (mEPSCs) and stimulus evoked EPSCs. Spontaneous events were presumably independent of stimulus-driven Ca2+ influx and were therefore termed asynchronous, whereas stimulus-evoked events presumably included responses synchronized with the intracellular Ca2+ concentration. A fluid jet directed toward the kinocilium (Fig. 1A) was used as the excitatory stimulus to deflect the bundle ∼1 μm within 20 ms of onset, with a rise time constant of ∼29 ms (Highstein et al. 2014). Example mEPSCs are shown in Fig. 1, C and E. EPSC event times, size (pA), recovery time constants (μs), and charge (fC) were determined by fitting an exponential rise-fall function to individual events (Fig. 1, D and E). EPSCs were recorded in calyces at rest and when stimulated by deflection of hair bundles associated with several different type 1 hair cells contacted by the terminal. The average EPSC rise time constant was 229 μs (n = 14 calyces; SD 26 μs) and exceeded the clamp speed of ∼150 μs by ∼2 SDs, indicating that the clamp was sufficiently fast to capture the rising phase of EPSCs in most cases.
The large membrane surface area and the complex morphology of calyceal endings led us to question whether perfect space clamp was achieved in the present experiments. Lucifer yellow dye delivered by the patch pipette revealed complex morphologies with single calyx endings enveloping multiple type 1 hair cells and collateral branches of the same afferent forming additional calyces enveloping other type 1 hair cells and/or forming bouton terminals contacting type 2 hair cells (Highstein et al. 2014). It has previously been shown using two-electrode recordings that voltage clamp at one site on a calyx is sufficient to space clamp the entire calyx terminal (Sadeghi et al. 2014). To determine if space clamp was also achieved in the present experiments, we examined the relationship between the size of the calyx ending and the capacitance recorded at the site of the electrode. Of 17 Lucifer yellow filled calyces, 4 were simple calyx endings enveloping 1 solitary hair cell, 4 were larger single calyces enveloping multiple hair cells, and 9 were complex calyces with collaterals contacting 1–4 additional hair cells. In endings lacking collaterals, the average capacitance recorded from calyx terminals enveloping one hair cell was 25 pF (SE 2.9, n = 7 calyces). In endings enveloping two hair cells, the average capacitance was 32 pF (SE 3.5, n = 4), and in calyces enveloping three hair cells, it was 49 pF (SE 10.2, n = 5). Consistent with an earlier report, the linear relationship between capacitance (Fig. 1F, black line) and number of hair cells enveloped by the calyx suggests space clamp of the terminal was achieved (the axon contributed ∼12 pF) (Sadeghi et al. 2014). However, two lines of evidence indicate that ideal space clamp did not always extend to collateral calyx terminals, collateral bouton terminals, or to the axon. Firstly, although capacitance at the recording site correlated strongly with the number of hair cells enveloped by the patched calyx (Fig. 1F), capacitance did not consistently increase when collateral terminals were present (Fig. 1F, n = 9, coll. cells), suggesting that the collateral calyces and boutons were not usually space clamped. Secondly, this caveat also applied to the axon. Although all EPSC data included in the present report were recorded at a −60 mV holding potential, we did confirm that action potentials could be evoked when the calyx was voltage clamped at depolarized levels >0 mV, thus demonstrating that remote nodes of Ranvier were not clamped.
To estimate what fraction of the EPSCs were well clamped, we constructed a two-dimensional histogram of mEPSC size vs. time constant for all spontaneous events (Fig. 1G). The histogram shows the probability of recording a mEPSC having amplitude A and decay time constant τ; gray denotes zero probability and white denotes maximum probability. Black contour lines show six equally spaced isoprobability curves. The black dots show the most probable time constant for the population of mEPSCs at each amplitude. Loss of space clamp would result in an inverse correlation between size and time constant. With the possible exception of very small mEPSCs, an inverse correlation was not obtained, indicating that most EPSC recordings were space clamped. The subset of calyces that were not space clamped might account for the small bulge (cross-correlation) in the histogram in Fig. 1G, bottom right (SC). The positive skewness (skew) of the distribution above the mean (SE) is due to large events and is consistent with a summation of simultaneous unitary quanta as described below.
We examined differences between spontaneous mEPSCs and evoked EPSCs by recording both events in individual calyces (n = 8 cells and k = 18,322 events). Spontaneous mEPSCs grouped together for all calyces had a mean size of 37.4 pA [SD = 21.1, skew = 1.44, and kurtosis (kurt) = 4.27], a mean decay time constant of 690 μs (SD = 393, skew = 1.23, and kurt = 3.32), and a mean rate of 51.8 s−1. EPSCs recorded in the same calyces during brief hair bundle displacements had a larger mean size of 45.8 pA (SD = 29.3, skew = 1.63, and kurt = 4.27), a nearly equivalent mean decay time constant of 697 μs (SD = 423, skew = 1.74, and kurt = 6.7), and a higher mean rate of 87 s−1. The fact that the average ESPC size increased during stimulation without a large increase in the decay time constant is consistent with the hypothesis that hair cell depolarization evoked simultaneous release of presynaptic vesicles (Glowatzki and Fuchs 2002; Li et al. 2014) or increased open time of a unitary fusion pore (Chapochnikov et al. 2014). Using Eq. 3, we estimated the unitary EPSC size as 23.3 pA and the unitary charge as 26 pC for this same group of cells. The unitary size implies spontaneous mEPSCs averaged 1.58 unitary quanta per event (avg. of ∼3 quanta for every 2 mEPSCs), while EPSCs during bundle displacement averaged 1.94 quanta per event (avg. of ∼2 quanta for each EPSC). The probability distribution of multiquantal events was positive skew normal, consistent with the skew of EPSC amplitude distributions discussed below.
To further examine the diversity of synaptic inputs from multiple hair cells to single calyx endings, we stimulated one hair cell at a time and compared spontaneous mEPSCs to evoked EPSCs (n = 9 calyces). In 56% of calyceal endings, there were only small differences in the EPSCs evoked by stimulation of different hair bundles (e.g., Fig. 2, A and B). The average EPSC size in this calyx was approximately twice as large as the unitary quantal size and exhibited similar kinetics for both spontaneous and evoked events. In 44% of calyces, evoked EPSCs showed diverse kinetics that depended strongly on which hair cell was stimulated. In Fig. 2, C and D, deflection of bundles 1 and 2 evoked nearly equivalent EPSCs (Fig. 2, C and D, red and green). Deflection of hair bundle 3, however, evoked much faster EPSCs in the same calyx with no change in the EPSC magnitude. In this calyx, the average EPSC size was nearly equivalent to the unitary quantal size in all three cases, implying that most EPSC events in this calyx were single quanta. In this recording, bundle 1 depolarized the hair cell immediately adjacent to the recording pipette, and therefore, EPSCs evoked by bundle 1 were likely well clamped. Bundles 2 and 3 resided on hair cells located 30–40 μm from the recording site, so EPSCs evoked by these bundles were most likely transmitted to the recording site via a collateral. Therefore, the faster EPSCs resulting from deflection of bundle 3 (Fig. 2D) cannot be explained by passive cable properties associated with possible lack of space clamp and require an alternative explanation. While one possibility is postsynaptic receptor diversity (Bonsacquet et al. 2006; Harms et al. 2014; Mayer 2011; Pei et al. 2007), another is that transmitter accumulation in the confined calyceal cleft prolongs EPSCs arising at specific synaptic sites (see Fig. 4 and discussion below) (Sadeghi et al. 2014). In the latter case, heterogeneity in the relative positions of synaptic ribbons and postsynaptic receptors as well as glutamate clearance could further contribute to the diversity in EPSC kinetics reported here (Dalet et al. 2012).
Fig. 2.
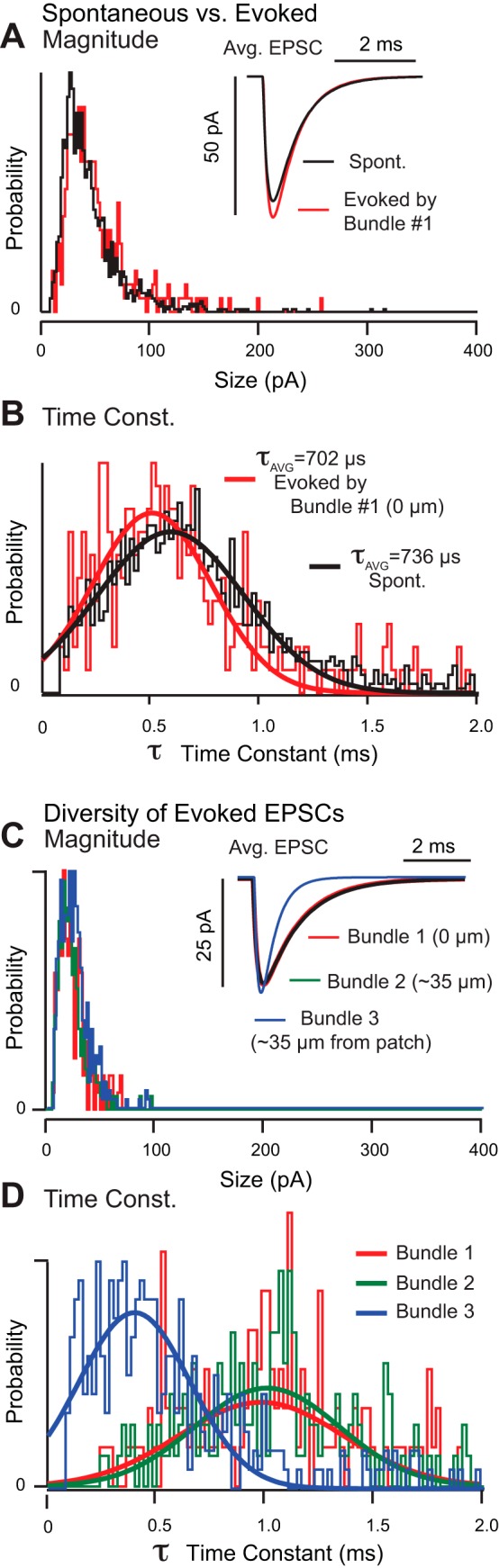
Spontaneous vs. evoked EPSC kinetics. A and B: probability histograms showing amplitudes (pA) and decay time constants (ms) for spontaneous EPSCs (black) and hair bundle deflection evoked EPSCs (red) recorded from a single calyx under whole cell voltage clamp. Average EPSCs are shown in the insets. C and D: stimulus evoked EPSCs recorded from a second calyx during deflection of 3 different hair bundles. EPSC size distributions in this calyx were relatively uniform regardless of which particular hair bundle was deflected, but decay time constants differed substantially. Bundle 1 stimulated the hair cell enveloped by the calyx at the recording site, while bundles 2 and 3 were located ∼30–40 μm from the recording site and presumably stimulated hair cells contacted via a collateral.
Fig. 4.
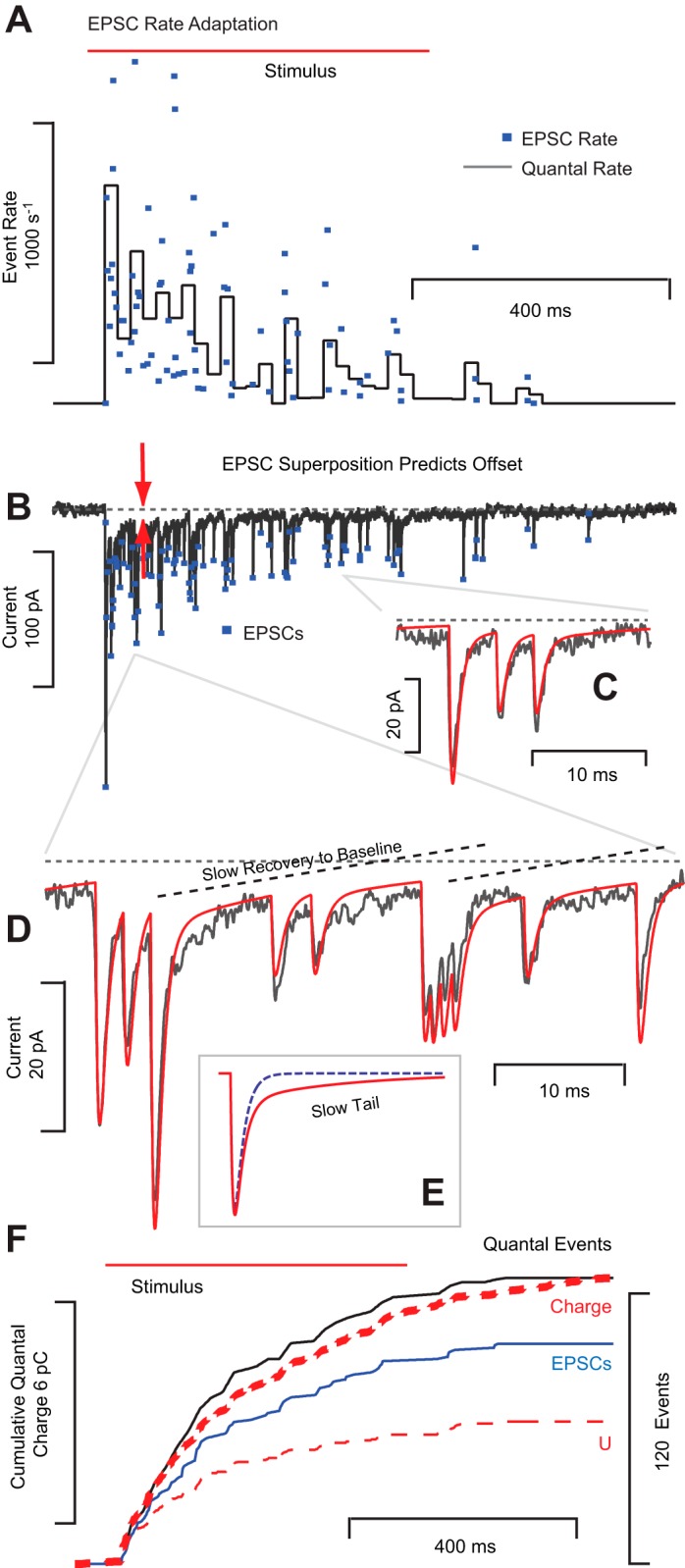
EPSC rate adapts during tonic hair bundle displacements. Example recordings from a single calyx. A: hair bundle deflection increased the rate of EPSCs from zero to >1,000 s−1 followed by a period of adaptation back to zero. Dots denote the instantaneous EPSC rate and the solid cityscape line (black) is an estimate of unitary quantal release rate reported in 20-ms bins. B: raw voltage-clamp data reveal adaptation of EPSC rate and amplitude with time. C: EPSC time constants did not change with amplitude, suggesting larger events at the onset of the stimulus arise from a higher probability of simultaneous quanta. D: data were well described by an integer convolution of unitary events combined with a model of transmitter clearance to capture the slow recovery to baseline. E: simulated EPSCs with (solid red) and without buildup (dashed) illustrating the slow tail that hypothetically could arise from glutamate buildup in the cleft. F: cumulative charge (thick red dashed) and cumulative quantal events (black) imply a unitary quantal size of ∼53 pC, including the slow tail. Neglecting the tail underpredicts the offset and charge (thin red dashed, U). Simultaneous release causes the cumulative number of quantal events (black) to exceed the number of EPSC events (blue).
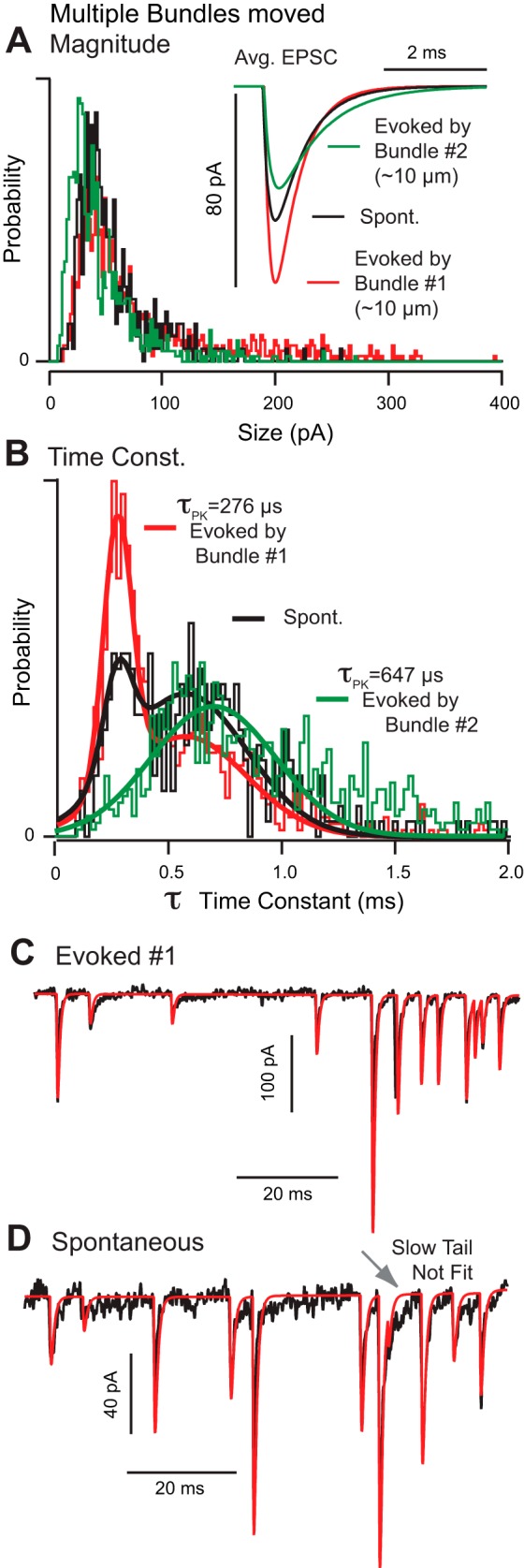
There were significant differences in EPSC size and kinetics depending on which particular type 1 hair cell was stimulated. Figure 3 provides a summary for a typical calyx enveloping multiple type 1 hair cells. The patch pipette was positioned on the calyx adjacent to hair cell 1 (∼10 μm distant from the recording site). Voltage-clamp recordings were collected at rest (spontaneous mEPSCs), during deflection of hair bundle 1, and during deflection of hair bundle 2 (adjacent to bundle 1). Based on the close vicinity of hair cells 1 and 2, it is likely that both cells were enveloped by a single voltage-clamped calyx. Deflection of hair bundle 1 evoked relatively large (85 pA +1.8 SE; k = 1,543 events) and fast (544 μs +9.4 SE) EPSC events at high rate (69 s−1), while deflection of bundle 2 evoked smaller (48 pA +2.1 SE; k = 288 events) and slower (922 μs +28 SE) EPSC events at lower rate (59 s−1). EPSCs evoked by bundle 2 were similar in size but slower than the population averages reported above, while EPSCs evoked by bundle 1 were larger in size and faster than the population averages reported above. Spontaneous mEPSC events were a mixture of these two evoked cases giving rise to a biomodal distribution of time constants (Fig. 3B, black; 58 pA +1.8 SE; 647 μs +20 SE; k = 980 events). Differences between all means were statistically significant (P < 0.05). Without question, EPSC recordings in this calyx strongly depended on which specific hair cell was stimulated; recording conditions were unchanged. Capacitance at the electrode site was 17 pF, raising the possibility that the calyx might not have been completely clamped. Filtering arising from loss of space clamp, however, is unlikely to explain the slow EPSC time constants evoked by bundle 2 in Fig. 3 and certainly cannot explain the slow EPSC time constants shown in Fig. 2 (Fig. 2D, bundles 2 and 3). Recordings in six calyces during movement of multiple type 1 hair cell bundles showed there was no correlation between EPSC kinetics or size and distance from the recording site. Therefore, it is difficult to explain EPSC diversity on the basis of space clamp alone.
To illustrate the difference between slow and fast EPSCs, we used the most probable EPSC decay time constant (276 μs) evoked by bundle 1 (Fig. 3) to reconstruct EPSCs in the time domain (Fig. 3C, red). When the same time constant was used to reconstruct the spontaneous events in the same calyx, the reconstruction failed to reproduce the “slow protracted tail” present in the raw data (Fig. 3D, arrow). This slow tail is the main feature distinguishing slow from fast EPSCs and is reflected in EPSC statistics as a rightward shift in the time constant probability histograms (Figs. 2, B and D, and 3B). Individual events had compact form with the rising phase described by a single exponential and the falling phase described by either one (Fig. 3C) or two exponential time constants (Fig. 3D, arrow, poor fit of single exponential). Clearly, heterogeneity of EPSC kinetics is due, at least in part, to mechanisms underlying heterogeneity in the slow tail. “Noncompact” EPSCs recorded in auditory inner hair cell synapses (Chapochnikov et al. 2014) were not common or easily identified in our preparation.
It has been argued that heterogeneity of EPSC size might arise in part from superposition of unitary quanta released from hair cells. This could be achieved by simultaneous release of multiple vesicles or, alternatively, by flickering release of various size from unitary vesicles (Chapochnikov et al. 2014; Glowatzki and Fuchs 2002; Li et al. 2009). Present results show that solitary EPSC events of differing size evoked by deflection of a single hair bundle all had the same kinetics (e.g., Figs. 1E and 4D). Moreover, we found no significant correlation between EPSC size and time constant (Fig. 1G). Allowing for simultaneous release, it was possible to fit the raw data as a convolution of unitary events. The red current traces in Figs. 3, 4, and 5 were obtained by deconvolution using identical rise and fall time constants for each EPSC in the record and adjusting only the EPSC amplitude as an integer sum of unitary quantal events. The mathematical optimization minimized the difference between the summated quantal events and the raw data while simultaneously maximizing the unitary quantal size, thus providing the maximum quantal size capable of fitting the data. The excellent correspondence between the data and the convolution support the existence of a unitary quantal event. However, the results cannot distinguish whether the unitary quantal event reflects complete release of a single vesicle or reflects quantized partial release. In this sense, the present data are consistent with both the synchronized multivesicular release hypothesis and a quantized version of the univesicular release hypothesis.
EPSC size and the implied number of simultaneous quantal events exhibited adaptive run down during maintained stimulation. This is illustrated in Fig. 4, where a step displacement of a single presynaptic hair cell bundle evoked a barrage of EPSCs that diminished in size and frequency over time. Figure 4A shows the instantaneous rate (dots, inverse of the inter-EPSC interval) and an estimate of the unitary event rate (gray histogram, reported in 20-ms bins; see methods). Note that the rates returned to prestimulus levels over time, a phenomenon termed rate adaptation herein. In addition to rate adaptation, the size of individual EPSCs reduced over time (Fig. 4B), implying that the probability of simultaneous events decreased during the maintained stimulus. As noted in a previous report (Highstein et al. 2014), the rate of EPSCs evoked by bundle displacements in the turtle lagena exhibits adaptive run down in 90% of cells tested, with an average time constant of 899 ms (115 minimum, 2,760 maximum). The magnitude of EPSC rate adaptation in these cells averaged 224 s−1 (0 min, 749 max, n = 10 calyces) and the extent averaged 72% (0 min, 100% max).
A simple rise-fall exponential was adequate to fit most solitary EPSC events but failed to reproduce the slow protracted recovery (Fig. 4, C and D) appearing in some records. Although the EPSCs in Fig. 4 had long tails that slowly decayed to baseline with a time constant of ∼15 ms (Fig. 3D), not all recordings exhibited the protracted tail. Therefore, this tail is unlikely to be a function of postsynaptic receptor kinetics. Alternatively, the tail could arise from transmitter clearance kinetics in the cleft, as suggested previously based on data in the rat semicircular canal crista (Sadeghi et al. 2014). If so, then differences in EPSC kinetics following stimulation of individual hair cells might primarily reflect differences in morphology of the calyx cleft, the relative positions of synaptic ribbons and receptors, and clearance protein expression (Dalet et al. 2012), rather than differences in postsynaptic receptor phenotype or attributes of presynaptic release. Present results are consistent with this interpretation. Deconvolution of the current trace using EPSC events that included the slow protracted tail reproduced the data (Fig. 4D), while neglecting the protracted tail (Fig. 4E, blue dashed, ϕ = 0 in Eq. 2) failed to reproduce the slow recovery to baseline.
From the standpoint of neurotransmission, the physiological consequence of the protracted EPSC tail is to increase the depolarizing charge delivered to the postsynaptic neuron, at the expense of temporal fidelity of the EPSC. This is illustrated in Fig. 4F, which shows the cumulative electrical charge (thick red dashed line) and the cumulative number of quantal events (black line). Dividing the final cumulative charge by the total number of quantal events provides ∼53 fC per unitary event, including the tail. In the absence of a tail, a simple exponential rise-fall EPSC estimates ∼27 fC per unitary event for this cell and substantially underpredicts the total cumulative depolarizing charge (U, thin dashed red line). This suggests that the protracted tail can nearly double the net depolarizing postsynaptic charge. In this cell (Fig. 4B), the current arises completely from summation of quantal events (there was no nqEPSC in this cell). For high EPSC rates, however, the slow component of the current summated to generate what resembled a tonic nonquantal current. In fact, all quantal events are blocked by compounds acting on glutamate receptors, thus proving that the long tail illustrated in this record arises from prolonged postsynaptic receptor opening and not from nonquantal synaptic transmission (Glowatzki and Fuchs 2002; Highstein et al. 2014; Holt et al. 2007). It interesting to note that the range of unitary quanta reported here in calyx terminals of the turtle lagena, with and without the protracted tail, bracket the 45 fC unitary charge reported previously in bouton terminals of the bullfrog amphibian papilla (Li et al. 2009), thus revealing similar unitary quantal charge across diverse hair cell synapses.
The main difference between EPSC kinetics reported here in calyx terminals of the turtle lagena and previous data in calyx terminals of the rat crista (Sadeghi et al. 2014) is the speed and prevalence of the slow protracted tail. In both preparations, the slow tail is most easily seen in small amplitude EPSCs (<50 pA) but the decay is faster (ϕ) and the size of the slow component (τS) is smaller in the turtle lagena relative to the rat crista. As a result, the protracted tail has very little impact on the correlation between EPSC size and time constant in the turtle lagena (Fig. 1G) but a large impact in the rat crista (Sadeghi et al. 2014). From a signal processing point of view, the faster decay in the lagena would sharpen the temporal fidelity of postsynaptic depolarizations relative to those in the crista and enhance the sensitivity to phasic signals such as sound or vibration. This is perhaps not surprising given the dual function of the lagena in auditory and vestibular sensation (Locke et al. 1999; Lu et al. 2003). The specific molecular mechanisms responsible for this difference are not known, but they could simply reflect differences in kinetics associated with cleft morphology and relative localization/expression of ribbons, receptors, and clearance proteins (Sadeghi et al. 2014).
Consistent with previous reports, quantal release was often accompanied by a nqEPSC, with some cells exhibiting only quantal events, some only nonquantal events, and others a mixture of the two (Highstein et al. 2014; Songer and Eatock 2013). Figure 5 provides an example of a calyx that responded to deflection of a single hair bundle with a barrage of EPSCs that adapted over time (Fig. 5, A and B) and with a nqEPSC that was maintained at an indefatigable tonic level throughout the duration of the stimulus. This demonstrates that a single hair-cell calyx synapse has the ability to signal tonic (nqEPSC) and phasic (quantal EPSCs) hair bundle displacements simultaneously, and suggests that in type 1 hair cells presynaptic vesicular release adapts while tonic nonquantal synaptic transmission is maintained.
DISCUSSION
The present report examined the quantal component of synaptic transmission from type 1 vestibular hair cells to postsynaptic calyx terminals in the vestibular lagena of the turtle. Both spontaneous and stimulus evoked EPSCs were examined. Results demonstrate that the size and kinetics of individual EPSC events vary depending on which particular presynaptic hair cell was activated. The data suggest the fundamental unitary quantal event is ∼26 fC and that individual EPSC events consist of a summation of precisely aligned unitary quanta. The average number of unitary quanta per EPSC increased during hair cell stimulation (e.g., Fig. 3, ∼1.5 to ∼2), thereby increasing the likelihood that a single EPSC event would evoke a phase locked action potential in the postsynaptic afferent neuron. The rate of stimulus evoked EPSCs showed adaptive run down over time (74% on average) suggesting that postsynaptic depolarization and phase locking would also run down with time. These features make the quantal component of transmission particularly sensitive to phasic stimuli and the onset of tonic stimuli. The unitary quantal charge was increased in some cells by a slow protraction of the EPSC tail. In these cells, stimulus evoked EPSCs were more likely to overlap in time to increase excitation of the postsynaptic afferent neuron (e.g., Fig. 4). Stimulus evoked quantal events were accompanied by indefatigable nonquantal transmission, affording the synapse a means to transmit tonic signals such as gravity or low frequency movements. This dual mode of synaptic transmission, using precisely timed EPSCs for phasic signals and nqEPSCs for tonic signals, could be particularly advantageous for organs such as the lagena and saccule, which convey both vestibular and auditory signals to the brain. These organs predate the mammalian cochlea, where inner hair cells have developed to exhibit very large multiquantal EPSCs specialized to transmit precisely timed auditory signals remotely reminiscent of the EPSCs reported here in the turtle lagena.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01-DC-008142 (to S. M. Highstein) and R01-DC-006685 (to R. D. Rabbitt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M.H., M.A.M., G.R.H., and R.D.R. conception and design of research; S.M.H., M.A.M., and R.D.R. performed experiments; S.M.H., M.A.M., G.R.H., and R.D.R. interpreted results of experiments; S.M.H. and R.D.R. drafted manuscript; M.A.M. and R.D.R. analyzed data; M.A.M., G.R.H., and R.D.R. edited and revised manuscript; M.A.M., G.R.H., and R.D.R. approved final version of manuscript; R.D.R. prepared figures.
REFERENCES
- Bonsacquet J, Brugeaud A, Compan V, Desmadryl G, Chabbert C. AMPA type glutamate receptor mediates neurotransmission at turtle vestibular calyx synapse. J Physiol 576: 63–71, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Macleod KM. Microseconds matter. PLoS Biol 8: e1000405, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapochnikov NM, Takago H, Huang CH, Pangrsic T, Khimich D, Neef J, Auge E, Gottfert F, Hell SW, Wichmann C, Wolf F, Moser T. Uniquantal release through a dynamic fusion pore is a candidate mechanism of hair cell exocytosis. Neuron 83: 1389–1403, 2014. [DOI] [PubMed] [Google Scholar]
- Contini D, Zampini V, Tavazzani E, Magistretti J, Russo G, Prigioni I, Masetto S. Intercellular K(+) accumulation depolarizes type I vestibular hair cells and their associated afferent nerve calyx. Neuroscience 227: 232–246, 2012. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175: 256–267, 2006. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res 210: 347–352, 2011. [DOI] [PubMed] [Google Scholar]
- Dalet A, Bonsacquet J, Gaboyard-Niay S, Calin-Jageman I, Chidavaenzi RL, Venteo S, Desmadryl G, Goldberg JM, Lysakowski A, Chabbert C. Glutamate transporters EAAT4 and EAAT5 are expressed in vestibular hair cells and calyx endings. PLoS One 7: e46261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR. Directional selectivity and frequency tuning of midbrain cells in the oyster toadfish, Opsanus tau. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189: 527–543, 2003. [DOI] [PubMed] [Google Scholar]
- Favre D, Sans A. Embryonic and postnatal development of afferent innervation in cat vestibular receptors. Acta Otolaryngol 87: 97–107, 1979. [DOI] [PubMed] [Google Scholar]
- Furukawa T. Sound reception and synaptic transmission in goldfish hair cells. Jpn J Physiol 36: 1059–1077, 1986. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Matsuura S. Adaptive rundown of excitatory post-synaptic potentials at synapses between hair cells and eight nerve fibres in the goldfish. J Physiol 276: 193–209, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5: 147–154, 2002. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. The Vestibular System: a Sixth Sense. New York: Oxford Univ. Press, 2012, p. xiii. [Google Scholar]
- Gulley RL, Bagger-Sjoback D. Freeze-fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea-pig vestibular system. J Neurocytol 8: 591–603, 1979. [DOI] [PubMed] [Google Scholar]
- Harms JE, Benveniste M, Kessler M, Stone LM, Arai AC, Partin KM. A charge-inverting mutation in the “linker” region of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors alters agonist binding and gating kinetics independently of allosteric modulators. J Biol Chem 289: 10702–10714, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held H. Die centrale Gehörleitung. Archiv Anatomie Physiologie/Anatomische Abteilung 201–248, 1893. [Google Scholar]
- Highstein SM, Holstein GR, Mann MA, Rabbitt RD. Evidence that protons act as neurotransmitters at vestibular hair cell-calyx afferent synapses. Proc Natl Acad Sci USA 111: 5421–5426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol 98: 1083–1101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol 472: 615–663, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell's afferent synapse. Proc Natl Acad Sci USA 103: 5537–5542, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci 17: 3312–3321, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Cho S, von Gersdorff H. Phase-locking precision is enhanced by multiquantal release at an auditory hair cell ribbon synapse. Neuron 83: 1404–1417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Keen E, Andor-Ardo D, Hudspeth AJ, von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse. J Neurosci 29: 7558–7568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Kindig AE, Donne SW, Callister RJ, Brichta AM. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp Brain Res 210: 607–621, 2011. [DOI] [PubMed] [Google Scholar]
- Locke R, Vautrin J, Highstein S. Miniature EPSPs and sensory encoding in the primary afferents of the vestibular lagena of the toadfish, Opsanus tau. Ann NY Acad Sci 871: 35–50, 1999. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Etudes sur l'onatomie et la physiologie du labyrinth de l'orielle et du VIII nerf, deuxiene partie. Travaux Lab Recherches Biologiques L'Universite Madrid 24: 53, 1926. [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Acoustic response properties of lagenar nerve fibers in the sleeper goby, Dormitator latifrons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189: 889–905, 2003. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Coding of acoustic particle motion by utricular fibers in the sleeper goby, Dormitator latifrons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190: 923–938, 2004. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Takumi Y, Nakagawa T, Usami S, Shinkawa H, Ottersen OP. Immunoelectron microscopy of AMPA receptor subunits reveals three types of putative glutamatergic synapse in the rat vestibular end organs. Brain Res 819: 58–64, 1999. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure 19: 1370–1380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ Jr. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci 14: 6058–6070, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res 103: 174–178, 1995. [DOI] [PubMed] [Google Scholar]
- Pei W, Huang Z, Niu L. GluR3 flip and flop: differences in channel opening kinetics. Biochemistry 46: 2027–2036, 2007. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Streeter MA. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol 95: 26–32, 2006. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehoörorgan der Wirbelthiere: Morphologisch-Histologische Studien/Das Gehoörorgan der Fische und Amphibien: Mit 35 Tafeln. Stockholm: Gedruckt in der Centraldruckerei: in Commission bei Samson & Wallin, 1881. [Google Scholar]
- Retzius G. Das Gehoörorgan der Wirbelthiere: Morphologisch-Histologische Studien/Das Gehoörorgan der Reptilien, der Voögel und der Saöugethiere. Stockholm: Gehoörorgan der Wirbelthiere, 1884. [Google Scholar]
- Rowe MH, Neiman AB. Information analysis of posterior canal afferents in the turtle, Trachemys scripta elegans. Brain Res 1434: 226–242, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J Comp Neurol 210: 239–257, 1982. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E. Glutamatergic signaling at the vestibular hair cell calyx synapse. J Neurosci 34: 14536–14550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schessel DA, Ginzberg R, Highstein SM. Morphophysiology of synaptic transmission between type I hair cells and vestibular primary afferents. An intracellular study employing horseradish peroxidase in the lizard, Calotes versicolor. Brain Res 544: 1–16, 1991. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Santos-Sacchi J, Castellano-Munoz M, Kong JH, Ricci AJ. Calcium-dependent synaptic vesicle trafficking underlies indefatigable release at the hair cell afferent fiber synapse. Neuron 70: 326–338, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH. Spontaneous synaptic potentials from afferent terminals in the guinea pig cochlea. Hear Res 59: 85–92, 1992. [DOI] [PubMed] [Google Scholar]
- Silver RA, Cull-Candy SG, Takahashi T. Non-NMDA glutamate receptor occupancy and open probability at a rat cerebellar synapse with single and multiple release sites. J Physiol 494: 231–250, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7: 826–833, 2004. [DOI] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Sewell WF. Neurotransmitter release from hair cells and its blockade by glutamate-receptor antagonists. Hear Res 52: 23–41, 1991. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeg MS, Fay RR, Bass AH. Directionality and frequency tuning of primary saccular afferents of a vocal fish, the plainfin midshipman (Porichthys notatus). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 631–641, 2002. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hear Res 68: 189–201, 1993. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ohmori H. Synaptic responses to mechanical stimulation in calyceal and bouton type vestibular afferents studied in an isolated preparation of semicircular canal ampullae of chicken. Exp Brain Res 80: 475–488, 1990. [DOI] [PubMed] [Google Scholar]
- Young ED, Fernandez C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol 84: 352–360, 1977. [DOI] [PubMed] [Google Scholar]
- Zhu H, Tang X, Wei W, Maklad A, Mustain W, Rabbitt R, Highstein S, Allison J, Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. J Assoc Res Otolaryngol 15: 73–86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


