Abstract
The fragile histidine triad (FHIT) gene is known to be a tumor suppressor gene and the abnormal methylation of FHIT has been identified in leukemia and several solid tumors. The transformation of the tumor F6 cell line from human fetal mesenchymal stem cells (FMSCs) was first reported in a previous study that also identified the presence of a population of cancer stem cells in the F6 cell line. However, the existence of the epigenetic changes during the transformation process have yet to be elucidated. To confirm the role of the FHIT gene in the transformation process of FMSC, the expression level and methylation status of the FHIT gene was examined in F6 tumor cells and FMSCs. Additionally, the alteration in cell morphology, the cell cycle and apoptosis in F6 cells following 5-Aza-CdR treatment was assessed. It was found that the FHIT gene was expressed in FMSCs, but not in F6 cells. The methylation-specific PCR results demonstrated that the promoter methylation of FHIT genes existed in the F6 cell line. Subsequent to treatment with 5-Aza-CdR the expression of FHIT genes was restored in F6 cells. In addition, the morphology of F6 cells was altered, and the cell cycle was arrested in the G2 phase, with the initiation of apoptosis. Overall, the present findings demonstrated that the FHIT gene was methylated in F6 cells and demethylation treatment lead to changes in the biological characteristics, thereby promoting the apoptosis of F6 cells. FHIT gene methylation may be one of the molecular events involved in the development and transformation of FMSCs into F6 tumor cells.
Keywords: DNA methylation, fragile histidine triad, fetal mesenchymal stem cells, F6 cells
Introduction
Mesenchymal stem cells (MSCs) are pluripotent adult stromal cells that possess the ability to differentiate into bone, cartilage and adipose tissues, and the cells exhibit promising potential for regenerative medicine (1). An increasing number of studies have demonstrated that MSCs demonstrate potential therapeutic effects for multiple diseases, including liver injury, ischemia/reperfusion-induced acute kidney injury and acute myocardial infarction (2–4). However, transplantation therapy using MSCs continues to be subject to investigation, particularly into the safety of MSCs in clinical application. In a previous study that aimed to determine the induction of MSC differentiation and immune function, tumorigenesis was observed unexpectedly and a cloned a novel tumor cell line, the F6 cell line, transformed from human fetal bone marrow MSCs (5). These findings initiated interest into investigating the mechanism of mutation in fetal MSCs (FMSCs).
Previous studies have demonstrated that epigenetic and genetic alterations markedly contribute to tumorigenesis. The hypermethylation of tumor suppressor genes has been detected in various types of tumors (6). Ohta et al first reported the tumor suppressor gene fragile histidine triad (FHIT), providing the molecular evidence for the association between fragile sites and cancer (7). The loss or reduction of FHIT expression has been revealed to be important in the initiation of tumorigenesis in a variety of tumors (8,9). It has also been reported that aberrant methylation of the FHIT gene occurs in certain types of cancer (10,11). Therefore, the methylation of the FHIT gene within F6 tumor cells may be a novel direction for the identification of the mechanism of transformation of FMSCs.
DNA methylation plays an important role in cancer development, and has also become a therapeutic target due to the reversibility of DNA methylation. The DNA methyltransferase (DNMT) inhibitor, 5-Aza-2′-deoxycytidine (5-Aza-CdR) is able to restore the expression of genes that induce growth arrest and apoptosis in tumor cells (12,13). In the F6 tumor cell line, the effect of 5-Aza-CdR on the cell cycle and cell apoptosis requires additional investigation.
The aim of the present study is to investigate the methylation status of the FHIT gene promoter in FMSC and F6 cells, and explore the effect of 5-Aza-CdR on the growth and apoptosis of FMSC and F6 cells. The present results revealed that methylation of the promoter of the FHIT gene occurred in the F6 cell line. The expression of the FHIT gene was restored in F6 cells, resulting in growth arrest and apoptosis, subsequent to treatment with 5-Aza-CdR. The present results indicated the significant role of FHIT in the malignant transformation of FMSCs and provided a potential target for tumor therapy.
Materials and methods
Cell culture
Human FMSCs and F6 cells were from our laboratory and cultured as previous described (5). FMSCs were derived from fetal bone marrow and the F6 cells were the mutated cell line. FMSCs and F6 cells were maintained in Dulbecco's modified Eagle's medium with low glucose (LG-DMEM; Gibco Life Technologies, Carlsbad, CA, USA) that was supplemented with 10% fetal bovine serum (FBS; Gibco Life Technologies), 100 units/ml penicillin and 100 µg/ml streptomycin. The cells were incubated at 37°C in a 5% CO2 atmosphere.
Reverse transcription polymerase chain reaction (RT-PCR)
The total RNA of the MSCs and F6 cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized using 4 mg of total RNA as the template and Oligo(dT) as the primer, with the SuperScript II RT kit (Invitrogen), according to the manufacturer's instructions. The cDNA was stored at −20°C until RT-PCR was performed. The primer sequences were designed as described in Table I.
Table I.
Primer sequences for the amplification of target genes.
| Genes | Primer sequence | Amplicon size, bp | Annealing temperature, °C |
|---|---|---|---|
| FHIT-M | F, 5′-AGGTACGGGGTTACGTTAGC-3′ | 133 | 60 |
| R, 5′-CAAACCTATTAAAACGAATTTTGC-3′ | |||
| FHIT-U | F, 5′-AATTGAGGTATGGGGTTATGTTAGT-3′ | 136 | 56 |
| R, 5′-AAACCTATTAAAACAAATTTTCACT-3′ | |||
| FHIT | F, 5′-CACGAAACTACCTTCAACTCC-3′ | 244 | 60 |
| R, 5′-AACTGTCCTTCGCTCTTGTG-3′ | |||
| β-actin | F, 5′-CACGAAACTACCTTCAACTCC-3′ | 265 | 56 |
| R, 5′-CATACTCCTGCTTGCTGATC-3′ |
M, methylation primer; U, forward primer for unmethylated genes; F, forward; R, reverse; FHIT, fragile histidine triad.
DNA isolation and bisulfite modification
DNA was isolated from F6 cells and FMSC using Universal Genomic DNA Extraction kit (Takara Bio, Inc., Otsu, Japan). Subsequently, 1 µg of genomic DNA was modified using the CpGenome DNA Modification kit (Chemicon, Temecula, CA, USA) according to the manufacturer's instructions. The modified DNA was resuspended in water and used immediately or stored at −70°C until use. Bisulfite treatment converted unmethylated cytosines to uracils, while leaving the methylated cytosines unaffected.
Methylation-specific PCR and sequencing
Methylation-specific PCR was performed as described by Lin et al (14). DNA methylation patterns in the CpG island of FHIT were determined by chemical treatment with sodium bisulfite and subsequent use of the aforementioned PCR procedure. Primer sequences for the methylated FHIT reaction (M) and primer sequences for the unmethylated FHIT reaction (U) are described in Table I. The reaction mixture (25 µl) contained 10X PCR buffer, 2.5 mmol/l MgCl2, 2.5 mmol/l deoxyribonucleotide triphosphates, 0.25 units Hot Start DNA polymerase (Takara Bio, Inc.) and 2 µl of modified DNA. Subsequent to the initial denaturation at 95°C for 10 min, 40 cycles of 45 sec at 94°C, 45 sec at 65°C (M) or 63°C (U) and 45 sec at 72°C were performed. These cycles were followed by a final 7 min elongation step at 72°C. DNA was treated in vitro with DNA methylase from Spiroplasma sp. strain MQ1, and untreated DNA was used as the positive control for the methylated DNA. Negative control samples that lacked DNA were included for each set of PCR. The PCR products were analyzed on 2% agarose gels and visualized under the Gene Genius imaging system (Synoptics Inc., Santa Clara, CA, USA) subsequent to ethidium bromide staining. Products of the methylation reaction of the FHIT gene DNA in F6 cells were cloned and sequenced (Shanghai Genecore Biotechnologies Co., Ltd., Shanghai, China).
Treatment with 5-Aza-CdR
The F6 cells or FMSCs were plated into six-well plates. After 24 h, 5-Aza-CdR was administered to each well at a concentration of 1, 5, 25, 50 or 100 µM, and the morphological changes were observed at 4, 8, 12 and 24 h. Subsequent to treatment with 5-Aza-CdR, the expression of the FHIT gene was detected by RT-PCR. Flow cytometry and Hoechst 33342 staining were used to detect DNA content and apoptosis. All images were obtained using a fluorescent microscope (Eclipse Ti-S; Nikon, Tokyo, Japan).
Statistical analysis. Differences between the groups were examined for statistical significance using Student's t-test and P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using GraphPad Prism 5 software (Graph Pad Software, Inc., La Jolla, CA, USA). All results were expressed as the mean ± standard deviation and all experiments were repeated at least three times.
Results
FHIT gene expression level in FMSCs and F6 cells
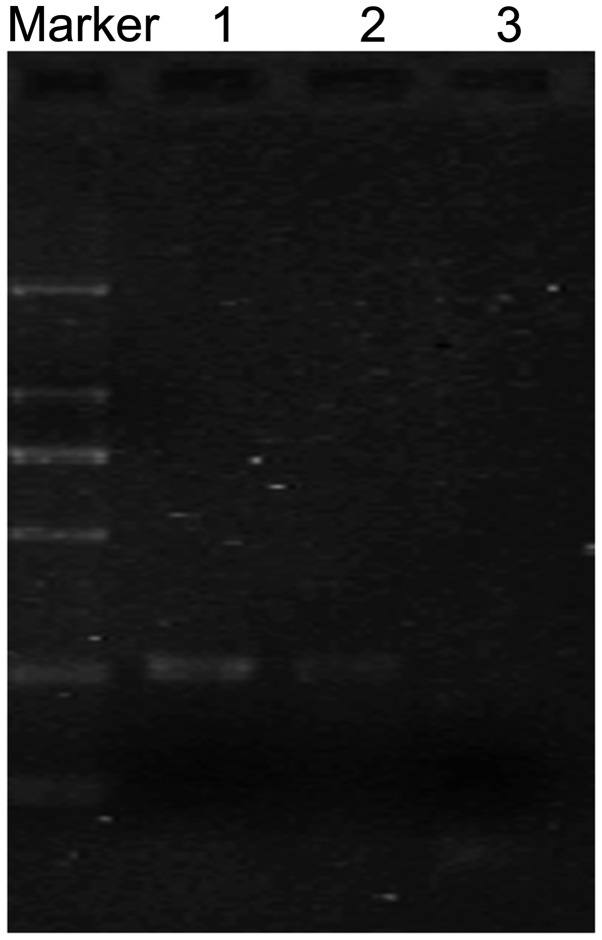
The expression of FHIT was initially detected in FMSCs and F6 cells by RT-PCR. The results revealed that FHIT mRNA was detectable in FMSCs, but not in F6 cells (Fig. 1).
Figure 1.
The expression of the FHIT gene in F6 cells and FMSCs. The mRNA levels of FHIT were determined by reverse transcription polymerase chain reaction. Marker, DL2000; lane 1, FMSCs; lane 2, F6 cells, lane 3, blank. FHIT, fragile histidine triad; FMSCs, fetal mesenchymal stem cells.
Methylation status of the FHIT gene in F6 cells
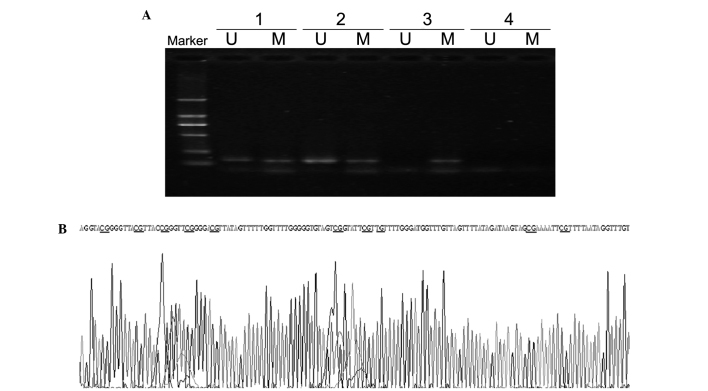
The methylation status of the FHIT gene in F6 cells was detected by methylation-specific PCR. The promoter region of FHIT contains numerous CpG islands. The results from methylation-specific PCR revealed that FMSCs were amplified by un-methylated and methylated primers, while F6 cells were amplified by methylated primers (Fig. 2A). Sequencing of bisulfite-treated DNA was performed to investigate the methylation status of the FHIT gene. The results of sequencing demonstrated that FHIT gene promoter methylation occurred in F6 cells (Fig. 2B).
Figure 2.
Methylation status of the FHIT gene in F6 cells and FMSCs. (A) Methylation-specific PCR of the FHIT gene. U and M represent PCR results by using primer sets for methylated and unmethylated FHIT gene, respectively. Lane 1, positive controls for methylation and unmethylation, comprising genomic DNA from normal bone marrow that was modified with or without DNA methylase from Spiroplasma sp. strain MQ1. Lane 2, FMSCs. Lane 3, F6 cells. Lane 4, double-distilled H2O control. Marker, DL2000 DNA ladder. (B) Sequencing of the methylated FHIT gene promoter in F6 cells. The CG sequences were not changed subsequent to bisulfite treatment. FHIT, fragile histidine triad; PCR, polymerase chain reaction; U, unmethylated; M, methylated; FMSCs, fetal mesenchymal stem cells.
Change in FHIT gene expression subsequent to treatment with 5-Aza-CdR
Subsequent to treatment with 50 µM 5-Aza-CdR for 8 h, the expression of the FHIT gene was detected in F6 cells by RT-PCR. The results demonstrated that the FHIT gene was expressed in F6 cells (Fig. 3).
Figure 3.
The expression of the FHIT gene in F6 cells induced by 5-Aza-CdR. Subsequent to treatment with 5-Aza-CdR for 8 h, the mRNA levels of FHIT were detected in F6 cells by reverse transcription polymerase chain reaction. Marker, DL2000; lane 1, fetal mesenchymal stem cells; lane 2, F6 cells; lane 3, double-distilled H2O control. FHIT, fragile histidine triad; 5-Aza-CdR, 5-Aza-2′-deoxycytidine.
Morphological characteristics and cell cycle
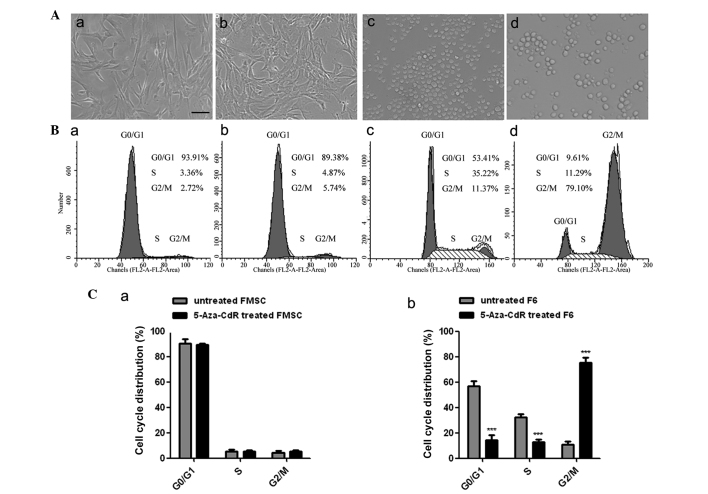
The cell morphology of F6 and FMSCs was assessed subsequent to treatment with 5-Aza-CdR for 8 h. It was found that treatment with 5-Aza-CdR inhibited the growth of F6 cells in vitro compared with the control. The F6 cells became enlarged, and the frequency of cell granulations increased. However, the morphology of FMSCs remained unchanged at the same concentration of 5-Aza-CdR treatment (Fig. 4A). Flow cytometry analysis revealed that the proportion of F6 cells in the G2 phase was markedly increased following treatment with 5-Aza-CdR, while the cell cycle of the FMSCs was not changed (Fig. 4B and C).
Figure 4.
Morphological characteristics and DNA content of F6 and FMSCs. (A) Cell morphology of (a) untreated FMSC, (b) 5-Aza-CdR-treated FMSC, (c) F6 and (d) 5-Aza-CdR-treated F6 (original magnification, ×200; scale bar, 100 µm). (B) Flow cytometry assay of (a) untreated FMSC, (b) 5-Aza-CdR-treated FMSC, (c) F6 and (d) 5-Aza-CdR treated F6. (C) Cell cycle distribution of (a) FMSC and (b) F6. The results were obtained from three independent experiments. ***P<0.001. FMSC, fetal mesenchymal stem cells; 5-Aza-CdR, 5-Aza-2′-deoxycytidine; F6, F6 cells.
Apoptosis in F6 cells treated with 5-Aza-CdR
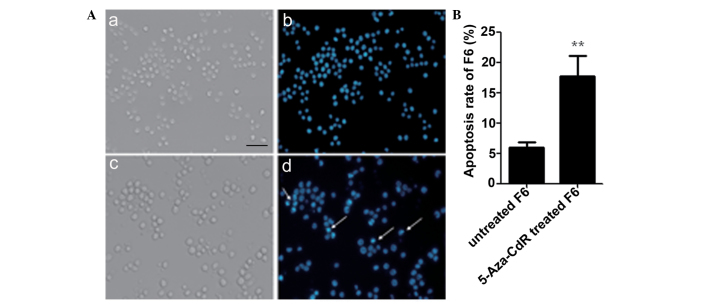
As demonstrated by the Hoechst 33342 staining results in Fig. 5, the proportion of apoptotic F6 cells was increased by 5-Aza-CdR. The nuclei of the apoptotic F6 cells were pyknotic with fragmentation that was stained bright blue (Fig. 5A). The apoptotic ratio of F6 cells was increased following 5-Aza-CdR treatment compared with the untreated cells (P<0.01; Fig. 5B).
Figure 5.
Apoptosis assay of F6 cells treated with 5-Aza-CdR and stained by Hoechst 33342. (A) Morphology of (a) untreated F6, (b) untreated F6 stained with Hoechst 33342, (c) F6 induced by 5-Aza-CdR, (d) F6 stained with Hoechst 33342 subsequent to treatment with 5-Aza-CdR (original magnification, ×200; scale bar, 100 µm). The arrows indicate the apoptotic cells in the field. (B) Apoptosis rate of F6. The results were obtained from three independent experiments. **P<0.01. 5-Aza-CdR, 5-Aza-2′-deoxycytidine; F6, F6 cells.
Discussion
Previously, the methylation of CpG islands in tumor suppressor genes during the initiation and development of tumors initiated general interest as a subject for studies. Methylation of the FHIT gene promoter plays an important role in several types of solid tumor and hematological malignancy (15,16). In the present study, the F6 cell line, a tumor cell line derived from mutant human FMSCs, was focused on. The methylation status of the FHIT gene was investigated, and the growth and apoptosis of F6 cells was observed following demethylation by treatment with 5-Aza-CdR. It was found that the promoter region of FHIT was methylated in the F6 cells. Treatment with 5-Aza-CdR induced an increase in the expression of FHIT and also promoted apoptosis in F6 cells.
Previous studies have indicated that the methylation status of FHIT may lead to the silencing of gene expression, promoting the occurrence and development of acute lymphoblastic leukemia (17). In the present study, the FHIT gene was found to be expressed in FMSCs, but not in F6 cells. To confirm that the silencing of gene expression was due to the methylation of FHIT during the transformation of FMSCs into F6 cells, methylation-specific PCR was used. The methylated FHIT gene was amplified using primers which only anneal to sequences that are methylated. To verify DNA integrity subsequent to bisulfite modification and to determine the specificity of PCR amplification, the products of PCR were cloned and sequenced. The results revealed that the CG sequence in the CpG island was not sulfurized, and the FHIT gene demonstrated complete methylation in F6 cells and partial methylation in FMSCs. These findings indicated that the FHIT gene in FMSCs may methylate progressively until the FMSCs undergo transformation into F6 tumor cells.
Clinically, 5-Aza-CdR is used for the treatment of myelodysplastic syndrome and demonstrates anti-leukemic activity against acute myeloid leukemia (AML). The clinical activity of 5-Aza-CdR against solid tumors is under investigation (18). To confirm the possibility that the silenced expression of the FHIT gene was caused by methylation, the present study examined whether 5-Aza-CdR was able to restore the expression of the FHIT gene. Subsequent to treatment with 5-Aza-CdR, the promoter region of the FHIT gene was identified as demethylated, and the expression of FHIT was increased at the mRNA levels. Following exposure to 5-Aza-CdR for 8 h, the cell cycle and apoptosis were analyzed by flow cytometry. In contrast to the results reported by Sard et al (19), the cell cycle of F6 cells was arrested in the G2 phase, but not in the G0 phase, and apoptosis in F6 cells occurred after 24 h. By contrast, the morphology and cell cycle of FMSCs was not changed throughout this process.
In the present study, 50 µM 5-Aza-CdR induced the expression of the FHIT gene, but when the concentration of 5-Aza-CdR was increased to 100 µM, FHIT gene expression was not restored. This result suggests that a low dose of 5-Aza-CdR affects the demethylation and growth of cells, but a high dose may result in severe side-effects, although a high dose may also increase the rate of apoptosis. Therefore, the concentration and exposure time of 5-Aza-CdR requires optimization in future studies and a reasonable dose schedule is extremely important for clinical application. A previous study revealed that the administration of 5-Aza-CdR in combination with tetrahydrouridine was effective in inhibiting the growth of murine melanoma tumors (20). Thus, 5-Aza-CdR in combination with an additional DNMT may decrease the side-effects of 5-Aza-CdR and provide a novel approach for tumor therapy.
The novel tumor cell line F6 was mutated from human FMSCs, and a previous study confirmed that F6 cells contain a population of cancer stem cells (CSCs) that contribute to the heterogeneity and tumorigenic potential of F6 cells (21). The present study revealed that FHIT gene promoter methylation occurred in F6 cells transformed from FMSCs. Additional studies are required to examine the biological characteristics of F6 cells and the associated pathways in this process. However, it is not clear whether other tumor suppressor genes have epigenetic alterations. The association between DNA methylation and F6 cells or CSCs requires additional investigation.
In summary, the present study demonstrates that the FHIT gene was methylated in F6 cells transformed from FMSCs, and that treatment with 5-Aza-CdR restored the expression of FHIT, altered the biological characteristics of F6 cells and induced apoptosis in F6 cells. The present results not only indicate that the silencing of the tumor suppressor gene FHIT was due to the DNA methylation in the transformed human mesenchymal stem cell F6 cell line, but also clarified the epigenetic mechanism of tumorigenesis and safety of MSCs in clinical application. Methylation of tumor suppressor genes may provide potential molecular targets for tumor therapy and also act as markers in the early detection of cancer.
Acknowledgements
The authors thank Qiaolin Chen for excellent assistance with flow cytometry analysis. This study was supported by the Key Project Supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (grant no., QYK11163).
References
- 1.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Qian H, Zhu W, Zhang X, Yan Y, Mao F, Wang M, Xu H, Xu W. Mesenchymal stem cells relieve fibrosis of Schistosoma japonicum-induced mouse liver injury. Exp Biol Med (Maywood) 2012;237:585–592. doi: 10.1258/ebm.2012.011362. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, Peng X, Li W, Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 4.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Qian H, Zhu W, Chen Y, Shao Q, Sun X, Hu J, Han C, Zhang X. A novel tumor cell line cloned from mutated human embryonic bone marrow mesenchymal stem cells. Oncol Rep. 2004;12:501–508. [PubMed] [Google Scholar]
- 6.Esteller M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 7.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/S0092-8674(00)81034-X. [DOI] [PubMed] [Google Scholar]
- 8.Connolly DC, Greenspan DL, Wu R, Ren X, Dunn RL, Shah KV, Jones RW, Bosch FX, Muñoz N, Cho KR. Loss of fhit expression in invasive cervical carcinomas and intraepithelial lesions associated with invasive disease. Clin Cancer Res. 2000;6:3505–3510. [PubMed] [Google Scholar]
- 9.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, McCue P, Huebner K. Concordant loss of fragile gene expression early in breast cancer development. Pathol Int. 2005;55:471–478. doi: 10.1111/j.1440-1827.2005.01855.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeong YJ, Jeong HY, Lee SM, Bong JG, Park SH, Oh HK. Promoter methylation status of the FHIT gene and Fhit expression: Association with HER2/neu status in breast cancer patients. Oncol Rep. 2013;30:2270–2278. doi: 10.3892/or.2013.2668. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R, Hussain S, Mehrotra R, Kumar RS, Kumar K, Pande P, Doval DC, Basir SF, Bharadwaj M. Kras gene mutation and RASSF1A, FHIT and MGMT gene promoter hypermethylation: Indicators of tumor staging and metastasis in adenocarcinomatous sporadic colorectal cancer in Indian population. PLoS One. 2013;8:e60142. doi: 10.1371/journal.pone.0060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JF, Zhang JG, Kuai XL, Zhang H, Jiang W, Ding WF, Li ZL, Zhu HJ, Mao ZB. Reactivation of the homeotic tumor suppressor gene CDX2 by 5-aza-2′-deoxycytidine-induced demethylation inhibits cell proliferation and induces caspase-independent apoptosis in gastric cancer cells. Exp Ther Med. 2013;5:735–741. doi: 10.3892/etm.2013.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HF, Dai ZJ, Bai HP, Lu WF, Ma XB, Bao X, Lin S, Wang XJ. RUNX3 gene promoter demethylation by 5-Aza-CdR induces apoptosis in breast cancer MCF-7 cell line. Onco Targets Ther. 2013;6:411–417. doi: 10.2147/OTT.S43744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Yao DM, Qian J, Wang YL, Han LX, Jiang YW, Fei X, Cen JN, Chen ZX. Methylation status of fragile histidine triad (FHIT) gene and its clinical impact on prognosis of patients with myelodysplastic syndrome. Leuk Res. 2008;32:1541–1545. doi: 10.1016/j.leukres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Cecener G, Tunca B, Egeli U, Bekar A, Tezcan G, Erturk E, Bayram N, Tolunay S. The promoter hypermethylation status of GATA6, MGMT, and FHIT in glioblastoma. Cell Mol Neurobiol. 2012;32:237–244. doi: 10.1007/s10571-011-9753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths EA, Gore SD, Hooker CM, Mohammad HP, McDevitt MA, Smith BD, Karp JE, Herman JG, Carraway HE. Epigenetic differences in cytogenetically normal versus abnormal acute myeloid leukemia. Epigenetics. 2010;5:590–600. doi: 10.4161/epi.5.7.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Zhang H, Li P, Yang Z, Qin L, Mo W. Gene expression of WWOX, FHIT and p73 in acute lymphoblastic leukemia. Oncol Lett. 2013;6:963–969. doi: 10.3892/ol.2013.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karahoca M, Momparler RL. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2′-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigenetics. 2013;5:3. doi: 10.1186/1868-7083-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo MP, Gramegna M, Croce CM, et al. The tumor-suppressor gene FHIT is involved in the regulation of apoptosis and in cell cycle control. Proc Natl Acad Sci USA. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcazar O, Achberger S, Aldrich W, Hu Z, Negrotto S, Saunthararajah Y, Triozzi P. Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo. Int J Cancer. 2012;131:18–29. doi: 10.1002/ijc.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Qian H, Zhu W, Zhang X, Yan Y, Wang M, Xu W. Isolation of cancer stem cells from transformed human mesenchymal stem cell line F6. J Mol Med Berl. 2010;88:1181–1190. doi: 10.1007/s00109-010-0659-5. [DOI] [PubMed] [Google Scholar]