Abstract
Infection of human neurons in vitro with varicella-zoster virus (VZV) at a low multiplicity of infection does not result in a cytopathic effect (CPE) within 14 days postinfection (dpi), despite production of infectious virus. We showed that by 28 dpi a CPE ultimately developed in infected neurons and that interferon gamma inhibited not only the CPE but also VZV DNA accumulation, transcription, and virus production, thereby prolonging the life of VZV-infected neurons.
TEXT
Varicella-zoster virus (VZV) is a neurotropic alphaherpesvirus that causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. As VZV-specific cell-mediated immunity declines with age and in immunocompromised individuals, VZV reactivates to cause zoster (shingles) and other neurologic diseases (1, 2).
Interferon gamma (IFN-γ) is a potent cytokine produced following primary VZV infection (3, 4). Nonhuman primates experimentally infected with simian varicella virus (SVV), the counterpart of VZV, produce IFN-γ 7 to 11 days postinfection (dpi) (5, 6). IFN-γ also inhibits herpes simplex virus 1 (HSV-1) infection in vitro (7) and in vivo (8–10). Furthermore, VZV reactivation correlates with a decline of VZV-specific IFN-γ-producing immune cells (11). VZV infection of human neurons in vitro is productive, although infected cells appear healthy 2 weeks later (12–14). Here, we tested whether a cytopathic effect (CPE) eventually develops in VZV-infected neurons and, if so, whether IFN-γ treatment suppresses viral growth and promotes neuronal survival after VZV infection.
Like ganglionic sensory neurons which express the IFN-γ receptor on the cell surface (15), immunofluorescent staining revealed that the induced pluripotent stem cell (iPSC)-derived neurons used in this study also expressed the IFN-γ receptor (data not shown). To analyze the antiviral effects of IFN-γ, cultures were either pretreated with tissue culture medium containing 10 ng/ml IFN-γ or left untreated, followed by infection the next day with VZV at a low multiplicity of infection (MOI; 0.001 to 0.0001; Zostavax; Merck) (14, 16, 17) for 3 h in the absence of IFN-γ. After infection, the inoculum was removed and replenished twice weekly for 14 days with medium containing or lacking 10 ng/ml IFN-γ. Immunofluorescence analysis for VZV immediate early (ORF63) and late (ORF68 and gE) proteins at 14 dpi revealed that IFN-γ greatly reduced VZV spread in neurons (Fig. 1).
FIG 1.
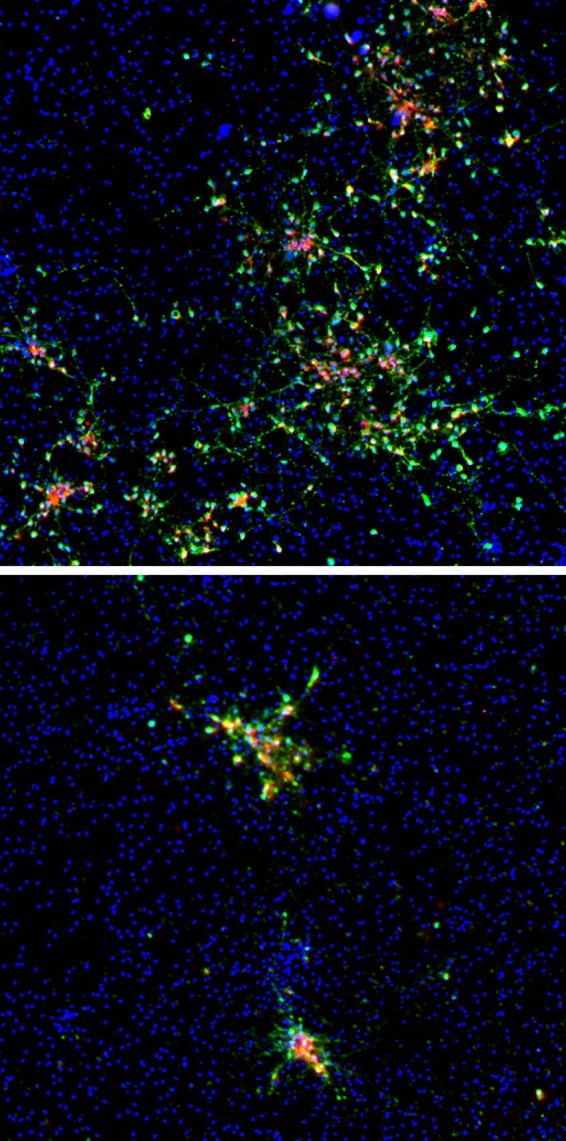
Inhibition of VZV spread in human neurons by IFN-γ. VZV-infected neurons were untreated (top) or treated with IFN-γ (bottom) for 14 dpi, fixed, and immunostained for an immediate early protein (VZV ORF63, green) and a late protein (VZV ORF68, red); 4′,6-diamidino-2-phenylindole staining is blue.
Neurons infected with VZV at a low multiplicity of infection (<0.001) do not develop a CPE 14 dpi and are indistinguishable from uninfected neurons (12, 14), despite extensive viral transcription (16), translation (12, 14), and production of infectious virus (17). However, by 28 dpi, a CPE did develop as evidenced by rounded cell bodies and retracted and fragmented neurites resembling a string of beads (Fig. 2, top panel). In contrast, cells that were pretreated with IFN-γ, infected, and maintained in the presence of IFN-γ for 28 days retained the healthy appearance of plump cell bodies and extended neurites (Fig. 2, bottom panel), features of uninfected neurons (14). Uninfected neurons treated with IFN-γ for 28 days also maintained a healthy appearance and were indistinguishable from untreated cultures (data not shown).
FIG 2.
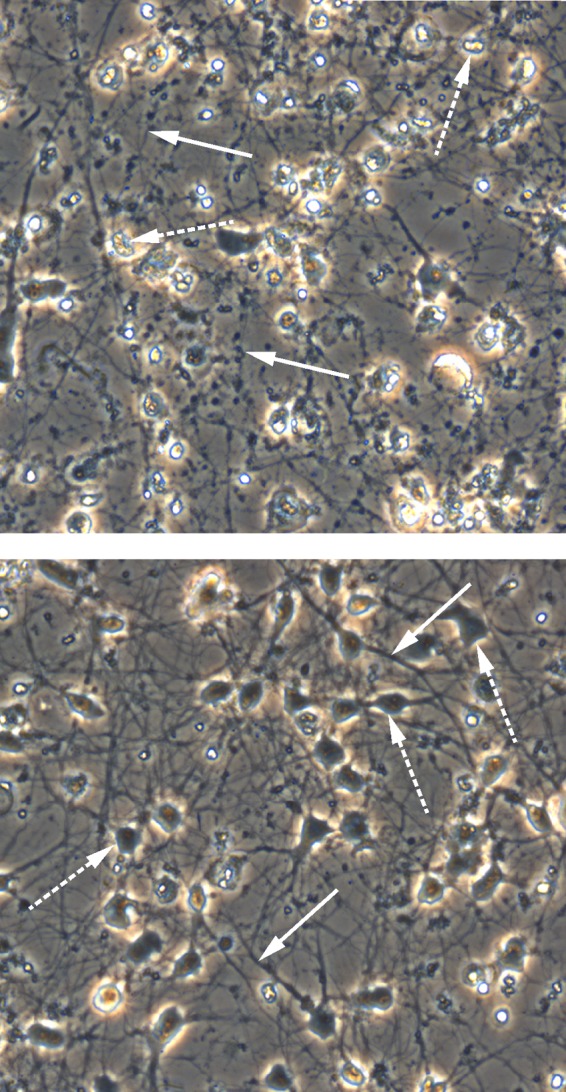
Prevention of VZV-induced CPE in human neurons by IFN-γ. At 28 dpi, CPE developed in VZV-infected neurons (top panel), characterized by rounding of cell bodies (dashed arrows) with retraction and fragmentation of neurites resembling a string of beads (solid arrows). VZV-infected neurons cultured in the presence of IFN-γ remained healthy at 28 dpi (bottom panel), exhibiting large cell bodies (dashed arrows) and extensive neurite outgrowth that forms a mesh throughout the culture (solid arrows).
The effects of IFN-γ treatment on VZV replication and transcription were examined. Untreated and treated cells were infected, maintained as described above, and harvested at 28 dpi. DNA was extracted (GenElute; Sigma, St. Louis, MO) for quantification of viral genomes in 10 ng of total DNA using TaqMan-based quantitative PCR (qPCR) targeted to a region within VZV ORF29 (Fig. 3, left panel) as described previously (17). At 28 dpi, IFN-γ-treated VZV-infected neurons contained 4.4-fold-fewer copies of VZV DNA than did untreated neurons. To analyze VZV transcripts, total RNA was harvested (mirVana; Ambion, Foster City, CA), and mRNA from 100 ng total RNA was bound to oligo(dT) beads (μMACS; Miltenyi, Bergisch Gladbach, Germany), DNase treated (DNase I; Ambion), eluted, and reverse transcribed (Transcriptor First cDNA synthesis kit; Roche, Basel, Switzerland). Compared to untreated cultures, IFN-γ treatment reduced the abundance of VZV transcripts ORF62 (5.4-fold), ORF63 (11.1-fold), ORF29 (5.6-fold), and ORF68 (7.3-fold), encompassing immediate early, early, and late kinetic classes (Fig. 3, right panel).
FIG 3.
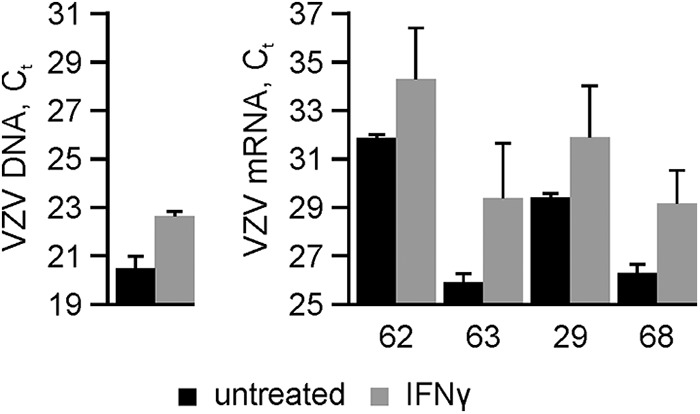
Inhibition of VZV DNA accumulation and viral transcription in VZV-infected human neurons by IFN-γ. VZV-infected human neurons were either untreated (black bars) or treated with IFN-γ (gray bars) for 28 days, when the abundances of viral DNA (left) and transcripts (right) corresponding to VZV ORF62, ORF63, ORF29, and ORF68 were determined. Data are mean cycle threshold (CT) values ± standard deviations from 2 independent cultures.
Production of infectious virus after IFN-γ treatment was examined by releasing treated and untreated VZV-infected neurons with trypsin at 28 dpi. Neurons were then cocultivated with uninfected fibroblasts and observed for plaque formation. At 28 dpi, when a CPE developed in untreated neurons, 69 ± 1 PFU were found, compared to 31 ± 5 PFU in IFN-γ-treated cultures (Fig. 4).
FIG 4.
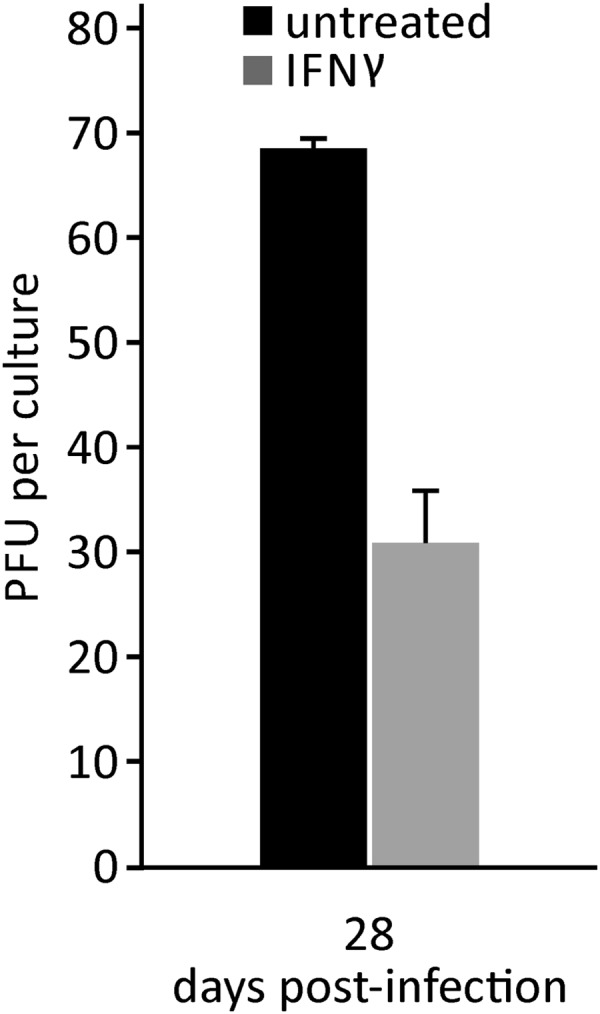
Reduced production of infectious VZV in human neurons by IFN-γ. VZV-infected human neurons were either untreated (black bar) or IFN-γ treated (gray bar) for 28 days. At 28 dpi, when a CPE developed in untreated neurons, PFU were determined. Data are means ± standard deviations from 2 independent cultures.
Overall, IFN-γ prolonged the life of VZV-infected neurons by inhibiting viral growth, reducing VZV genome content and transcript abundance, and decreasing production of infectious virus. To develop an in vitro model of VZV latency, future studies will examine the effects of other interferons (alpha and beta) as well as additional cytokines on VZV-infected neurons.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AG006127 (D.G.), AG032958 (D.G. and R.J.C.), and NS082228 (R.J.C.) from the National Institutes of Health. N. L. Baird was supported by training grant NS007321 to D. Gilden from the National Institutes of Health.
We thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
REFERENCES
- 1.Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. 2011. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol 37:441–463. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA, Gilden D. 2013. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol 15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins DE, Redman RL, Lam EM, Liu C, Lin I, Arvin AM. 1998. Interleukin (IL)-10, IL-12, and interferon-gamma production in primary and memory immune responses to varicella-zoster virus. J Infect Dis 178:940–948. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 4.Torigoe S, Ihara T, Kamiya H. 2000. IL-12, IFN-gamma, and TNF-alpha released from mononuclear cells inhibit the spread of varicella-zoster virus at an early stage of varicella. Microbiol Immunol 44:1027–1031. doi: 10.1111/j.1348-0421.2000.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 5.Haberthur K, Meyer C, Arnold N, Engelmann F, Jeske DR, Messaoudi I. 2014. Intrabronchial infection of rhesus macaques with simian varicella virus results in a robust immune response in the lungs. J Virol 88:12777–12792. doi: 10.1128/JVI.01814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traina-Dorge V, Sanford R, James S, Doyle-Meyers LA, de Haro E, Wellish M, Gilden D, Mahalingam R. 2014. Robust pro-inflammatory and lesser anti-inflammatory immune responses during primary simian varicella virus infection and reactivation in rhesus macaques. J Neurovirol 20:526–530. doi: 10.1007/s13365-014-0274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feduchi E, Alonso MA, Carrasco L. 1989. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol 63:1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin E, Tanamachi B, Openshaw H. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol 73:3418–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin EM, Hinton DR, Chen J, Openshaw H. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol 69:4898–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol 162:2895–2905. [PubMed] [Google Scholar]
- 11.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, Shaw A, Silber JL, Schlienger K, Chalikonda I, Vessey SJ, Caulfield MJ. 2003. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis 188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 12.Grose C, Yu X, Cohrs RJ, Carpenter JE, Bowlin JL, Gilden D. 2013. Aberrant virion assembly and limited glycoprotein C production in varicella-zoster virus-infected neurons. J Virol 87:9643–9648. 14. doi: 10.1128/JVI.01506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloutskin A, Kinchington PR, Goldstein RS. 2013. Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose. Virology 443:285–293. doi: 10.1016/j.virol.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Seitz S, Pointon T, Bowlin JL, Cohrs RJ, Jonjic S, Haas J, Wellish M, Gilden D. 2013. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J Neurovirol 19:75–81. doi: 10.1007/s13365-012-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. 1997. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med 186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird NL, Bowlin JL, Cohrs RJ, Gilden D, Jones KL. 2014. Comparison of varicella-zoster virus RNA sequences in human neurons and fibroblasts. J Virol 88:5877–5880. doi: 10.1128/JVI.00476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baird NL, Bowlin JL, Yu X, Jonjic S, Haas J, Cohrs RJ, Gilden D. 2014. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 458-459:1–3. doi: 10.1016/j.virol.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


