ABSTRACT
The four serotypes of dengue virus (DENV) cause the most important and rapidly emerging arboviral diseases in humans. The recent phase 2b and 3 studies of a tetravalent dengue vaccine reported a moderate efficacy despite the presence of neutralizing antibodies, highlighting the need for a better understanding of neutralizing antibodies in polyclonal human sera. Certain type-specific (TS) antibodies were recently discovered to account for the monotypic neutralizing activity and protection after primary DENV infection. The nature of neutralizing antibodies after secondary DENV infection remains largely unknown. In this study, we examined sera from 10 vaccinees with well-documented exposure to first and second DENV serotypes through heterotypic immunization with live-attenuated vaccines. Higher serum IgG avidities to both exposed and nonexposed serotypes were found after secondary immunization than after primary immunization. Using a two-step depletion protocol to remove different anti-envelope antibodies, including group-reactive (GR) and complex-reactive (CR) antibodies separately, we found GR and CR antibodies together contributed to more than 50% of neutralizing activities against multiple serotypes after secondary immunization. Similar findings were demonstrated in patients after secondary infection. Anti-envelope antibodies recognizing previously exposed serotypes consisted of a large proportion of GR antibodies, CR antibodies, and a small proportion of TS antibodies, whereas those recognizing nonexposed serotypes consisted of GR and CR antibodies. These findings have implications for sequential heterotypic immunization or primary immunization of DENV-primed individuals as alternative strategies for DENV vaccination. The complexity of neutralizing antibodies after secondary infection provides new insights into the difficulty of their application as surrogates of protection.
IMPORTANCE The four serotypes of dengue virus (DENV) are the leading cause of arboviral diseases in humans. Despite the presence of neutralizing antibodies, a moderate efficacy was recently reported in phase 2b and 3 trials of a dengue vaccine; a better understanding of neutralizing antibodies in polyclonal human sera is urgently needed. We studied vaccinees who received heterotypic immunization of live-attenuated vaccines, as they were known to have received the first and second DENV serotype exposures. We found anti-envelope antibodies consist of group-reactive (GR), complex-reactive (CR), and type-specific (TS) antibodies, and that both GR and CR antibodies contribute significantly to multitypic neutralizing activities after secondary DENV immunization. These findings have implications for alternative strategies for DENV vaccination. Certain TS antibodies were recently discovered to contribute to the monotypic neutralizing activity and protection after primary DENV infection; our findings of the complexity of neutralizing activities after secondary immunization/infection provide new insights for neutralizing antibodies as surrogates of protection.
INTRODUCTION
Dengue virus (DENV) belongs to the Flavivirus genus of the Flaviviridae family. DENV comprises four distinct serotypes (DENV1, DENV2, DENV3, and DENV4) which circulate in tropical and subtropical regions and cause the most common and significant arboviral diseases in humans (1). It was reported recently that approximately 390 million DENV infections, with 25% apparent infections, occur annually, including dengue fever and the severe forms of disease, dengue hemorrhagic fever and dengue shock syndrome (1–3). Despite tremendous progress in dengue vaccine development, no licensed DENV vaccine is currently available (4). Several DENV candidate vaccines have advanced to clinical trials: a previous phase 2b trial of Sanofi Pasteur's live-attenuated chimeric yellow fever-dengue (CYD) tetravalent vaccine demonstrated an efficacy of 30.2% (9.2% against DENV2), and recent reports of phase 3 trials of the same vaccine revealed efficacies of 56.5 to 60.8% (35.0 to 42.3% against DENV2), highlighting the need for a better understanding of immune responses and their correlation with protection (5–10).
DENV contains a positive-sense single-stranded RNA genome which is translated into one polyprotein containing three structural proteins, the capsid, precursor membrane (prM), and envelope (E), and seven nonstructural proteins (11). As the major surface protein on virions, the E protein participates in receptor binding and membrane fusion and is the main target of neutralizing antibodies (Abs) (4, 11). The ectodomain of E proteins contains three domains. Domain I (DI) is located in the center, domain II (DII), an elongated domain containing the fusion loop (FL) at its tip, is involved in dimerization and membrane fusion, and domain III (DIII), an immunoglobulin-like domain, is involved in receptor binding and stabilization of trimers during fusion (11–14).
There are several serocomplexes in the Flavivirus genus, including the DENV serocomplex, Japanese encephalitis virus (JEV) serocomplex, tick-borne encephalitis virus serocomplex (TBEV), and yellow fever virus (YFV). The amino acid sequence homology of the E protein is about 39 to 49% between different serocomplexes, 63 to 78% between DENV serotypes, and up to 96 to 97% between genotypes within each DENV serotype (15). Based on the binding specificity, three categories of anti-E Abs have been identified. Anti-E Abs that recognize members of different serocomplexes, or members within the same serocomplex, or a single member, are called group-reactive (GR), complex-reactive (CR), and type-specific (TS) Abs, respectively (16). Previous studies have demonstrated that different categories of anti-E monoclonal antibodies (MAbs) have different epitopes and neutralizing potencies. Studies of mouse MAbs have revealed that the GR MAbs primarily recognize the highly conserved residues in the FL of DII, whereas CR and TS MAbs recognize different but slightly overlapping residues in DIII (17–21). TS MAbs are generally more potent neutralizing MAbs than CR or GR MAbs (19, 21). Recent studies of human MAbs revealed that GR MAbs recognize either FL or both FL and bc loop residues in DII (22–24); TS MAbs recognize epitopes in DIII, interdomain residues, or the DI/DII hinge region (25–29); CR MAbs recognize DIII or E dimer involving FL and other residues (25, 26, 30).
It is known that after primary DENV infection, individuals develop monotypic neutralizing Abs against the infecting serotype, which correlates with lifelong protection against that serotype (31–34). After secondary DENV infection, individuals develop multitypic neutralizing Abs not only against the exposed serotypes but also against serotypes to which they have not yet been exposed (“nonexposed” serotypes) (32, 35). Such heterotypic neutralizing Abs are thought to contribute to protection against the nonexposed serotypes during a third or fourth DENV infection, as suggested by the lower rates of hospital admissions (36) and reduced risk of symptomatic DENV infection in humans (37) as well as lower viremia in monkeys (38–41). However, the nature of these neutralizing Abs remains elusive.
Previous studies of polyclonal Abs in human sera after DENV infection revealed that the majority of anti-E Abs were cross-reactive, including both GR and CR Abs, whereas only a minor proportion was TS Abs (17, 21, 42, 43). Our recent studies showed that GR anti-E MAbs derived from patients after secondary DENV infection had higher binding avidities and neutralizing potencies than those from patients after primary DENV infection (23). Moreover, the concentrations of GR Abs in sera correlated with the neutralizing titers against the nonexposed serotypes after secondary DENV infection (44). Based on these observations, we hypothesize that cross-reactive anti-E Abs (including GR and CR Abs) generated after secondary DENV infection have high avidity and potent neutralizing actviites, and they contribute to the neutralizing activities against the nonexposed serotypes. Since the DENV infection history is important to an Ab response, we investigated vaccinees with well-documented first and second DENV serotype exposures through heterotypic immunization of two monovalent live-attenuated vaccines (45), as well as patients with confirmed secondary DENV infection. Our in-depth analyses of anti-E Abs in polyclonal sera, including binding specificity, avidity, composition of Abs, and neutralization before and after depletion experiments, revealed that cross-reactive anti-E Abs contributed significantly to neutralizing activities against both exposed and nonexposed DENV serotypes after secondary DENV immunization/infection. This information has implications for alternative strategies of DENV vaccine development and adds to our understanding of the complexity of neutralizing Abs after secondary DENV infection.
MATERIALS AND METHODS
Dengue virus immune sera.
Sera collected at day 42 postimmunization from 10 vaccinees who received heterotypic immunization of two monovalent live-attenuated DENV vaccines were studied (45). There were 4 vaccinees with the immunization sequence of DENV4 then DENV1 (DENV4 → DENV1), 4 vaccinees who received DENV4 → DENV2, and 2 who received DENV2 → DENV1 (Table 1). Sera from three patients collected at 1 to 3 months after confirmed secondary DENV1 infection were also studied (46). Pooled sera comprised a mixture of convalescent-phase sera from four confirmed dengue patients, described previously (21).
TABLE 1.
Basic information and serum neutralization titers of vaccinees in this study
| Vaccinee IDa | Interval (yrs)b | Primary and secondary immunization serotypes | Time of sampling (day)c | FRNT50 titerd |
|||
|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | ||||
| 1 | 1.3 | D4, primary | 42 | 60 | <40 | 40 | 640 |
| D1, secondary | 42 | 2,560 | 960 | 1,280 | 2,560 | ||
| 4 | 1.4 | D4, primary | 42 | <40 | <40 | <40 | >1,280 |
| D1, secondary | 42 | 2,560 | 1,280 | 640 | 640 | ||
| 5 | 7.4 | D4, primary | 42 | <40 | <40 | <40 | >1,280 |
| D1, secondary | 42 | 480 | 640 | 640 | 960 | ||
| 6 | 1.9 | D4, primary | 42 | 60 | 80 | <40 | >1,280 |
| D1, secondary | 42 | 640 | 1,280 | 640 | 1,920 | ||
| 11 | 2.7 | D2, primary | 42 | 160 | >1,280 | 60 | 160 |
| D1, secondary | 42 | 1,920 | 5,120 | 240 | 320 | ||
| 12 | 2.1 | D2, primary | 42 | <40 | 1,920 | <40 | 160 |
| D1, secondary | 42 | 240 | 1,920 | 240 | 320 | ||
| 16 | 4.0 | D4, primary | 42 | <40 | <40 | <40 | >1,280 |
| D2, secondary | 42 | 480 | 6,400 | 240 | 1,920 | ||
| 19 | 5.9 | D4, primary | 42 | <40 | <40 | <40 | 1,920 |
| D2, secondary | 42 | 1,280 | 1,280 | 960 | 1,920 | ||
| 20 | 6.4 | D4, primary | 42 | <40 | <40 | <40 | 960 |
| D2, secondary | 42 | 960 | 1,280 | 640 | 1,280 | ||
| 22 | 6.6 | D4, primary | NA | ||||
| D2, secondary | 42 | 2,560 | 1,920 | 1,280 | 7,680 | ||
Ten vaccinees who received heterotypic immunization with live-attenuated DENV vaccine were included in this study (45).
The interval (in years) between the first and second immunizations (45).
The day after the first or second immunization (45). NA, not available.
The neutralizing titers (FRNT50) on Vero cells (23). D1, DENV1; D2, DENV2; D3, DENV3; D4, DENV4. Data are mean results of duplicates from one of two experiments.
Ethics statement.
The 10 adult vaccinees, who were flavivirus naive at baseline, were enrolled in a previously described randomized, double-blind, placebo-controlled study of heterotypic immunization at the Center for Immunization Research at the John Hopkins Bloomberg School of Public Health under an investigational new drug application approved by the U.S. Food and Drug Administration (45, 47). All study documents were approved by the Western Institutional Review Board (IRB). Informed consent was obtained in accordance with the Code of Federal Regulations (Title 21, Part 50). The three adult dengue patients were from the Kaohsiung Medical University Hospital in Kaohsiung, Taiwan, between 2002 and 2009. With the approval of the Kaohsiung Medical University IRB, informed consent was obtained. All serum samples involved in this study were coded for anonymity, and their analysis was approved by the IRB of the University of Hawaii at Manoa (CHS17568).
Western blot analysis and dot blot assay.
Western blot (WB) analysis was performed as described previously (42). Vero cells infected with DENV1 (Hawaii), DENV2 (New Guinea C), DENV3 (H87 strain), DENV4 (H241 strain), or West Nile virus (WNV; NY99 strain) or mock infected were lysed in 1% NP-40 lysis buffer. The cell lysates were then prepared in nonreducing sample buffer, boiled, and separated by 12% polyacrylamide gel electrophoresis under 2% SDS, followed by transfer to a nitrocellulose (NC) membrane (Hybond-C Extra; GE Healthcare, United Kingdom), blocking, incubation with primary Abs (from vaccinee or patient sera) and secondary Abs, and detection with enhanced chemiluminescence reagents (PerkinElmer Life Science, Boston, MA) (21, 42). For the dot blot (DB) assay, virus-infected Vero cell lysates were diluted in 1× phosphate-buffered saline (PBS) and dot blotted using a 96-formatted dot blotter (Labrepco) and an NC membrane as described previously (21).
Virion ELISA and IgG avidity assay.
DENV virions derived from ultracentrifugation of culture supernatants of virus-infected Vero cells or pellets of mock-infected Vero cells were UV inactivated (48), diluted in coating buffer, and loaded on a flat-bottom 96-well plate at 4°C overnight, followed by blocking and incubation with primary Abs (serum at a 1:2,000 dilution) and secondary Abs. After a final wash and incubation with tetramethylbenzidine substrate and stop solution, the optical density at 450 nm (OD450) was read, with a reference wavelength of 650 nm (23, 44). IgG avidity was determined in a modified enzyme-linked immunosorbent assay (ELISA) with an additional washing step with 8 M urea (49–51). After incubation with primary Abs (serum at a 1:100 dilution) and washing, 100 μl of 8 M urea was added to each well at room temperature for 10 min, followed by washing 4 times and incubation with secondary Abs. The avidity index was calculated as the OD450 with urea divided by the OD450 without urea. The concentration of 8 M urea was chosen since the avidity indices based on 8 M urea correlated well with those observed with 6 M and 9 M urea (data not shown).
Focus reduction neutralization test.
The flat-bottom 96-well plate was seeded with Vero cells (3 × 104 per well) 24 h prior to infection. Serial 2-fold dilutions of serum were mixed with 50 focus-forming units of DENV1 (Hawaii strain), DENV2 (NGC strain), DENV3 (CH53489), or DENV4 (H241 strain) at 37°C for 1 h; the mixtures were added to each well, followed by an overlay, incubation, removal of the overlay, fixation, and staining (17, 23). The foci were counted with an ImmunoSpot reader (Cellular Technology, OH). For undepleted vaccinee serum (starting from a dilution of 1:40), the 50% focus reduction neutralization test (FRNT50) titer was determined based on the dilution of serum that inhibited 50% of foci compared with virus-only wells. For patient serum and depleted serum (starting from a 1:80 dilution), the FRNT50 titer was determined by nonlinear regression analysis (GraphPad Prism 5.0).
Depletion of cross-reactive Abs.
To deplete GR Abs, sera (1:20 dilutions in 1× PBS) were incubated with NC membrane precoated with lysates of 293T cells transfected with a prM/E expression plasmid of WNV or mock transfected in microcentrifuge tubes (0.9 ml) at room temperature for 2 h, followed by incubation with WNV virus-like particles (VLPs) or pellets derived from culture supernatants of mock-transfected 293T cells in 1× PBS at 37°C for 1 h. The supernatant mixtures then underwent ultracentrifugation at 150,000 × g and 4°C for 1 h to remove bound GR Abs. VLPs were prepared as described previously (21). Postdepletion sera were examined by WB analysis, DB assay, virion ELISA, and FRNT. The percent reduction in FRNT50 titer by GR Abs was equal to [1 – (FRNT50 of WNV-depleted serum)/(FRNT50 of mock-depleted serum)] × 100. To further deplete CR Abs, WNV-depleted serum was incubated with NC membrane coated with lysates of DENV3 (a nonexposed serotype)-infected or mock-infected Vero cells, followed by incubation with UV-inactivated DENV3 virions or pellets derived from culture supernatants of mock-infected Vero cells; next, ultracentrifugation at 150,000 × g at 4°C for 1 h was employed to remove bound CR Abs. Compared with mock-mock-depleted sera, the percent reduction in the FRNT50 titer by GR and CR Abs was calculated via the following equation: [1 – (FRNT50 of WNV-DENV3-depleted serum)/(FRNT50 of mock-mock-depleted serum)] × 100. Compared with WNV-depleted sera, the percent reduction in the FRNT50 titer by CR Abs was calculated as follows: [1 – (FRNT50 of WNV-DENV3-depleted serum)/(FRNT50 of WNV-depleted serum)] × 100.
Determination of the percentages of GR Abs and TS Abs.
Fourfold serial dilutions of postdepletion serum were subjected to virion ELISAs (of each of the four DENV serotypes); the endpoint titers were determined based on the reciprocal of the highest dilutions of postdepletion serum that reached an OD450 value greater than the cutoff, which was equal to 3 standard deviations of the mean OD450 value of pooled preimmune sera of vaccinees (at a 1:500 dilution) using four-parameter nonlinear regression analysis (GraphPad Prism 5.0). After WNV depletion, the percent GR Abs was calculated as follows: [1 – (endpoint titer of WNV-depleted serum)/(endpoint titer of mock-depleted serum)] × 100. After WNV-DENV3 depletion, the percent TS Abs was calculated as follows: [(endpoint titer of WNV-DENV3-depleted serum)/(endpoint titer of mock-mock-depleted serum)] × 100. For an OD450 value (at a 1:500 dilution) less than the cutoff after WNV-DENV3 depletion, the endpoint titer was <500 and the percent TS Abs was estimated was <500/endpoint titer of the mock-mock-depleted serum. The proportion of CR Abs was calculated as follows: 100 – (percent GR Abs) – (percent TS Abs).
Elution of GR Abs from NC membranes.
After the depletion of GR Abs, the NC membranes with bound Abs were washed with 1× PBS twice, followed by incubation with 0.1 M glycine–HCl (pH 2.7) at room temperature for 10 min, and immediate addition of 1 M Tris (pH 8.0) at 1/10 of the total volume, to neutralize the acidic pH. Eluted Abs (adjusted to the original volume) were tested for binding avidity in an IgG avidity assay (at a 1:100 dilution, the same dilution tested for serum) and epitope mapping by DB assay and capture ELISA using wild-type (WT) and mutant VLPs (21).
Statistical analysis.
The two-tailed Mann-Whitney test was used to determine the difference in avidity indices, percent GR Abs, percent TS Abs, and the reduction in the FRNT50 titer between two groups. The two-tailed Spearman correlation test was used to determine the relationship between avidity index and FRNT50 using GraphPad Prism 5.0.
RESULTS
Binding specificity and neutralizing patterns of vaccinee sera.
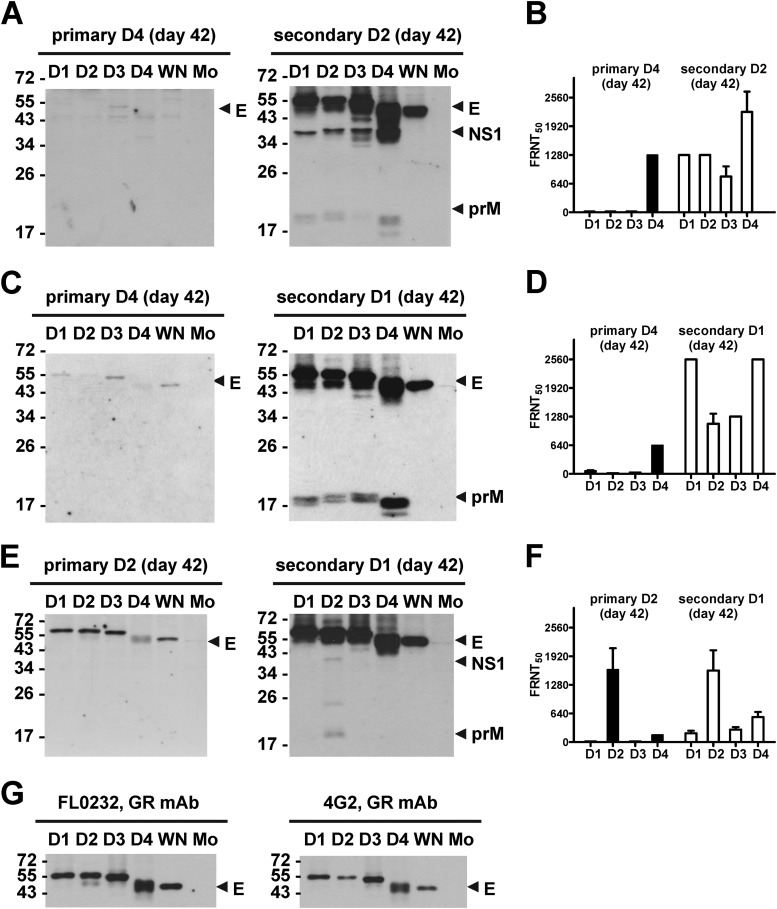
The basic information for the 10 vaccinees, including DENV immunization serotypes, sequences, and sampling times, is summarized in Table 1. We first examined the binding specificity of day 42 serum from a vaccinee, who had primary DENV4 and secondary DENV2 immunizations (designated DENV4 → DENV2). WB analysis revealed detectable anti-E Abs against four DENV serotypes and WNV, a member of the JEV serocomplex, after primary immunization, and strong anti-E Abs with similar spectra and moderate to weak anti-prM Abs against four DENV serotypes after secondary immunization (Fig. 1A). A similar trend was observed in vaccinees (Fig. 1C and E) representing the other two sequences of DENV immunizations in this study (Table 1). This trend is in agreement with the notion that anti-E Abs are the major Ab response and GR anti-E Abs are generated after primary and secondary DENV infections (17, 21, 42, 44). Notably, the strongest anti-E or anti-prM Abs after secondary immunization were against the primary immunization serotype in all vaccinees tested (Fig. 1 and data not shown). In addition, a monotypic neutralizing pattern against the exposed serotype after primary immunization and a multitypic neutralizing pattern against both exposed and nonexposed serotypes after secondary immunization were observed (Fig. 1B, D, and F and Table 1).
FIG 1.
Binding specificity and neutralizing activities of vaccinee sera. (A to F) Day 42 sera from three vaccinees (ID19 [A and B]; ID1 [C and D]; ID12 [E and F]) after primary and secondary immunizations were subjected to WB analysis at 1:500 dilution (A, C, and E) and FRNT titer determinations (B, D, and F). (G) Previously described mouse GR anti-E MAbs (FL0232, 4G2) (21) were used to verify that comparable amounts of DENV and WNV antigens were loaded. Data are means and standard deviations of duplicates from one of two experiments. WN, WNV; Mo, mock.
GR anti-E Abs contribute to neutralizing activities against four serotypes after secondary immunization.
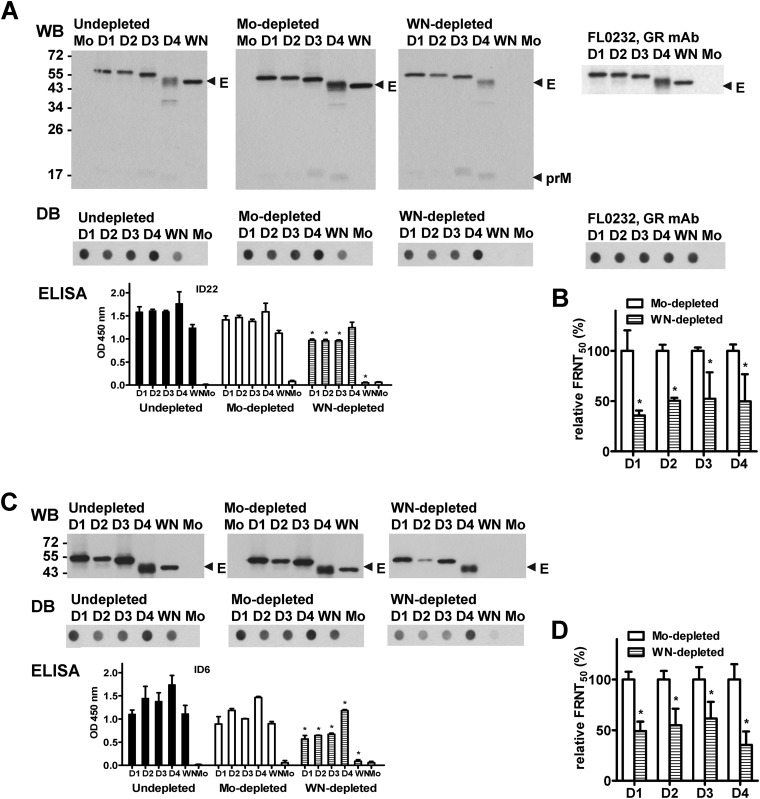
To investigate whether GR anti-E Abs contribute to the multitypic neutralizing activities after secondary DENV immunization, we performed a depletion experiment to deplete GR anti-E Abs in two vaccinees' sera by incubation with WNV antigen (including cell lysates containing WNV prM/E proteins and WNV VLPs) and examined the neutralizing activities based on the FRNT. Compared with nondepleted and mock-depleted sera, the E-binding signals to WNV in WNV-depleted sera were undetectable or at background levels, as shown by WB analysis, DB assay, and ELISA (Fig. 2A and C), suggesting nearly complete depletion of GR anti-E Abs. Since anti-prM Abs after secondary DENV infection recognized the prM protein of DENV but not of WNV, there are no GR anti-prM Abs (Fig. 1). Thus, the Abs depleted by WNV antigen were GR anti-E Abs (Fig. 2A).
FIG 2.
Contribution of GR anti-E Abs to neutralizing activities after secondary immunization. Day 42 sera from two vaccinees (ID22, A and B; ID6, C and D) after secondary immunization were depleted with WNV antigen or mock treatment to remove GR Abs, as described in Materials and Methods. Postdepletion sera were examined by WB analysis, DB assay, virion ELISA at a 1:2,000 dilution (A and C), and neutralizing titers (FRNT50) (B and D). A previously described mouse GR anti-E MAb (FL0232) (21) was used to verify that comparable amounts of DENV and WNV antigens were loaded for the WB analysis and DB assay (A, right). Data are means and standard deviations of two experiments, each with duplicates. WN, WNV; Mo, mock. *, P = 0.03, two-tailed Mann-Whitney test, comparing OD450 values (A and C) and relative FRNT50 titers (B and D) between WNV- and mock-depleted sera for each DENV serotype or WNV.
We next examined the effect of depletion on neutralizing activities. In vaccinee ID 22 (sequence of immunization, DENV4 → DENV2), WNV-depleted sera showed reductions in FRNT50 titers to both exposed serotypes (DENV2 and DENV4) and nonexposed serotypes DENV1 and DENV3, compared to titers of mock-depleted sera (Fig. 2B). A similar trend was observed in another vaccinee (ID 6) (Fig. 2D). The percent reductions in FRNT50 titers against four DENV serotypes in eight vaccinees are summarized in Table 2 (P < 0.0001 for each serotype; two-tailed Mann-Whitney test), suggesting that GR anti-E Abs in polyclonal human sera contribute significantly to the neutralizing activities against four serotypes after secondary DENV immunization.
TABLE 2.
Reduction in FRNT50 titers after depletion of GR anti-E Abs by use of WNV antigen
| Vaccinee IDa | Immunization serotypes (primary, secondary) | % reduction in FRNT50 titerb |
|||
|---|---|---|---|---|---|
| D1c | D2c | D3c | D4c | ||
| 1 | D4 → D1 | 22.5 | 52.5 | 40.7 | 53.3 |
| 4 | D4 → D1 | 28.8 | 51.6 | 45.8 | 52.3 |
| 5 | D4 → D1 | 53.4 | 37.3 | 55.7 | 34.2 |
| 6 | D4 → D1 | 50.9 | 45.1 | 38.6 | 64.5 |
| 16 | D4 → D2 | 54.6 | 77.5 | 14.3 | 36.1 |
| 19 | D4 → D2 | 51.0 | 55.1 | 14.3 | 22.7 |
| 20 | D4 → D2 | 46.7 | 16.0 | 40.0 | 35.3 |
| 22 | D4 → D2 | 64.0 | 50.9 | 25.0 | 73.1 |
Vaccinees received heterotypic immunization with live-attenuated DENV vaccine (45).
The neutralizing titers (FRNT50) on Vero cells were determined (23). D1: DENV1; D2: DENV2; D3: DENV3; D4: DENV4. Day 42 sera after secondary immunization were depleted of GR anti-E Abs. The % reduction in FRNT50 titers, determined as described in Methods, were presented as mean from two experiments, each with duplicates.
P <0.0001, two-tailed Mann-Whitney test, comparing the percent reduction in FRNT50 titer between WNV-depleted and mock-depleted sera (all data points of 8 vaccinees) for each serotype.
Higher serum IgG avidity to exposed and nonexposed DENV serotypes after secondary immunization than after primary immunization.
We next investigated serum IgG avidity by a modified virion ELISA using 8 M urea (49–51). We included serotypes DENV1, DENV2, and DENV4 in the avidity assay, since they represent both the exposed and nonexposed serotypes of the three immunization sequences in this study (Table 1). As shown in Fig. 3D to F, the IgG avidity to both exposed and nonexposed serotypes, regardless of the sequences of immunization, was significantly higher after secondary immunization than after primary immunization. Notably, comparing sera after secondary immunization, the IgG avidity to the primary immunization serotype (DENV4) was significantly higher than that to the secondary immunization serotype (DENV2 or DENV1) in subgroups with immunization sequences of DENV4 → DENV1 and DENV4 → DENV2 (Fig. 3D and F).
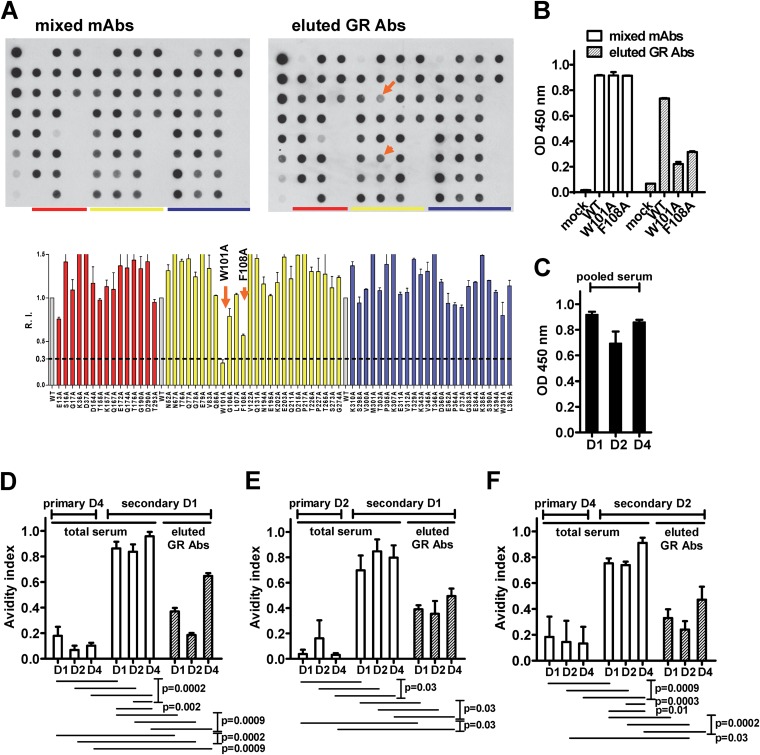
FIG 3.
Epitopes and IgG avidities of eluted GR Abs after secondary immunization, compared with total serum IgG avidity after primary and secondary immunization. (A and B) Day 42 serum from vaccinee ID 20 after secondary DENV2 immunization was depleted with WNV antigen; GR anti-E Abs were eluted from an NC membrane as described in Materials and Methods and subjected to epitope mapping by DB assay (A) and capture ELISA using WT and mutant VLPs containing key FL mutations (B) (21). (C to E) Avidity indices of eluted GR anti-E Abs and total serum were determined as described in Materials and Methods. Vaccinees were subgrouped by sequence of immunization, including DENV4 → DENV1 (ID1, ID4, ID5, and ID6 [D]), DENV2 → DENV1 (ID11 and ID12 [E]), and DENV4 → DENV2 (ID16, ID19, and ID20 [F]). A pooled DENV-immune human serum was used to verify that comparable amounts of DENV were loaded in the virion ELISA (C). Data are means and standard deviations of duplicates from one of two experiments (A to C) or means and standard deviations of duplicates from two experiments for all individuals within each subgroup (D to E). The P values were determined by a two-tailed Mann-Whitney test.
To characterize the GR Abs absorbed by WNV antigen in the depletion experiment, we eluted the GR Abs from NC membranes and examined their epitope and binding avidities. As shown in Fig. 3A and B, the eluted GR Abs from vaccinee ID 20 showed greatly reduced binding to FL mutants (W101A plus F108A) in the DB assay and VLP capture ELISA, suggesting that the eluted GR Abs recognized these FL residues, which are two common epitope residues of human GR anti-E Abs reported previously (22–24). For vaccinees with the immunization sequence DENV4 → DENV1, the avidity indices of the eluted GR Abs (ranging from 0.19 to 0.65) were higher than those of total serum after primary immunization (P = 0.0002, 0.0002, and 0.0009 for DENV1, DENV2, and DENV4, respectively; two-tailed Mann-Whitney test), but lower than those of total serum after secondary immunization (Fig. 3D) (P = 0.0009 for each serotype; two-tailed Mann-Whitney test), suggesting that GR Abs together with other Abs, such as CR or TS Abs, contribute to the increased avidity of total serum after secondary immunization. A similar trend was observed for the eluted GR Abs from sera of vaccinees with the other two sequences of immunization (Fig. 3E and F), though the avidity indices of eluted GR Abs were significantly higher than those of total serum after primary immunization only in one or two serotypes, probably due to the small sample size.
CR anti-E Abs contribute to multitypic neutralizing activities after secondary immunization.
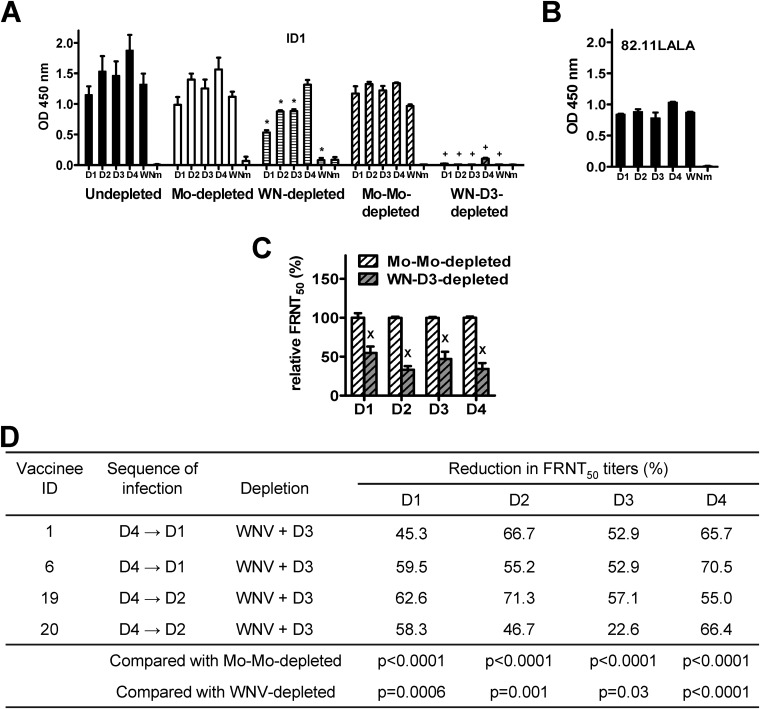
To investigate if CR Abs also contribute to the neutralizing activities after secondary immunization, we used DENV3 (a nonexposed serotype) antigen to additionally deplete CR Abs in WNV-depleted sera from vaccinee ID 1 (DENV4 → DENV1). Compared with mock-mock-depleted serum, WNV-DENV3-depleted serum showed loss of binding to DENV2 and DENV3 (the nonexposed serotypes), suggesting a complete depletion of CR Abs (Fig. 4A). The remaining binding signals to DENV4 (the primary immunization serotype) and relatively low signals to DENV1 (the secondary immunization serotype) represent the TS Abs (Fig. 4A and 5C, serum dilutions of 1:2,000 and 1:500, respectively). A similar trend was observed in another vaccinee, ID 6 (DENV4 → DENV1) (data not shown). Analysis of the neutralizing activities in WNV-DENV3-depleted sera, compared with mock-mock-depleted serum, revealed reductions in FRNT50 titers against each serotype (Fig. 4C). The percent reductions in FRNT50 titers after depletion of GR and CR Abs in four vaccinees are summarized in Fig. 4D (in comparison with WNV-depleted sera, P = 0.0006, 0.001, 0.03, and <0.0001 for DENV1, DENV2, DENV3, and DENV4, respectively; two-tailed Mann-Whitney test). These findings suggest that CR Abs contribute to neutralizing activities against DENV after secondary immunization.
FIG 4.
Contribution of CR anti-E Abs to neutralizing activities after secondary immunization. (A to C) Day 42 serum from vaccinee ID 1 after secondary immunization was depleted with WNV antigen and WNV plus DENV3 antigens to remove GR Abs and GR plus CR Abs, respectively, as described in Materials and Methods. Depletion with mock and mock-mock sera served as controls. Postdepletion sera were examined by virion ELISA at a 1:2,000 dilution (A) and by FRNT (C). A human anti-E GR MAb (82.11LALA) was used to verify that comparable amounts of DENV and WNV antigens were loaded (B) (25, 44). Data are means and standard deviations from two experiments, each with duplicates. *, +, and x, P = 0.03, two-tailed Mann-Whitney test, comparing OD450 values between WNV- and mock-depleted sera (*) or between WNV-D3- and mock-mock-depleted sera (+) to each serotype or WNV (A), and comparing the relative FRNT50 between WNV-D3- and mock-mock-depleted sera (x) to each serotype (C). (D) Summary of the percent reduction in FRNT50 titer after depletion with WNV plus DENV3 antigens in four vaccinees. Data are means from two experiments, each with duplicates. The P value was determined by comparing the percent reduction in FRNT50 titers (all data points of 4 vaccinees) against each serotype between WNV-D3- and mock-mock-depleted or WNV-depleted sera (two-tailed Mann-Whitney test). WN, WNV; Mo, mock.
FIG 5.
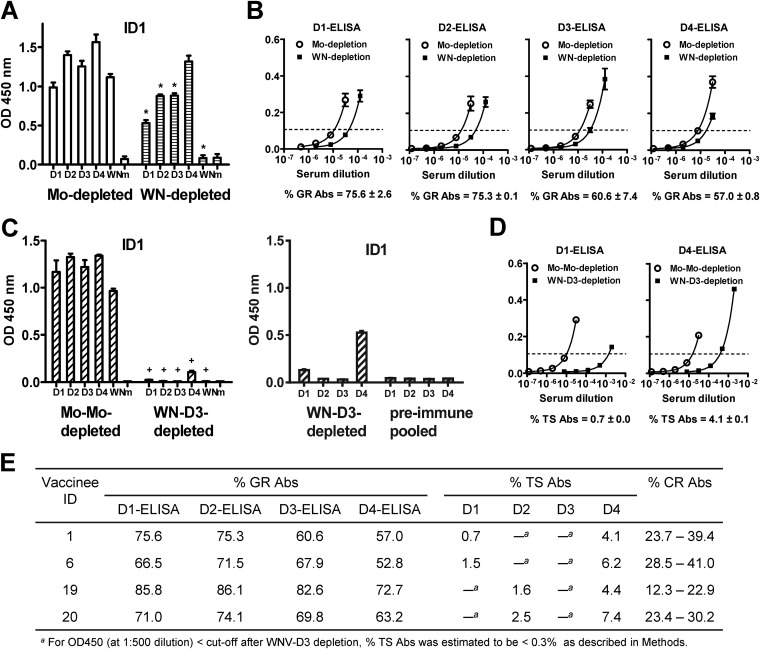
Composition of anti-E Abs in sera after secondary immunization. (A and B) Day 42 serum from vaccinee ID 1 after secondary (DENV4 → DENV1) immunization was depleted with WNV antigen and examined by virion ELISA at a 1:2,000 dilution (A). Fourfold serial dilutions of the WNV- and mock-depleted sera were subjected to virion ELISA of each serotype to determine the endpoint titers and percent GR Abs (B), as described in Materials and Methods. (C and D) WNV-depleted serum was further depleted with DENV3 (a nonexposed serotype) antigen and examined by virion ELISA at 1:2,000 (C, left graph) and 1:500 (C, right graph). Fourfold serial dilutions of the WNV-DENV3- and mock-mock-depleted sera were subjected to virion ELISA for DENV1 and DENV4 to determine the endpoint titers and percentages of TS Abs (D), as described in Materials and Methods. (E) Summary of the composition of anti-E Abs in sera of four vaccinees. The percent CR Abs was calculated as follows: 100 – percent GR Abs – percent TS Abs. * and +, P = 0.03 (two-tailed Mann-Whitney test) for OD450s between WNV- and mock-depleted sera (*) or between WNV-D3- and mock-mock-depleted sera (+) to each DENV serotype or WNV. Data are means and standard deviations of duplicates from two experiments. WN, WNV; Mo, mock.
Determining the proportion of GR Abs and TS Abs in sera.
Since our depletion experiment with WNV antigen and WNV plus DENV3 antigens resulted in nearly complete depletion of GR Abs and CR Abs, respectively, we sought to use the endpoint titers of each postdepletion serum to determine the percent GR Abs and percent TS Abs in sera. For vaccinee 1 (DENV4 → DENV1), the percentages of GR Abs were 75.6%, 75.3%, 60.6%, and 57% based on DENV1, DENV2, DENV3, and DENV4 virion ELISAs, respectively; the percentages of TS Abs to DENV1 and DENV4 were 0.7% and 4.1%, respectively (Fig. 5A to D). Figure 5E summarizes the composition of anti-E Abs in four vaccinees' sera. The percentages of GR Abs ranged from 52.8% to 86.1%. The percentages of TS Abs for the previously exposed serotypes ranged from 0.7% to 7.4%. Interestingly, the percentage of TS Abs to the primary immunization serotype (DENV4) was higher than that to the secondary immunization serotypes (DENV1 or DENV2) (P = 0.0002; two-tailed Mann-Whitney test). Via subtraction, the percent CR Abs thus calculated ranged from 12.3% to 41.0%.
GR anti-E Abs contribute to multitypic neutralizing activities in patients after secondary DENV infection.
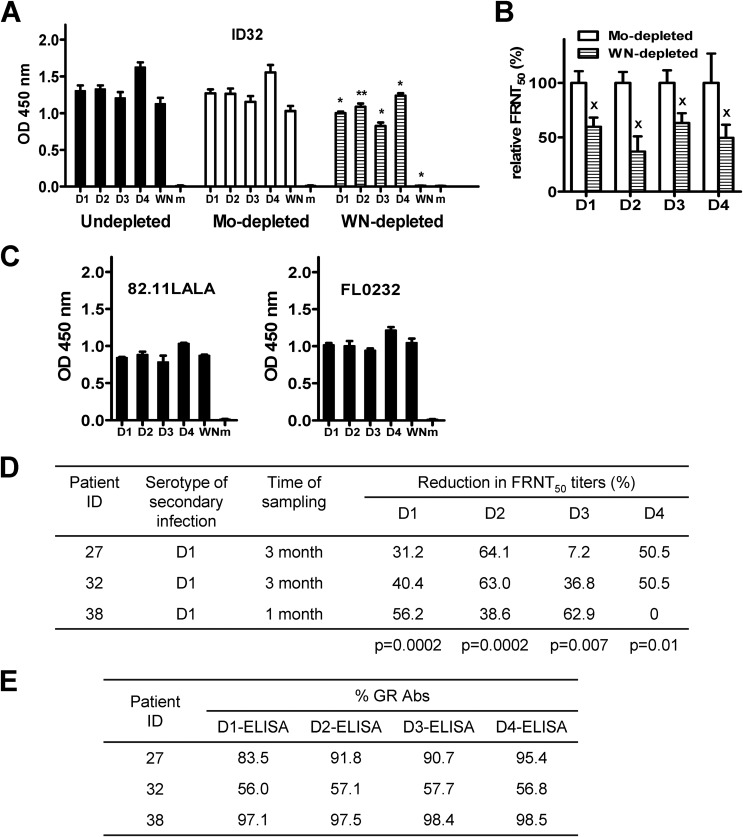
To investigate if GR anti-E Abs contribute to neutralizing activities after natural secondary DENV infection, we examined sera from three patients 1 to 3 months after secondary DENV1 infection. Compared with mock-depleted serum, WNV-depleted serum of patient ID 32 showed loss of binding to WNV (Fig. 6A), suggesting a complete depletion of GR Abs. Furthermore, WNV-depleted serum showed reductions in FRNT50 titers against four serotypes (Fig. 6B). Figure 6D summarizes the reductions in FRNT50 titers in three patients (P = 0.0002, 0.0002, 0.007, and 0.01 for DENV1, DENV2, DENV3, and DENV4, respectively; two-tailed Mann-Whitney test). These findings suggest that GR Abs contribute to neutralizing activities against multiple DENV serotypes after a secondary DENV infection.
FIG 6.
Contribution of GR anti-E Abs to neutralizing activities in patients after secondary DENV infection. (A and B) Sera from patient ID 32 at 3 months after secondary DENV1 infection were depleted with WNV antigen or mock depleted. Postdepletion sera were examined by virion ELISA at a 1:2,000 dilution (A) and by FRNT (B). A mouse (FL0232) and human (82.11LALA) GR anti-E MAb were used to verify that comparable amounts of DENV and WNV antigens were loaded in the virion ELISA (25, 44) (C). Data are means and standard deviations of two or three experiments, each with duplicates. *, P = 0.002; **, P = 0.009; x, P = 0.03 (two-tailed Mann-Whitney test, comparing OD450 values [* and **]) (A) and relative FRNT50 (x) (B) between WNV- and mock-depleted sera to each serotype or WNV. (D) Summary of the percent reduction in FRNT50 titers against 4 serotypes after depletion with WNV antigen in three patients. Data are means from two experiments, each performed in duplicate. The P value was determined by comparing the percent reduction in FRNT50 titers (all data points of 3 patients) between WNV- and mock-depleted sera to each serotype (two-tailed Mann-Whitney test). (E) Fourfold serial dilutions of WNV- and mock-depleted sera were subjected to virion ELISA of each serotype to determine the endpoint titers (as described for Fig. 5). The percentages of GR Abs in three patients' sera after secondary DENV1 infection are summarized. Data are means of duplicates from one of two experiments. WN, WNV; Mo, mock.
Based on the endpoint titers of postdepletion sera in the virion ELISAs, we determined the percentages of GR Abs in these patients (data not shown). As summarized in Fig. 6E, the percentages of GR Abs ranged from 56.0% to 98.5%, which were in the same range as those in vaccinees. Together, our findings suggest that vaccinees receiving heterotypic immunization with two monovalent live-attenuated vaccines generate predominant cross-reactive Abs with compositions and contributions to neutralizing activities similar to those in patients after natural secondary DENV infection.
Heterogeneity of neutralizing Abs after secondary immunization.
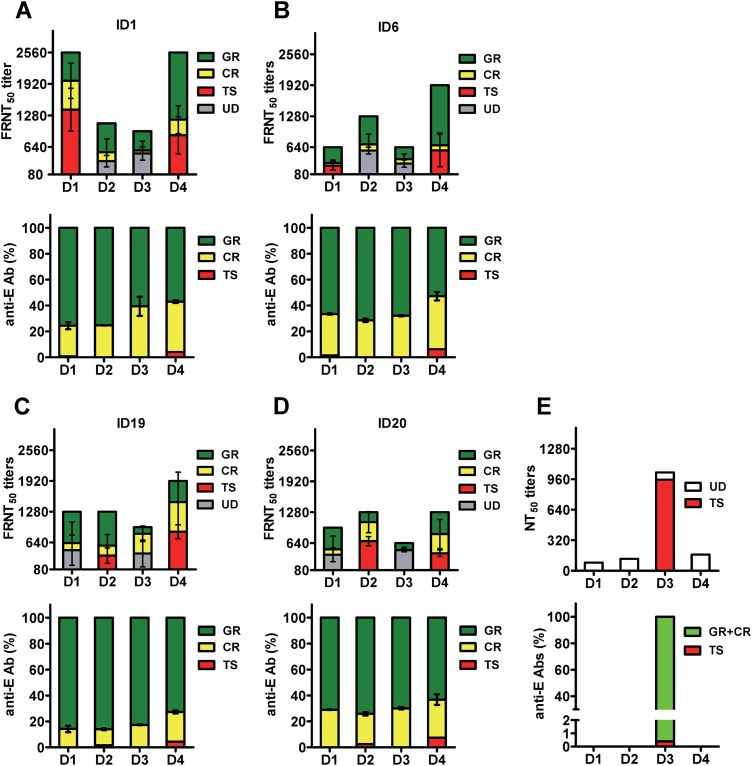
Figure 7A to D summarizes the proportion of different categories of anti-E Abs in four vaccinees after secondary immunization and the contribution of the Abs to neutralizing activities. As a comparison, the proportion of TS anti-E Abs and its contribution to the monotypic neutralizing activity of a previously reported case of primary DENV3 infection are presented in Fig. 7E (27, 43). In contrast to the scenario after primary DENV infection, where TS anti-E Abs constitute a small percentage of total anti-E Abs but account for the majority of neutralizing activities (27, 43), our results revealed that GR and CR Abs predominate and together contribute to more than 50% of the neutralizing activities against two to four serotypes, suggesting the complexity of neutralizing Abs after secondary immunization with monovalent live-attenuated DENV vaccine.
FIG 7.
Composition of anti-E Abs and contribution of different anti-E Abs to neutralizing activities in four vaccinees after secondary immunization. (A to D) The proportion of different anti-E Abs (percentages of GR, CR, and TS Abs) were based on data shown in Fig. 5E, and the contributions of GR and CR Abs to neutralizing activities are based on the percent reductions of FRNT50 titers in Table 2 and Fig. 4D for four vaccinees (ID1 [A], ID6 [B], ID19 [C], and ID20 [D]). After depletion of GR and CR Abs, the remaining neutralizing activities to previously nonexposed serotypes were designated undetermined (UD). (E) As a comparison, data from a previously reported case of primary DENV3 infection were summarized. Shown are the percentages of TS anti-E Abs, estimated based on endpoint titers in recombinant DIII and virion ELISAs (43), and their contribution to the monotypic neutralizing activity (27).
DISCUSSION
In this study, we carried out an in-depth analysis of anti-E Abs in polyclonal sera from vaccinees and patients after secondary DENV immunization/infection and found that cross-reactive anti-E Abs, including GR and CR Abs, contributed significantly to neutralizing activities against three or four DENV serotypes. To our knowledge, this is the first report demonstrating the complexity of neutralizing Abs after secondary DENV immunization/infection (32, 35). These findings, combined with our recent report of high-avidity and potent neutralizing human GR MAbs derived from patients after secondary infection (23), have implications for sequential heterotypic immunization or primary immunization of DENV-primed individuals. In light of the recent discovery of certain TS Abs contributing to monotypic neutralizing activity and protection after primary DENV infection (52, 53), our findings of the complexity of neutralizing Abs after secondary immunization/infection provide new insights into the difficulty of using neutralizing Abs as surrogates of protection in individuals receiving DENV vaccines (5–7).
We developed a two-step depletion protocol for polyclonal serum to remove GR and CR anti-E Abs separately, and we examined the effects on neutralizing activities. The observations that GR and CR Abs contributed to neutralizing activities against both nonexposed serotypes and exposed serotypes suggested that heterotypic immunization can generate heterotypic neutralizing activities through GR and CR Abs and boost homotypic neutralizing activities through GR and CR Abs as well. It is likely that during acute secondary DENV infection, memory B cells recognizing the conserved epitopes expand rapidly and generate higher-avidity and cross-reactive neutralizing Abs through affinity maturation, as evidenced by higher levels and rapid increases of serum avidity, cross-reactive memory B cells, and plasmablasts compared with primary infection (49–51, 54–56). It is worth noting that the contributions of GR or CR Abs to neutralization against each serotype varied (Table 2; Fig. 4D). Studies of human MAbs have shown that many GR and CR MAbs do not neutralize the four DENV serotypes equally (23–25, 30); a potent DENV3 TS neutralizing human MAb (5J7) has been reported to bind all four serotypes (CR Abs in binding) (57). Similarly, several DENV2 TS and DENV1 TS neutralizing human MAbs can bind multiple serotypes (26, 29). These may explain different degrees of reductions in neutralizing activities among different serotypes following depletion. This also suggests that the neutralizing Abs against nonexposed serotypes generated through heterotypic immunization might vary both in their titer and duration. Whether a third dose of heterotypic immunization can generate stronger GR and CR Abs remains to be investigated. After depletion of GR and CR Abs, considerable amounts of neutralizing activities remained and were designated undetermined (UD) neutralizing Abs to the nonexposed serotypes (Fig. 7). One possibility is that during the depletion of GR Abs with WNV antigens (cell lysates followed by VLPs), some GR neutralizing Abs that bind to epitopes present only on virions are not depleted by the WNV VLPs and thus contribute greatly to the UD neutralizing Abs and some fraction of TS neutralizing Abs against the exposed serotypes as well. Another possibility is that some potent neutralizing CR Abs that bind better to mature than immature virions are not completely depleted by the DENV3 virions derived from Vero cells, where immature and partially immature virions constitute a large proportion (data not shown). These are supported by recent reports that the symmetry of TBEV VLPs is different from that of virions (58), and several CR potent neutralizing human MAbs recognize E-dimer epitopes on mature virions (30, 59). Third, the postdepletion serum was heated at 56°C for 30 min before the FRNT but not heated before the virion ELISA, which may have resulted in a lower OD value (60, 61); some undetected neutralizing Abs would contribute to FRNT titers. Together, these three possibilities might account for the UD neutralizing Abs.
Based on the endpoint titers in virion ELISAs, anti-E Abs recognizing the exposed serotypes consist of GR, CR, and TS Abs, whereas anti-E Abs recognizing the nonexposed serotypes consist of GR and CR Abs (Fig. 5E). Interestingly, the percentage of TS Abs recognizing the second exposure serotype was lower than that recognizing the first exposure serotype (Fig. 5E). It is conceivable that compared with the predominant and overwhelming memory B cells recognizing the conserved GR and CR epitopes during secondary DENV infection (49, 55, 56), the proportion of B cells recognizing the new TS epitope is relatively small, resulting in fewere TS Abs recognizing the secondary infection serotype. This finding suggests that sequential heterotypic immunization can generate TS Abs against the second, third, and fourth serotypes, but the proportions of such TS Abs might be small.
Previous studies of serum IgG avidity to DENV commonly tested one, two, or mixed serotypes as antigens and have not yet addressed the avidities to different serotypes (49–51). In our avidity assay, we tested DENV1, DENV2, and DENV4, which represented both the exposed and nonexposed serotypes of the three immunization sequences in this study (Table 1), to examine if avidity to nonexposed and exposed serotypes differed. In agreement with previous reports (49–51), serum IgG avidity was significantly higher after secondary immunization than after primary immunization (Fig. 3). Moreover, IgG avidity to each serotype correlated with the neutralizing titers against that serotype for DENV1 and DENV2 but not for DENV4 (r = 0.85, P < 0.0001 [DENV1]; r = 0.54, P = 0.016 [DENV2]; r = 0.38, P = 0.12 [DENV4]; two-tailed Spearman correlation test) (data not shown).
Consistent with the principle of original antigenic sin described for DENV infection (62), the strongest anti-E or anti-prM Abs band after secondary immunization was against the primary immunization serotype, based on WB analysis (Fig. 1 and data not shown). However, higher neutralizing Ab titers against the primary immunization serotype were found in only 6 of 10 vaccinees (Table 1), which is in agreement with a previous report (63). This is probably because the neutralizing Abs represented only a small subset of total anti-E Abs in polyclonal sera and could have been affected by DENV strains used, whereas the majority of anti-E Abs are cross-reactive and can be detected by DENV antigens derived from different strains in most binding assays. Consistent with this interpretation, we found a higher serum IgG avidity to the primary immunization serotype than to the secondary immunization serotype (Fig. 3D and F). A recent study reported that serum IgG avidity to the primary infection serotype was higher during the acute phase but lower than that observed for the secondary infection serotype after 3 to 18 months, suggesting a possible replacement of secondary serotype-specific long-lived plasma cells in the bone marrow (64). In this regard, variation in the timing of such a replacement in bone marrow may account for the different results in our study, which was based on day 42 sera.
There are several limitations of this study. First, the sample size was small, especially when only 3 patients were analyzed. It is likely that several variables might affect the Ab response, such as the sequence of infecting serotypes, time interval between infections, host genetic differences, etc. Second, while we showed the percentages of GR Abs and their contributions to neutralizing activities in patients were similar to those in vaccinees, the percentages of CR Abs and TS Abs and their contributions to neutralizing activities after natural secondary DENV infection remain unknown. This is due to lack of information on the primary infection serotype and thus the nonexposed serotypes to select as the antigen in our depletion experiments. Future studies of larger sample sizes and of well-documented cases with known primary, secondary, and/or tertiary infection serotypes from prospective cohort studies are needed to further our understanding of Ab responses during DENV natural infection. Third, we studied cross-reactive Abs from day 42 to 3 months after secondary DENV exposure. Whether these cross-reactive Abs are present in sufficient amounts during the acute viremic stage of infection to prevent severe disease or in suboptimal concentrations and thus contributing to antibody-dependent enhancement (ADE) remains to be investigated. Previous studies have shown that maternal neutralizing Ab titers correlate with the age at onset of DHF/DSS in infants (65, 66), suggesting that the timing and concentration of cross-reactive Abs during DENV infection are critical for disease pathogenesis. In addition, future studies on B-cell populations will complement our analysis on the circulating Abs, as a recent study using deep sequencing showed increased expansion of B-cell clones with consistent Ab sequence features in the complementarity-determining region 3 after acute DENV infection (67).
To explain the low efficacy (overall, 30.2%; DENV2, 9.2%) despite the presence of neutralizing Abs in Sanofi Pasteur's phase 2b vaccine trial, several possibilities have been proposed, including the need for detailed analysis of pre- and postvaccination samples on an individual level for those with breakthrough infection, the lack of DENV nonstructural proteins in the CYD vaccine to induce protective T-cell responses (68), the possible antigenic mismatch of circulating genotypes and vaccine strains, and the overestimation of neutralizing Ab titers using non-FcγR-bearing cell-based neutralization assays (8–10). With regard to the relevance of current neutralization assays, it is worth noting that neutralizing titers higher than 1:10 have been reliably used as a surrogate of protection in two successful human flaviviral vaccines, JEV and YFV vaccines, each targeting a single member in the Flavivirus genus (69–71). In the case of primary DENV infection, the presence of monotypic neutralizing activity correlates with lifelong protection against that serotype (31–35). Such monotypic neutralizing activity can be depleted by DENV antigen of the same serotype but not by that of nonexposed serotypes, suggesting that only TS Abs, rather than cross-reactive GR or CR Abs, contribute to such monotypic neutralizing activity (27). The epitopes recognized by the TS neutralizing Abs after primary DENV3 infection were recently reported to be residues in the DI/DII hinge region, while two residues proximal to the DI/DII hinge region comprised the epitope following immunization of monovalent DENV1 live-attenuated vaccine (52, 53), suggesting the importance of TS Abs recognizing such epitopes to confer protection. Our findings suggest that GR and CR Abs contribute to the neutralizing activities against the nonexposed serotypes after secondary DENV immunization/infection and may mask the detection of such protective TS neutralizing Abs. Since about 25% and 30% of monovalent and bivalent responses, respectively, were elicited after the first dose of CYD vaccine (72), it is conceivable that these individuals would experience secondary DENV infection during the second or third dose and generate neutralizing Abs to the third or fourth serotypes, of which some are contributed by GR and CR Abs rather than TS neutralizing Abs. This may explain why neutralizing Abs against all four serotypes achieved after three doses of CYD vaccine do not correlate with protection against certain serotypes. Our findings also suggest that in future assays to detect “protective” TS neutralizing Abs in polyclonal sera of vaccinees receiving tetravalent live-attenuated vaccine, GR and CR Abs may need to be depleted to avoid confounding effects.
ACKNOWLEDGMENTS
This work was supported by grants P20GM103516 from the National Institute of General Medical Sciences, NIH, R01AI110769-01 from the National Institute of Allergy and Infectious Diseases, NIH, and in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
We thank Eva Harris at the University of California Berkeley for kindly providing human MAb 82.11LALA and Saguna Verma at the University of Hawaii at Manoa for kindly providing lysates of WNV-infected cells.
REFERENCES
- 1.Simmons CP, Farrar JJ, Nguyen V, Wills B. 2012. Dengue. N Engl J Med 366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2009. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 3rd ed WHO, Geneva, Switzerland. [Google Scholar]
- 4.Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 5.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 6.Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A, CYD14 Study Group . 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 7.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Montoya MC, Supelano MC, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F. CYD15 Study Group. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. 2012. Defeating dengue: a challenge for a vaccine. Nat Med 18:1622–1623. doi: 10.1038/nm.2997. [DOI] [PubMed] [Google Scholar]
- 9.Wilder-Smith A. 2014. Dengue vaccines: dawning at last? Lancet 384:1327–1329. doi: 10.1016/S0140-6736(14)61142-9. [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB. 2013. Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 31:4501–4507. doi: 10.1016/j.vaccine.2013.06.079. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbach BD, Murray CL, Thiel HJ, Rice CM. 2013. Flaviviridae, p 712–747. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott William & Wilkins, Philadelphia, PA. [Google Scholar]
- 12.Modis Y, Ogata S, Clements D, Harrison SC. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 13.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M, Sánchez-San Martín C, Zheng A, Kielian M. 2010. In vitro reconstitution reveals key intermediate states of trimer formation by the dengue virus membrane fusion protein. J Virol 84:5730–5740. doi: 10.1128/JVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiasny K, Kiermayr S, Holzmann H, Heinz FX. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol 80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 17.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Sukupolvi-Petty S, Austin K, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha B, Brien JD, Sukupolvi-Petty S, Austin K, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. 2010. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 6:e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, Wu YC, Lai CY, Lu CH, Huang JH, Chang GJ, Wu HC, Wang WK. 2012. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high-throughput assay. PLoS Negl Trop Dis 6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang ST, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. 2013. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 87:52–66. doi: 10.1128/JVI.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai WY, Lai CY, Wu YC, Lin HE, Edwards E, Jumnainsong A, Kliks S, Halstead S, Mongkolsapaya J, Screaton GR, Wang WK. 2013. High avidity and potent neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J Virol 87:12562–12575. doi: 10.1128/JVI.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE Jr. 2013. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 4(6):e00873–13. doi: 10.1128/mBio.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey RA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teoh EP, Kukkaro PP, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 29.Smith SA, de Alwis AR, Kose N, Jadi RS, de Silva AM, Crowe JE Jr. 2014. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol 88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinsky A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabin AB. 1952. Research on dengue during World War II. Am J Trop Med Hyg 1:30–50. [DOI] [PubMed] [Google Scholar]
- 32.Innis BL. 1997. Antibody responses to dengue virus infection, p 221–244. In Gubler DJ, Kuno G (ed), Dengue and dengue hemorrhagic fever. CAB International, Cambridge, MA. [Google Scholar]
- 33.Guzman MG, Alvarez M, Rodriguez-Roche R, Bernardo L, Montes T, Vazquez S, Morier L, Alvarez A, Gould EA, Kouri G, Halstead SB. 2007. Neutralizing antibodies after infection with dengue 1 virus. Emerg Infect Dis 13:282–286. doi: 10.3201/eid1302.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imrie A, Meeks J, Gurary A, Sukhbaatar M, Truong TT, Cropp CB, Effler P. 2007. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol 20:672–675. doi: 10.1089/vim.2007.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halstead SB. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467. doi: 10.1016/S0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP Jr, Srikiatkhachorn A. 2007. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 77:910–913. [PubMed] [Google Scholar]
- 37.Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST. 2013. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 208:1026–1033. doi: 10.1093/infdis/jit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halstead SB, Shotwell H, Casals J. 1973. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis 128:7–14. [DOI] [PubMed] [Google Scholar]
- 39.Scherer WF, Breakenridge FA, Dickerman RW. 1972. Cross-protection studies and search for subclinical disease in new world monkeys infected sequentially with different immunologic types of dengue viruses. Am J Epidemiol 95:67–79. [DOI] [PubMed] [Google Scholar]
- 40.Price WH. 1968. Sequential immunization as a vaccination procedure against dengue viruses. Am J Epidemiol 88:392–397. [DOI] [PubMed] [Google Scholar]
- 41.Whitehead RH, Chaicumpa V, Olson LC, Russell PK. 1970. Sequential dengue virus infections in the white-handed gibbon (Hylobates lar). Am J Trop Med Hyg 19:94–102. [DOI] [PubMed] [Google Scholar]
- 42.Lai CY, Tsai WY, Lin SR, Kao CL, Hu SP, King CC, Wu HC, Wang WK. 2008. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82:6631–8843. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai CY, Williams KL, Wu YC, Knight S, Balmaseda A, Harris E, Wang WK. 2013. Analysis of cross-reactive antibodies recognizing the fusion loop of envelope protein and correlation with neutralizing antibody titers in Nicaraguan dengue cases. PLoS Negl Trop Dis 7:e2451. doi: 10.1371/journal.pntd.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durbin AP, Schmidt A, Elwood D, Wanionek KA, Lovchik J, Thumar B, Murphy BR, Whitehead SS. 2011. Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis 203:327–334. doi: 10.1093/infdis/jiq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu PY, Ke GM, Chen PC, Liu LT, Tsai YC, Tsai JJ. 2013. Spatiotemporal dynamics and epistatic interaction sites in dengue virus type 1: a comprehensive sequence-based analysis. PLoS One 8:e74165. doi: 10.1371/journal.pone.0074165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McArthur JH, Durbin AP, Marron JA, Wanionek KA, Thumar B, Pierro DJ, Schmidt AC, Blaney JE Jr, Murphy BR, Whitehead SS. 2008. Phase I clinical evaluation of rDEN4Δ30-200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg 79:678–684. [PMC free article] [PubMed] [Google Scholar]
- 48.Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, Luo H, Nakatsuka A, Nerurkar VR. 2009. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood-brain barrier. Virology 385:425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zompi S, Montoya M, Pohl O, Balmaseda A, Harris E. 2012. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl Trop Dis 6:e1568. doi: 10.1371/journal.pntd.0001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Souza VA, Tateno AF, Oliveira RR, Domingues RB, Araújo ES, Kuster GW, Pannuti CS. 2007. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol 39:230–233. doi: 10.1016/j.jcv.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Matheus S, Deparis X, Labeau B, Lelarge J, Morvan J, Dussart P. 2005. Discrimination between primary and secondary dengue virus infection by an immunoglobulin G avidity test using a single acute-phase serum sample. J Clin Microbiol 43:2793–2797. doi: 10.1128/JCM.43.6.2793-2797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. 2013. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog 9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messer WB, de Alwis R, Yount BL, Royal SR, Huynh JP, Smith SA, Crowe JE Jr, Doranz BJ, Kahle KM, Pfaff JM, White LJ, Sariol CA, de Silva AM, Baric RS. 2014. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proc Natl Acad Sci U S A 111:1939–1944. doi: 10.1073/pnas.1317350111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL. 2011. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis 204:1514–1522. doi: 10.1093/infdis/jir607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. 2012. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol 86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Hadinoto V, Appanna R, Joensson K, Toh YX, Balakrishnan T, Ong SH, Warter L, Leo YS, Wang CI, Fink K. 2012. Plasmablasts generated during repeated dengue infection are virus glycoprotein-specific and bind to multiple virus serotypes. J Immunol 189:5877–5885. doi: 10.4049/jimmunol.1201688. [DOI] [PubMed] [Google Scholar]
- 57.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE Jr. 2012. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol 86:2665–2675. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferlenghi I, Clarke M, Ruttan T, Allison SL, Schalich J, Heinz FX, Harrison SC, Rey FA, Fuller SD. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol Cell 7:593–602. doi: 10.1016/S1097-2765(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 59.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Desprès P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA. 2015. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 60.Morse SS. 1990. Critical factors in an enzyme immunoassay (ELISA) for antibodies to mouse thymic virus (MTLV). Lab Anim 24:313–320. doi: 10.1258/002367790780865985. [DOI] [PubMed] [Google Scholar]
- 61.Namekar M, Kumar M, O'Connell M, Nerurkar VR. 2012. Effect of serum heat-inactivation and dilution on detection of anti-WNV antibodies in mice by West Nile virus E-protein microsphere immunoassay. PLoS One 7:e45851. doi: 10.1371/journal.pone.0045851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halstead SB, Rojanasuphot S, Sangkawibha N. 1983. Original antigenic sin in dengue. Am J Trop Med Hyg 32:154–156. [DOI] [PubMed] [Google Scholar]
- 63.Kuno G, Gubler DJ, Oliver A. 1993. Use of ‘original antigenic sin’ theory to determine the serotypes of previous dengue infections. Trans R Soc Trop Med Hyg 87:103–105. doi: 10.1016/0035-9203(93)90444-U. [DOI] [PubMed] [Google Scholar]
- 64.Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. 2013. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl Trop Dis 7:e2274. doi: 10.1371/journal.pntd.0002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38:411–419. [DOI] [PubMed] [Google Scholar]
- 66.Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, Capeding RZ. 2009. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med 6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parameswaran P, Liu Y, Roskin KM, Jackson KK, Dixit VP, Lee JY, Artiles KL, Zompi S, Vargas MJ, Simen BB, Hanczaruk B, McGowan KR, Tariq MA, Pourmand N, Koller D, Balmaseda A, Boyd SD, Harris E, Fire AZ. 2013. Convergent antibody signatures in human dengue. Cell Host Microbe 13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. 2013. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 110:E2046–53. doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gotuzzo E, Yactayo S, Córdova E. 2013. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. 2005. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine 23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Roehrig JT, Hombach J, Barrett AD. 2008. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol 21:123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 72.Dayan GH, Thakur M, Boaz M, Johnson C. 2013. Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 31:5047–5054. doi: 10.1016/j.vaccine.2013.08.088. [DOI] [PubMed] [Google Scholar]