ABSTRACT
Monocyte-derived dendritic cells (MDDC) stimulate CD8+ cytotoxic T lymphocytes (CTL) by presenting endogenous and exogenous viral peptides via major histocompatibility complex class I (MHC-I) molecules. MDDC are poorly susceptible to HIV-1, in part due to the presence of SAMHD1, a cellular enzyme that depletes intracellular deoxynucleoside triphosphates (dNTPs) and degrades viral RNA. Vpx, an HIV-2/SIVsm protein absent from HIV-1, antagonizes SAMHD1 by inducing its degradation. The impact of SAMHD1 on the adaptive cellular immune response remains poorly characterized. Here, we asked whether SAMHD1 modulates MHC-I-restricted HIV-1 antigen presentation. Untreated MDDC or MDDC pretreated with Vpx were exposed to HIV-1, and antigen presentation was examined by monitoring the activation of an HIV-1 Gag-specific CTL clone. SAMHD1 depletion strongly enhanced productive infection of MDDC as well as endogenous HIV-1 antigen presentation. Time-lapse microscopy analysis demonstrated that in the absence of SAMHD1, the CTL rapidly killed infected MDDC. We also report that various transmitted/founder (T/F) HIV-1 strains poorly infected MDDC and, as a consequence, did not stimulate CTL. Vesicular stomatitis virus glycoprotein (VSV-G) pseudotyping of T/F alleviated a block in viral entry and induced antigen presentation only in the absence of SAMHD1. Furthermore, by using another CTL clone that mostly recognizes incoming HIV-1 antigens, we demonstrate that SAMHD1 does not influence exogenous viral antigen presentation. Altogether, our results demonstrate that the antiviral activity of SAMHD1 impacts antigen presentation by DC, highlighting the link that exists between restriction factors and adaptive immune responses.
IMPORTANCE Upon viral infection, DC may present antigens derived from incoming viral material in the absence of productive infection of DC or from newly synthesized viral proteins. In the case of HIV, productive infection of DC is blocked at an early postentry step. This is due to the presence of SAMHD1, a cellular enzyme that depletes intracellular levels of dNTPs and inhibits viral reverse transcription. We show that the depletion of SAMHD1 in DCs strongly stimulates the presentation of viral antigens derived from newly produced viral proteins, leading to the activation of HIV-1-specific cytotoxic T lymphocytes (CTL). We further show in real time that the enhanced activation of CTL leads to killing of infected DCs. Our results indicate that the antiviral activity of SAMHD1 not only impacts HIV replication but also impacts antigen presentation by DC. They highlight the link that exists between restriction factors and adaptive immune responses.
INTRODUCTION
HIV-1-specific CD8+ cytotoxic T lymphocyte (CTL) activity strongly correlates with control of viremia during acute infection and progression to disease (1). Depletion of CD8+ T cells in rhesus macaques infected with SIVmac leads to a failure to control viral loads (2, 3). However, this antiviral response is not capable of completely eliminating HIV-1. Viral replication persists, leading to the establishment of reservoirs and progression to disease in the absence of treatment. Understanding the requirements for an optimal CD8+ T cell response is key for the development of vaccines and strategies to eliminate HIV-1-infected cells.
Dendritic cells (DC) are the most potent antigen-presenting cells. In their immature form, they capture pathogens in peripheral tissues. Within the lymph nodes, matured DC present processed antigens to CD8+ T cells, which in turn respond by killing infected cells and inhibiting infection through the release of cytokines and gamma interferon (IFN-γ). Immature DC can present major histocompatibility complex class I (MHC-I) epitopes derived from captured HIV-1 in the absence of productive infection (4, 5). Nevertheless, stimulation of CTL through this pathway likely is less efficient than stimulation by endogenous antigens, even when viral entry is enhanced through vesicular stomatitis virus (VSV) pseudotyping of HIV-1 particles (4, 6). DC do not get readily infected by HIV-1, in large part due to the presence of SAMHD1, an antiviral protein that blocks infection at an early postentry step (7, 8). SAMHD1 is a dNTP hydrolase that depletes the intracellular pool of dNTPs in myeloid cells, limiting the availability of substrates for viral DNA synthesis (9–12). SAMHD1 also binds and degrades incoming HIV-1 RNA through its RNase activity (13). The relative contribution of each of these two functions to HIV-1 restriction has yet to be clarified. In any case, inhibition of SAMHD1 enhances productive infection of DCs by cell-free and cell-associated HIV-1 (8, 14). Moreover, upon HIV-1 exposure, the absence of SAMHD1 leads to maturation of DCs and secretion of type I IFN (14, 15). Maturation of DCs also leads to the priming of CD8+ T cells (15). Altogether, SAMHD1 seems to have both beneficial and detrimental effects on HIV-1 replication.
HIV-1 has evolved to circumvent both restriction factors and innate and adaptive immunity. Vpu, for example, downmodulates Tetherin, which senses and retains viral particles at the cell surface (16–18). Nef downmodulates MHC molecules to limit recognition of viral peptides by adaptive immunity (19–22). APOBEC3G introduces lethal mutations into HIV-1 DNA (23–25). APOBEC3G-edited genomes generate truncated forms of viral proteins, which are efficiently processed for antigen presentation, enhancing stimulation of HIV-1-specific CTL (26). By degrading APOBEC3G, Vif enhances viral replication and limits antigen presentation. In the case of SAMHD1, HIV-2 targets SAMHD1 for degradation through Vpx, whereas this function is lacking in HIV-1. An attractive hypothesis as to why HIV-1 does not block SAMHD1 activity involves the necessity to bypass immune detection. HIV-1 may maintain the antiviral immune response at a low level in DCs at the expense of high replication levels.
The role of SAMHD1 in HIV-1 antigen presentation and cross-presentation has not been investigated extensively. To address this question, we studied the ability of monocyte-derived DC (MDDC), treated with Vpx or left untreated and exposed to HIV-1, to stimulate HIV-1-specific CD8+ T cell clones. We found that with Vpx, HIV-1-exposed MDDC strongly enhanced stimulation of CTL. This stimulation leads to inhibition of viral replication and rapid killing of infected MDDC by CTLs. Vpx enhanced presentation of viral peptides through the endogenous pathway but had no effect on exogenous antigen presentation. Finally, we demonstrate that MDDCs are poorly sensitive to infection by R5-tropic transmitted/founder (T/F) HIV-1 strains (27) and do not present antigen derived from these viruses. Vpx is capable of enhancing infection and antigen presentation of T/F viruses only if viral entry is optimized by vesicular stomatitis virus glycoprotein (VSV-G) pseudotyping. Altogether, our results demonstrate that SAMHD1 limits HIV-1 antigen presentation by DC.
MATERIALS AND METHODS
Cells and viruses.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient from blood of healthy human donors purchased from the Etablissement Français du Sang (Paris, France). PBMC were screened by flow cytometry for expression of HLA-A2 (BD Pharmingen) or HLA-B27 (One Lambda). Purified CD14+ monocytes were positively selected from PBMC using anti-CD14 magnetic beads (Miltenyi Biotec). CD14+ cells were differentiated into monocyte-derived dendritic cells (MDDC) by culture in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 10 ng/ml of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Miltenyi Biotec), and 50 ng/ml recombinant human interleukin-4 (IL-4; Immunotools). Fresh medium with cytokines was added at day 3, and cells were infected at day 5. Primary autologous CD4+ T cells were purified from PBMC using anti-CD4 magnetic beads (Miltenyi Biotec) and activated with phytohemagglutinin (PHA; 1 μg/ml; Remel Europe Ltd.) for 24 h. Cells were cultured in IL-2-containing RPMI medium for 5 days. The HLA-A2-restricted HIV-1-specific CTL clone EM40F21 is specific for the SL9 epitope (SLYNTVATL; amino acids 77 to 85 in Gag p17) and has been described previously (28). The HLA-B27-restricted KK10 CTL clone is specific for the KK10 epitope (KRWIILGLNK; amino acids 263 to 272 in Gag p24 capsid) and also has been described previously (29).
VSV-G-pseudotyped HIV-1 strains, wild-type (WT) HIV-1, and SIVmac virus-like particles (VLP) which either express Vpx or do not express Vpx were produced as previously described (8, 14). HIV-1 virus strains used were SF2, NL4-3, NL(AD8) (an NL4-3 strain which contains an R5-tropic envelope), and T/F HIV-1 strains (WITO, RHPA, CH040, CH077, and THRO), obtained from NIH AIDS Reagent Program. NL4-3-IRES-GFP has been described previously (30).
MDDC infection and intracellular staining.
MDDC were incubated for 2 h with SIVmac VLP and then plated at 106 cells in 500 μl of medium in 24-well plates. Various amounts of virus (ranging from 5 to 50 ng of p24) were added into wells and incubated for 2 h at 37°C. Medium was replaced by fresh RPMI containing IL-4 and GM-CSF, and cells were incubated for 24 h. Where indicated, raltegravir (RAL) was added at a final concentration of 1 μM. Cells were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde and then permeabilized with PBS containing 1% bovine serum albumin (BSA) and 0.5% Triton X-100 for 15 min at 4°C. Cells were washed with PBS plus 1% BSA and then stained for Gag (KC57 clone; phycoerythrin [PE]; Beckman Coulter) and SAMHD1 (I19-18 clone; Alexa Fluor 488; generated by the Institut Pasteur antibody production core facility). For surface staining of HLA-A2 (BD Pharmingen) and MHC-I (Sigma), antibodies were incubated with cells before fixing with paraformaldehyde (PFA). Cells were washed and analyzed using a FACSCanto II flow cytometer (Becton Dickinson).
T cell activation assays. (i) ELISPOT assay.
A total of 105 MDDC, infected as described above, were cocultured with 2,500 EM40F21 CTL for 18 h in 96-well enzyme-linked immunosorbent spot (ELISPOT) assay plates (MSIPS4W10; Millipore). When indicated, 1 μM RAL was added to cultures. As a positive control, MDDC were incubated with 1 μM cognate peptide (SL9) for 3 h before addition of CTL. IFN-γ production was measured as previously described (4). Background IFN-γ secretion by CTL cultured with uninfected MDDC are included.
(ii) Flow cytometry.
A total of 5 × 104 HLA-B27+ MDDC, treated with SIVmac VLP, were infected with 50 ng/ml p24 SF2(VSV) for 24 h and then cocultured with 5 × 104 KK10 CTL clones in round-bottom 96-well plates. When indicated, 1 μM RAL was added in cultures. As a positive control, MDDC were pulsed with 0.1 μM KK10 peptide for 2 h at 37°C. After 1 h of coculture, 5 μg/ml brefeldin A (Molecular Probes, Life Technologies) was added into wells, and incubation was continued for a further 6 h at 37°C. Cells then were stained for CD8 (Miltenyi) and live/dead marker (Invitrogen) at room temperature for 10 min. After fixing with PBS containing 4% paraformaldehyde at 4°C, cells were washed and permeabilized in PBS containing 0.1% BSA and 0.05% saponin and then stained for MIP1-β (R&D Systems Europe), IFN-γ (BioLegend), tumor necrosis factor alpha (TNF-α) (BioLegend), and IL-2 (BioLegend) for 30 min at 4°C. Samples were analyzed using a FACSCanto II flow cytometer (Becton Dickinson). Activated CTL corresponded to the percentage of CD8-positive cells, gated on live cells, simultaneously expressing MIP1-β, IFN-γ, TNF-α, and IL-2.
siRNA treatment.
Small interfering RNA (siRNA) specific for SAMHD1 (M-013950-00) and an siRNA control (D-001206-14-05) were purchased from Dharmacon. MDDC were transfected with 100 nM siRNA using HiPerFect reagent (Qiagen) in 12-well plates, as previously described (14). Cells were cultured in complete medium supplemented with IL-4 and GM-CSF. At 5 days posttransfection, SAMHD1 silencing was assessed by flow cytometry. Cells were infected with HIV-1 SF2 for 48 h and cocultured with EM40F21 CTL for 18 h in an ELISPOT assay.
Viral inhibition assays.
Indirect killing of MDDC was assessed by monitoring the disappearance of Gag-positive MDDC in cocultures with CTL. MDDC were labeled with DDAO far-red dye (Life Technologies) and treated with SIVmac VLP. A total of 5 × 104 MDDC were plated into round-bottom 96-well plates and infected with 0.5 ng of p24 for 2 h at 37°C. Medium was replaced and cells were incubated further for 24 h. After washing, 2.5 × 104 CTL clones were added into wells, rapidly centrifuged at 1,000 rpm, and incubated for 24 h. In experiments where CD8+ T cell degranulation was analyzed, infected unlabeled MDDC were incubated for 48 h, and CD107a antibody (BD Bioscience) was added directly into the medium together with CTL. Cells were collected at 2 h, 5 h, or 24 h postaddition of CTL and stained for surface DC-Sign (R&D Systems Europe) and CD8 (IoTest). Cells were fixed with PBS containing 4% paraformaldehyde and then stained for intracellular Gag (KC57 clone; Beckman Coulter) in PBS containing 1% BSA and 0.05% saponin and analyzed by flow cytometry.
Viral transfer assays.
MDDC were infected as described above for the viral inhibition assays. After 24 h of culture, 5 × 104 autologous CD4+ T cells and 2.5 × 104 CTL clones were added into wells and cocultured for 96 h in IL-2-containing medium. When indicated, RAL was added at the same time as CD4+ T cells and CTL. Cells were stained for surface DC-Sign (R&D Systems) and CD4 (Miltenyi), and intracellular Gag then was analyzed by flow cytometry.
Time-lapse imaging of MDDC killing.
MDDC were treated with SIVmac VLP and infected with VSV-pseudotyped NL4.3-IRES-GFP (50 ng of p24 per 106 cells) for 48 h. A total of 2.5 × 103 MDDC were mixed with 7.5 × 103 CTL in complete medium and plated onto fibronectin-coated multiexperiment U-dishes (Hi-Q4; Nikon). 4′,6-Diamidino-2-phenylindole (DAPI) reagent was added directly into the medium to monitor for cell death. As a positive control, MDDC were labeled with CMRA (Molecular Probes, Life Technologies) and pulsed with 1 μM SL9 peptide for 3 h before culture with CTL. Images were acquired every 3 min using a Nikon Biostation IMQ. Cell death was determined as morphological change and the appearance of DAPI in infected MDDC following contact with CTL. Image analysis and tracking were performed using ImageJ (FIJI).
Statistical analyses.
Statistical tests were performed with GraphPad Prism 5.
RESULTS
Downmodulation of SAMHD1 by Vpx or by siRNAs increases stimulation of CTL by HIV-1-infected MDDC.
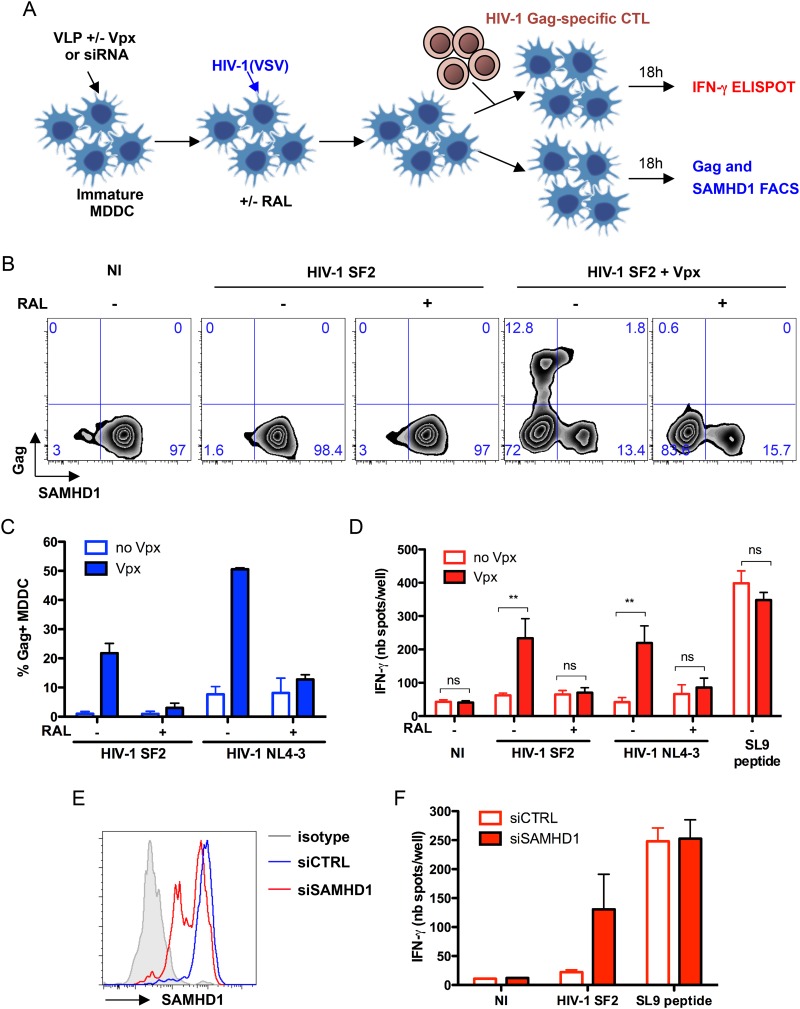
We first investigated whether SAMHD1 influences HIV-1 antigen presentation. To this end, immature MDDC were preincubated for 2 h with VSV-G-pseudotyped SIVmac VLPs which either express Vpx or do not express Vpx (31). The cells then were infected with two different HIV-1 strains, SF2 or NL4-3, pseudotyped with VSV-G (Fig. 1A). After 24 h, MDDC were either maintained alone or cultured for 18 h with an HIV-1-specific CD8+ CTL clone, EM40F21 (28). EM40F21 was derived from an HIV-1-infected patient and recognizes the immunodominant Gag p17 epitope, SL9, presented by HLA-A2. As expected, preincubation of MDDC with VLPs containing Vpx leads to SAMHD1 degradation and a strong increase in Gag levels detected by flow cytometry (Fig. 1B and C). Raltegravir (RAL), an integrase inhibitor, decreased the Gag signal, indicating that it corresponded to newly synthesized molecules. After coculture, CTL stimulation was analyzed by measuring IFN-γ production in an ELISPOT assay. In the presence of SAMHD1, HIV-1-exposed MDDC induced weak secretion of IFN-γ, close to background levels obtained with noninfected cells (Fig. 1D). This was consistent with the small amount of intracellular Gag (Fig. 1B and C). Depletion of SAMHD1 in MDDC infected with SF2 or NL4-3 induced a 14- or 9.5-fold increase in IFN-γ levels, respectively. RAL reduced IFN-γ secretion to almost background levels, indicating that at the viral inoculum used, stimulation of CTL was due primarily to endogenous peptides. Similar results were obtained in MDDC infected with nonpseudotyped HIV-1 (see Fig. S1A in the supplemental material). We did not observe a difference in the amount of surface MHC-I and HLA-A2 in cells infected in the presence or absence of Vpx (see Fig. S1B). Therefore, the enhanced CTL activation likely resulted from an increase in the amount of Gag synthesized in MDDC and not from a higher MHC-I surface density. Moreover, Vpx did not influence presentation of the control SL9 peptide, excluding a role of Vpx on CTL activation (Fig. 1D).
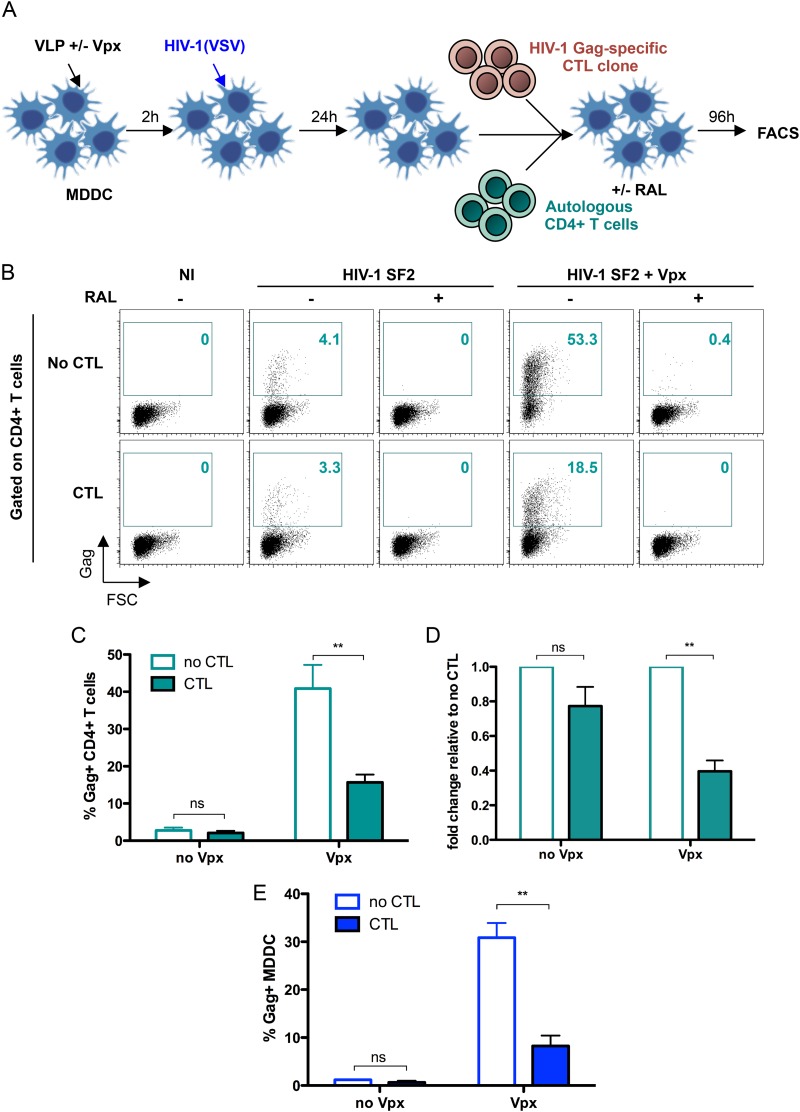
FIG 1.
Infection of MDDC in the absence of SAMHD1 enhances IFN-γ secretion by HIV-1-specific CTL. (A) A total of 106 monocyte-derived dendritic cells (MDDC) were pretreated for 2 h with SIVmac VLP, which either contain or are devoid of Vpx, and were infected with VSV-G-pseudotyped HIV-1 SF2 or NL4-3 strain (20 ng p24 and 100 ng p24, respectively). Where indicated, raltegravir (RAL) was added to the medium at the same time as the virus. Cells were washed after 2 h and cultured in medium alone or in medium with RAL. After 24 h, 105 MDDC were cultured alone for 18 h and monitored for expression of Gag and SAMHD1 by flow cytometry. In parallel, MDDC were cultured with EM40F21 CTL at a 40:1 ratio for 18 h in an IFN-γ ELISPOT assay. (B) Dot plots show percentages of MDDC expressing Gag and SAMHD1 from one representative experiment out of five. (C) The mean (± standard deviation [SD]) percentages of Gag+ MDDC from three independent experiments are indicated on the graph. (D) The number of IFN-γ spots per well (nb spots/well) is shown on the graph. As a positive control, noninfected (NI) MDDC were incubated with 1 μM SL9 cognate peptide for 3 h before culture with CTL. Data are means (± SD) of duplicates from at least two independent experiments. Statistical significance was assessed by a Mann-Whitney test (**, P < 0.005; ns, nonsignificant [P > 0.05]). (E) MDDC were treated with either control siRNA (siCTRL) or SAMHD1-specific siRNA (siSAMHD1). Histograms show expression of SAMHD1, monitored by flow cytometry, 5 days after treatment in one representative experiment. (F) Five days after siRNA treatment, MDDC were infected with VSV-G-pseudotyped HIV-1 SF2 for 48 h. Cells were cocultured with EM40F21 CTL for 18 h in an IFN-γ ELISPOT assay. Data are means (± SD) of duplicates from two independent experiments.
We next silenced SAMHD1 in MDDC using siRNA. SAMHD1-directed siRNA, but not control siRNA, decreased the levels of the protein in a fraction of MDDC, as assessed by flow cytometry (Fig. 1E). As expected (8, 14), SAMHD1 silencing led to the appearance of 6- to 10-fold more Gag-expressing cells after HIV-1 infection (not shown). Moreover, in SAMHD1-silenced cells, this enhanced sensitivity to HIV-1 infection was associated with a significant increase in the activation of HIV-1-specific CTL (Fig. 1F).
Together, these experiments indicate that depletion of SAMHD1 by Vpx or by siRNAs enhanced antigen presentation upon HIV-1 exposure of MDDCs.
Depletion of SAMHD1 in MDDC does not affect exogenous antigen presentation.
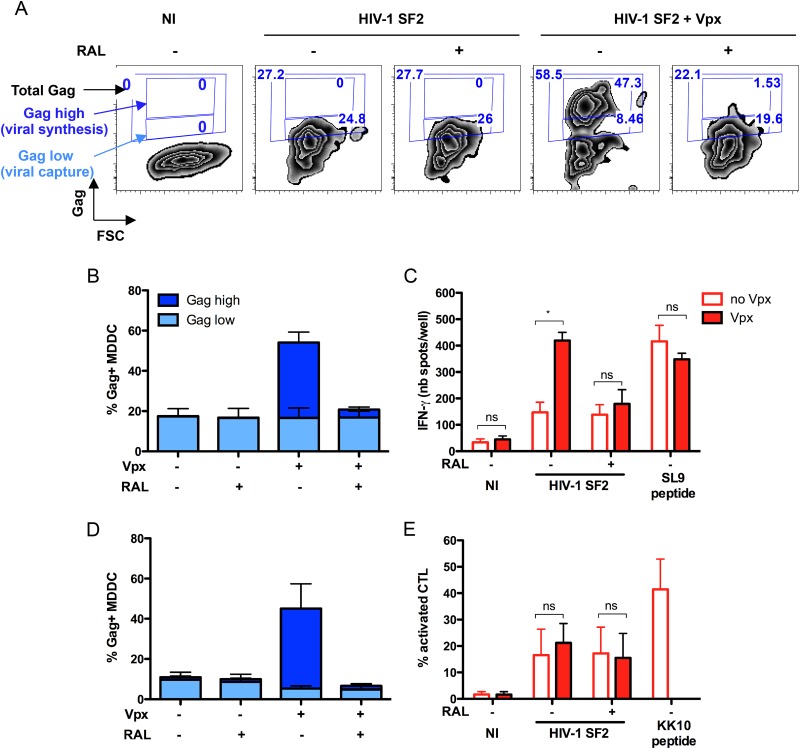
We asked whether SAMHD1 could modulate exogenous presentation of HIV-1, a phenomenon that does not require productive infection of MDDC. We exposed MDDC to high viral loads of VSV-G-pseudotyped SF2 in order to increase the amount of incoming viral material. In this setting, the total number of Gag-expressing cells detected by flow cytometry after 48 h could be separated into two populations depending on the mean fluorescence intensity (MFI) of the staining (Fig. 2A and B): a “Gag-high” population (∼40% of total cells) and a “Gag-low” population (∼20% of total cells). The Gag-high population appeared in the presence of Vpx and was sensitive to RAL, indicating that it corresponded to newly synthesized viral proteins. The Gag-low population was detected with or without Vpx and was resistant to RAL. Inhibiting reverse transcription with nevirapine did not diminish the percentage of Gag-low cells (not shown). Furthermore, the Gag-low population was detected as soon as 2 to 6 h after viral exposure (see Fig. S2 in the supplemental material), confirming that this population harbored captured virions. When cultured with EM40F21 CTL in an IFN-γ ELISPOT assay, MDDC exposed to high viral loads were capable of stimulating CTLs in the absence of Vpx (Fig. 2C). As with the low virus inocula, depletion of SAMHD1 led to an increase in T cell activation (3-fold). This increase resulted from productive infection of MDDC, since RAL diminished levels of IFN-γ. Moreover, Vpx did not significantly decrease IFN-γ secretion when cells were infected in the presence of RAL, suggesting that SAMHD1 has no effect on HIV-1 presentation of incoming virions.
FIG 2.
SAMHD1 does not modulate HIV-1 cross-presentation in the absence of productive infection. (A) A total of 106 MDDC were exposed to SIVmac VLP and infected for 2 h with a high dose of VSV-G-pseudotyped SF2 (100 ng p24). Cells were either treated with RAL or left untreated. Cells were cultured with or without EM40F21 CTL, and flow cytometry and ELISPOT analysis were carried out as described for Fig. 1A. Dot plots show the percentage of cells with a high mean fluorescence intensity (MFI) of Gag (viral synthesis) and cells with a low Gag MFI (viral capture), gated on total cells, in one representative experiment out of three. (B) The graph shows mean (± SD) percentages of cells expressing high and low Gag MFI in MDDC cultured alone from three independent experiments. (C) The graph represents mean (± SD) numbers of IFN-γ spots per well of duplicates from three independent experiments in cocultures of MDDC and CTL. As a positive control, noninfected (NI) MDDC were incubated with 1 μM SL9 cognate peptide for 3 h before culture with CTL. (D) HLA-B27+ MDDC were infected as described for panel A. After 24 h, 5 × 104 cells were cultured alone for 6 h, and mean (± SD) percentages of cells expressing high and low Gag MFI was determined in three independent experiments. (E) MDDC were cultured with HLA-B27-restricted KK10 CTL at a 1:1 ratio for 6 h. As a positive control, noninfected MDDC were incubated with 0.1 μM KK10 peptide for 3 h before culture with CTL. The percentage of activated CTL was determined as CD8+ cells simultaneously expressing IFN-γ, TNF-α, MIP1-β, and IL-2. Dead cells were excluded by staining with Aqua Vivid reagent. Data represent mean (± SD) values from three independent experiments, except NI, which represents mean (± SD) values from two experiments. Statistical significance was assessed by a Mann-Whitney test (*, P < 0.05; ns, nonsignificant [P > 0.05]).
To further confirm these results, we cocultured infected HLA-B27+ MDDC with an HLA-B27-restricted CD8+ T cell clone specific for the HIV-1 KK10 Gag p24 epitope. The KK10-specific clone efficiently recognizes the epitope when it is derived from incoming viral particles (29). After 24 h of infection, MDDC were cultured with KK10 CTL for 6 h, a setting at which the KK10 clone is stimulated mainly by exogenous peptides (29). As with HLA-A2+ MDDC, infection of HLA-B27+ MDDC resulted in the detection of a Gag-high and Gag-low population (Fig. 2D). Activation of the KK10 clone was monitored by flow cytometry for IFN-γ, TNF-α, MIP1-β, and IL-2 expression. In the absence of Vpx, MDDC induced activation of 16% of KK10-specific CTL (Fig. 2E). When cells were treated with RAL, the Gag-low population was not diminished and T cell activation was maintained at 17% (Fig. 2E). Thus, the KK10 CTL response was induced predominantly by presentation of antigens derived from incoming viral capsid and minimally by endogenous antigens. Pretreatment of MDDC with Vpx induced a 5-fold increase in Gag+ cells but only a 1.3-fold increase in stimulation of the KK10 clone (∼20% of activated CTL). In Vpx-treated MDDC, RAL strongly diminished the Gag-high population while maintaining the Gag-low population. With RAL, the CTL were similarly activated in the presence or in the absence of Vpx (Fig. 2E). Increasing the amount of incoming virions led to similar results but with a higher percentage of activated T cells, indicating that the lack of variation observed was not due to saturation of CTL stimulation (not shown).
Therefore, our results, obtained with two different CTL clones, indicate that SAMHD1 does not influence the exogenous presentation of HIV-1 antigens in the absence of productive infection.
HIV-1 replication in Vpx-treated MDDC is abrogated in the presence of CTL.
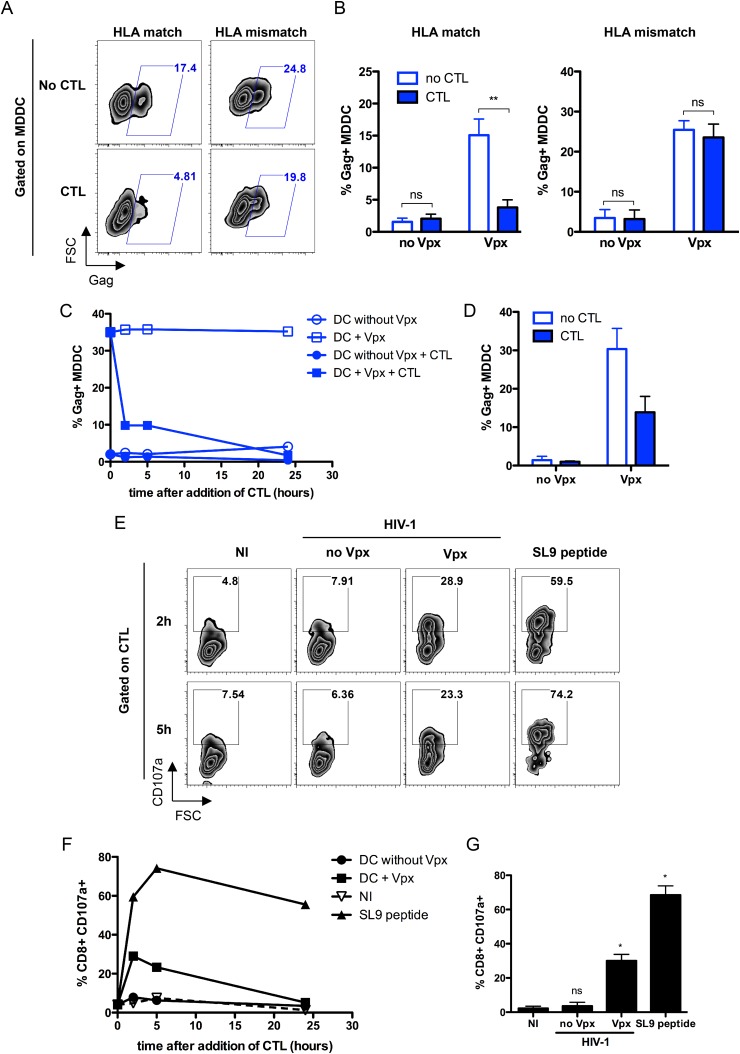
Activated CTL eliminate infected target cells by direct lysis or by releasing antiviral factors and chemokines that block spreading of HIV-1 (32–35). We examined the outcome of SAMHD1 depletion on viral replication in MDDC in the presence of Gag-specific CTLs. MDDC were infected with VSV-G-pseudotyped HIV-1 in the presence or absence of Vpx for 24 h and then cultured for a further 24 h with or without EM40F21 CTL. When cultured alone, 15% to 25% of MDDC were Gag+ with Vpx (Fig. 3A, top left, and B, left). Coculture of infected HLA-matched MDDC with CTL diminished the amount of Gag+ MDDC to levels approaching those observed in the absence of Vpx, ∼3.5 and 1.5% Gag+ cells, respectively (Fig. 3A, bottom left, and B, left). Under these conditions, SAMHD1 depletion did not confer an advantage to HIV-1 replication in the presence of activated CTL. Clearance of infection was HLA dependent, since the amount of Gag in HLA-mismatched MDDC was similar both in the presence and absence of CTLs (Fig. 3A and B, right). Furthermore, we observed that while Gag expression remained constant over 24 h in MDDC cultured alone, the amount of Gag+ MDDC rapidly declined over time in the presence of CTL (Fig. 3C). A 2.2-fold decrease in the amount of infected MDDC occurred as early as 2 h after culture with CTL (Fig. 3D).
FIG 3.
HIV-1-specific CTL inhibit Vpx-mediated infection of MDDC through their cytotoxic activity. (A) Far-red-labeled HLA-A2+ (match) or HLA-A2− (mismatch) MDDC were pretreated with SIVmac VLP and exposed for 2 h to VSV-G-pseudotyped HIV-1 SF2 (2 ng p24/105 cells). After 24 h, cells were washed and incubated with either EM40F21 CTL at a 2:1 ratio or with medium alone for 24 h and then analyzed by flow cytometry. Plots are gated on far red+ cells, and values in each panel represent the percentage of Gag+ cells in Vpx-treated MDDC. One representative experiment out of three is shown. (B) Graphs show mean numbers (± SD) of Gag+ MDDC from three independent experiments. Statistical significance was assessed by a two-way analysis of variance (ANOVA) test (**, P < 0.005; ns, nonsignificant [P > 0.05]). (C) MDDC were infected as described for panel A and incubated with either EM40F21 CTL or with medium alone. Cells were monitored for Gag expression by flow cytometry in DC-Sign+ cells after 2 h, 5 h, and 24 h of coculture. One representative experiment out of three is shown. (D) Mean numbers (± SD) of Gag+ MDDC after 2 h of culture with CTL are represented from two independent experiments. (E and F) MDDC were treated as described for panel C, and CD107a antibody was added directly into wells during coculture with CTL. MDDC loaded with SL9 peptide were used as a positive control. Cells were collected after 2 h, 5 h, and 24 h of culture and analyzed by fluorescence-activated cell sorting (FACS) for CD8, DC-Sign, and Gag expression. Plots are gated on CD8+ T cells, and values in each panel represent the percentage of CD107a+ cells. The graph represents percentages of CD8+ CD107a+ cells in one representative experiment out of three. (F) Mean (± SD) percentages of CD8+ cells expressing CD107a after 2 h of culture with MDDC are represented from three independent experiments. Statistical significance was assessed by a Mann-Whitney test (*, P < 0.05; ns, nonsignificant [P > 0.05]).
We next investigated whether inhibition of viral infection in MDDC was due to CTL-mediated cell lysis. VLP-treated MDDC infected with VSV-G-pseudotyped HIV-1 were cocultured with CTL in the presence of CD107a antibody, a marker for CTL degranulation (36). CD107a expression at the surface of CD8+ cells was monitored over time. In the absence of Vpx, a CD107a signal was detected, similar to the results for noninfected conditions (Fig. 3E and F, compare no Vpx and NI). In the presence of Vpx, infected MDDC strongly induced the expression of CD107a, indicating that activated CTL displayed enhanced cytotoxic functions. CTL degranulation occurred as soon as 2 h after exposure to MDDC (Fig. 3G) and correlated with the decrease in the amount of Gag+ MDDC detected in cultures at the same time points (Fig. 3C and F). Levels of CD107a decreased over time, likely due to antibody internalization.
Altogether, these results indicate that depletion of SAMHD1 increases the amount of intracellular Gag, enabling CTLs to efficiently recognize MDDC and to inhibit viral replication.
CTL rapidly lyse MDDC productively infected with HIV-1.
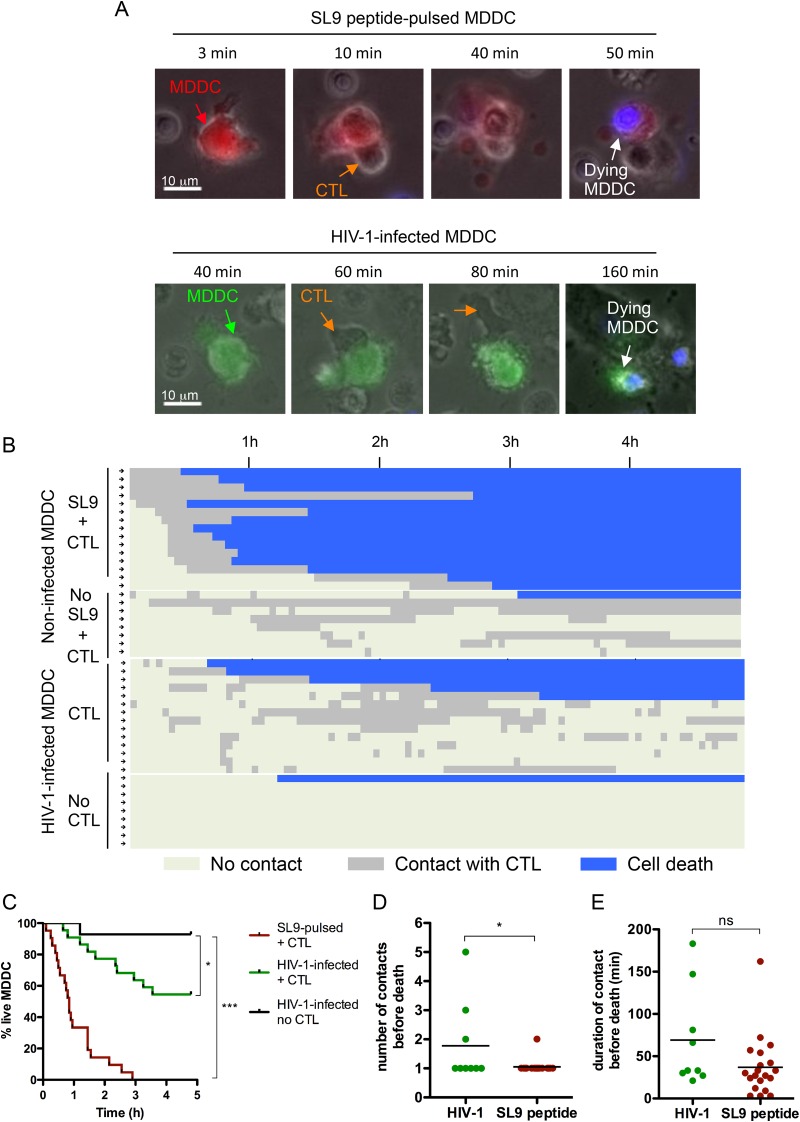
We next asked whether CTL activation leads to killing of infected MDDC. To this aim, we infected Vpx-treated MDDC with NL4-3 HIV-1, encoding internal ribosome entry site-green fluorescent protein (IRES-GFP), and analyzed MDDC-CTL cultures using time-lapse microscopy. Adding DAPI, a nonpermeable cell marker that stains the nucleus of dying or dead cells, to the medium allowed us to monitor in real time the death of GFP-expressing cells. One image was recorded every 3 min for 5 h.
In the absence of virus, only rare MDDC were killed after entering into contact with CTLs (Fig. 4B; also see Fig. S3B in the supplemental material). As a control for CTL-induced cell death, we preexposed MDDC, labeled with a red dye (CMRA), to the Gag SL9 peptide before culture with CTL (Fig. 4A; also see Movie S1 in the supplemental material). Peptide-pulsed MDDC rapidly changed morphology and stained for DAPI following interaction with CTL. The majority of SL9 peptide-loaded MDDC were killed after a single and short contact with CTL (Fig. 4B, C, D, and E). The whole-cell population was destroyed by 3 h postcoculture (Fig. 4B and C).
FIG 4.
Vpx promotes killing of infected MDDC by CTL. (A, upper) Uninfected CMRA-labeled MDDC (red cells) were exposed to the SL9 peptide for 3 h. (Lower) Vpx-treated MDDC were infected with VSV-G-pseudotyped NL4-3 encoding IRES-GFP (5 ng p24/3 × 105 cells) for 48 h. MDDC were plated with EM40F21 CTL at a 1:3 ratio on U dishes coated with fibronectin. To distinguish dead cells, DAPI dye was added into the medium and cells were imaged by time-lapse microscopy. Green cells represent infected live MDDC, and blue cells represent dead cells. (B) Individual CMRA+ or GFP+ cells were tracked, and the times of contact with CTL (in gray) and cell death (in blue) were quantified for each cell. Each line/arrow corresponds to one MDDC monitored for 5 h. (C) The graph represents the mean percentages of live GFP+ MDDC from two independent donors after addition of CTL to cultures. Statistical significance was assessed using a log-rank test (*, P < 0.05; **, P < 0.0001). The number of CTL contacts (D) and the duration of contacts (E) before death were quantified for HIV-1-infected MDDC or SL9 peptide-pulsed MDDC that were killed. Statistical significance was assessed by a Mann-Whitney test (*, P < 0.05; ns, nonsignificant [P > 0.05]).
We then analyzed the behavior of HIV-1-infected MDDC from two independent donors. Upon productive infection, MDDC established contact with CTL, which led to morphological changes and the death of the infected cell (Fig. 4A; also see Movie S2 in the supplemental material). We analyzed 22 infected cells for time of contact with CTL and CTL-mediated cell death (Fig. 4B; also see Fig. S3B). About 45% of infected MDDC were dead at the end of the 5-h video. This quantification was consistent with a flow cytometry analysis of cells recovered after filming (see Fig. S3C). Analysis of the video revealed various modes of interaction and duration of contacts between MDDC and CTL (Fig. 4D and E). In most cases, MDDC interacted with one CTL that maintained contact until the target started dying (Fig. 4D). In other cases, multiple CTL contacts were necessary for the killing of infected MDDC. Some CTL killed their targets after only 30 to 40 min of contact, while other CTL maintained contact for 1 to 3 h before killing (Fig. 4E).
Together, the results indicate that in the presence of HIV-1-specific CTL, Vpx-mediated HIV-1 infection of MDDC leads to a rapid lysis of infected cells.
Killing of MDDC by CTL inhibits transmission of HIV-1 to CD4+ T cells.
We next asked if CTL inhibits HIV-1 transmission from MDDC to CD4+ T cells. Infected MDDC were cultured with autologous CD4+ T cells in the presence or absence of CTL for 96 h and then analyzed for Gag expression (Fig. 5A). Without Vpx, in the absence of CTL, low levels of Gag (about 2%) were detected in CD4+ T cells (Fig. 5B and C). RAL abrogated Gag expression in CD4+ T cells. CTL induced only a 1.3-fold decrease in HIV-1 transmission (Fig. 5B and C). With Vpx-treated MDDC, up to 40% of CD4+ T cells were infected in the coculture. Under these conditions, the addition of CTL led to a 3-fold decrease in HIV-1 transmission to CD4+ T cells (Fig. 5D). The residual viral transmission likely was due to the fact that CTL did not completely eliminate infected MDDC in our settings (Fig. 5E). These results demonstrate that killing of infected MDDC in the presence of Vpx inhibits HIV-1 transmission to CD4+ T cells.
FIG 5.
CTL inhibit Vpx-mediated HIV-1 transmission from MDDC to CD4+ T cells. (A) MDDC were treated with SIVmac VLP for 2 h and infected with VSV-G-pseudotyped HIV-1 SF2 for 24 h (1 ng p24/2 × 105 cells). Cells were washed and cultured with autologous CD4+ T cells and EM40F21 CTL at a 2:2:1 ratio (MDDC:CD4+ T cells:CTL). Under conditions with RAL, the drug was added at the beginning of the coculture. After 96 h of culture, cells were monitored by flow cytometry for expression of CD8, CD4, DC-Sign, and Gag. (B) Dot plots show percentages of Gag-expressing CD4+ T cells in one representative experiment. Mean (± SD) percentages of Gag in CD4+ T cells (C) and DC-Sign+ cells (E) from three independent experiments are indicated on graphs. (D) Mean (± SD) fold changes of Gag in CD4+ T cells, relative to results under conditions without CTL, from three independent donors are shown. Statistical significance was assessed by a two-way ANOVA test (**, P < 0.01; ns, nonsignificant [P > 0.05]).
Vpx rescues infection and antigen presentation of VSV-G-pseudotyped T/F HIV-1.
T/F viruses preferentially exhibit R5 tropism but have been shown to poorly infect macrophages and dendritic cells (27, 37, 38). Vpx increases infection of macrophages by T/F HIV-1 (39). Therefore, we explored if SAMHD1 depletion could rescue infection of T/F viruses and enhance viral antigen presentation in MDDC.
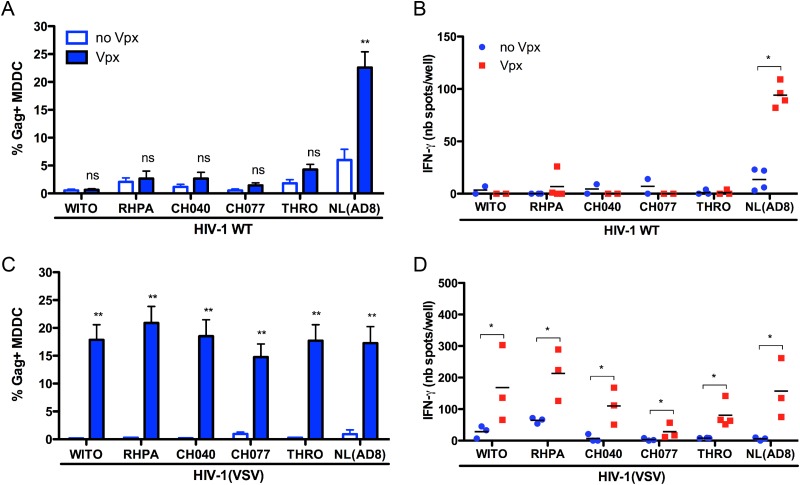
We used a panel of five previously described T/F viruses (WITO, RHPA, CH040, CH077, and THRO) (27) and, as a control, the NL(AD8) R5-tropic strain. NL(AD8) poorly infected MDDC (5% of Gag+ cells at 96 h pi) (Fig. 6A). As expected, Vpx increased NL(AD8) infection, reaching ∼22% of Gag+ cells. This increase was associated with an enhancement of SL9-specific CTL stimulation (Fig. 6B). In contrast, Vpx modestly increased infection by T/F viruses, with a maximum of 4% Gag+ cells infected with THRO and less than 3% for the four other T/F viruses (Fig. 6A). IFN-γ secretion was undetectable in ELISPOT assays using MDDC infected with T/F HIV-1 and was not enhanced by Vpx (Fig. 6B).
FIG 6.
Vpx increases antigen presentation of R5-tropic transmitted/founder HIV-1 in an entry-dependent manner. A total of 106 VLP-treated MDDC were infected with 250 ng of the indicated WT strains (A) or 25 ng of VSV-G-pseudotyped strains (C) of HIV-1. Cells were washed after 2 h and cultured for 96 h and 72 h, respectively. Data represent mean (± SD) percentages of Gag from five independent experiments. (B and D) A total of 105 cells infected with WT HIV-1 for 72 h and VSV-G-pseudotyped strains for 24 h were used to stimulate 2,500 EM40F21 CTL in an IFN-γ ELISPOT assay. Data are the means (± SD) from duplicates and represent at least two donors. Statistical significance was assessed by a Mann-Whitney test (**, P < 0.005; *, P < 0.05; ns, nonsignificant [P > 0.05]).
We next addressed whether Vpx increases infection and antigen presentation of VSV-G-pseudotyped T/F HIV-1. As expected, MDDC were poorly susceptible to the pseudotyped viruses without Vpx (∼1.5% of Gag+ cells) (Fig. 6C). Addition of Vpx triggered infection of MDDC (up to 40% of Gag+ cells), indicating that upon entry into MDDC, pseudotyped T/F HIV-1 strains are sensitive to SAMHD1 (Fig. 6C). When cultured with EM40F21 CTL, Vpx increased CTL stimulation by VSV-G-pseudotyped T/F viruses (Fig. 6D). We observed different levels of CTL activation depending on the T/F virus. WITO, RHPA, and CH040 induced a strong response, whereas CH077 and THRO were poorly recognized by CTL (Fig. 6D). Differences in the ability of T/F viruses to stimulate CTL may result from differential antigen processing due to variations in epitope-flanking sequences (40), since CH077 and THRO encode SL9 sequences identical to those of CH040 and NL(AD8), respectively (see Fig. S4 in the supplemental material). IFN-γ secretion by CTL was associated with a CTL-induced inhibition of infection (see Fig. S4).
Taken together, the results indicate that T/F viruses poorly infect MDDC because of an entry defect. Pseudotyping T/F viruses with VSV-G allowed viral entry and productive infection only when SAMHD1 was degraded by Vpx. Moreover, SAMHD1 limits antigen presentation by MDDC infected with pseudotyped T/F viruses.
DISCUSSION
While the role of SAMHD1 in restriction and sensing of HIV-1 was described previously, its impact in the adaptive immune response remains less characterized. We investigated the role of SAMHD1 in MHC-I-restricted viral antigen presentation by MDDC. We analyzed the activation of two HIV-1-specific CTL clones cocultured with MDDC exposed to different viruses in the presence or absence of Vpx. CTL activation was assessed by measuring IFN-γ by ELISPOT assay or cytokine production by flow cytometry. CTL degranulation was monitored by CD107a surface expression. To study the effect of CTLs on HIV-1 infection, we monitored the disappearance of Gag+ MDDC and visualized death in real time using time-lapse microscopy.
We show that degradation of SAMHD1 by Vpx in HIV-1-infected MDDC leads to a strong increase in MHC-I-restricted endogenous presentation of viral peptides. However, Vpx had no effect on HIV-1 exogenous presentation. CTL induced the disappearance and killing of Vpx-treated Gag+ MDDC. This killing was antigen specific, since CTL also briefly contacted uninfected targets but did not eliminate them. Of note, we did not observe a downmodulation of MHC-I in productively infected MDDC and found that, in agreement with previous studies (41, 42), the stimulation of CTL was similar whether or not the virus expressed Nef (not shown).
The specific CTL response has been associated with immune control of HIV-1 infection, strongly suggesting that elimination of infected cells limits viral spread and progression to disease (43). To our knowledge, no real-time visualization of the killing of HIV-1-infected MDDC by CTL has been described previously. Here, we show that when MDDC are sufficiently infected following SAMHD1 degradation, the levels of antigens generated lead to efficient recognition by CTL and death. This process occurred between 30 min and 3 h after interaction with CTL, and sometimes several attempts were necessary before contact led to killing. In contrast, the majority of MDDC pulsed with a high dose of SL9 peptide was killed after a single and short (about 30 min) contact with CTL. This is in agreement with previous studies demonstrating that when target cells display strong antigenic stimuli, CTL maintain strong contact until the death of the target (44, 45). HIV-1-infected MDDC likely present different levels of epitopes, depending, for example, on the stage of infection, which may in turn affect the ability and rapidity of CTL to recognize these targets. We hypothesize that the killing of MDDC, at least with the EM40F21 CTL clone tested, resulted mainly from the recognition of newly synthesized viral molecules, since we did not observe an effect of Vpx on the efficiency of early exogenous antigen presentation. In the absence of Vpx, antigen levels are low, and although CTL stimulation can occur through exogenous presentation of antigens, this process is less efficient than endogenous presentation (4, 6, 28). Thus, by not having a vpx gene, HIV-1 may avoid high antigen expression and presentation, favoring viral spread. In agreement with this, we observed a strong inhibition of HIV-1 transmission from Vpx-exposed MDDC to autologous CD4+ T cells in the presence of CTL, whereas the transmission of HIV-1 from untreated MDDC to CD4+ T cells was minimally affected by CTL. Further studies will help to determine whether other CTL clones, targeting different viral epitopes and displaying various avidities (45), recognize and kill infected DCs with different efficacies. Future work also will help to assess whether the maturation state of DCs affects the efficiency of SAMHD1-mediated enhancement of antigen presentation.
It has been reported that the initial antigen-specific CTL response to T/F viruses contributes to the control of acute viremia in infected individuals (46, 47). How T/F viruses interact with MDDCs and induce antigen presentation is not fully characterized. In vitro, DCs bind T/F HIV-1 and efficiently transfer these viruses to CD4+ T cells (37). We further show here that T/F HIV-1 poorly infected MDDC, even in the presence of Vpx; hence, it did not promote stimulation of HIV-1-specific CTL. The inability of T/F viruses to infect MDDC was due to inefficient viral entry, since VSV-G pseudotyping rescued infection and antigen presentation in the presence of Vpx. Therefore, Vpx fails to confer a replicative advantage to nonpseudotyped T/F HIV-1 in MDDC. This suggests that Vpx is not essential for myeloid-dependent viral propagation during primo-infection. In favor of this hypothesis, a recent study demonstrated that myeloid cells localized in mucosal sites from SIVsm-infected primates contained very small amounts of viral DNA (48). Myeloid cells in lymphoid tissues, however, contained viral DNA, but this was not dependent on the presence of Vpx. Rather, viral DNA in myeloid cells arose from phagocytosis of infected T cells (48, 49). The phagocytosis of living or dying infected cells also may represent a source of exogenous viral antigens (50, 51). This further suggests that in vivo, HIV-1 avoids productive infection of DCs.
Several studies reported slower viral replication and progression to AIDS in primates infected with a vpx-deleted simian immunodeficiency virus mutant (48, 52–54). This may result from the ability of Vpx to increase infection in resting CD4+ T cells, a population which is highly refractory to HIV-1 (55–57). Further work will help to determine if SAMHD1, by controlling HIV-1 infection of resting CD4+ T cells (55, 56), also impacts viral antigen presentation by these cells upon cell activation. It also will be interesting to investigate the role of Vpx in HIV-2 antigen presentation. We recently observed that primary HIV-2 strains do not productively infect MDDC, even though they naturally encode Vpx (58). Thus, HIV-2, like the laboratory-adapted and T/F HIV-1 strains described here, may limit infection of DCs to lower immune detection. Overall, our results highlight how the antiviral activity of restriction factors affects the adaptive immune response. Our results also suggest that SAMHD1 inhibitors, by enhancing viral antigen presentation by DCs, are interesting candidates to manipulate the immune response to HIV-1.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Virus & Immunity Unit for discussions and critical readings of the manuscript.
D.A. was supported by a postdoctoral fellowship from ANRS and the FP7 HIT Hidden HIV program. The work was supported by grants from the ANRS, SIDACTION, AREVA Foundation, the Labex IBEID program, the FP7 program, HIT Hidden HIV (Health-F3-2012-305762), and Institut Pasteur, as well as by the Vaccine Research Institute, Investissements d'Avenir program (ANR-10-LABX-77).
We thank the Programme EVA Centre for AIDS Reagents and the NIH AIDS Reagent Program for the gifts of reagents.
We have no competing financial interests to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00069-15.
REFERENCES
- 1.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 4.Buseyne F, Le Gall S, Boccaccio C, Abastado JP, Lifson JD, Arthur LO, Riviere Y, Heard JM, Schwartz O. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat Med 7:344–349. doi: 10.1038/85493. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Plata MT, Urrutia A, Cardinaud S, Buzon MJ, Izquierdo-Useros N, Prado JG, Puertas MC, Erkizia I, Coulon PG, Cedeno S, Clotet B, Moris A, Martinez-Picado J. 2012. HIV-1 capture and antigen presentation by dendritic cells: enhanced viral capture does not correlate with better T cell activation. J Immunol 188:6036–6045. doi: 10.4049/jimmunol.1200267. [DOI] [PubMed] [Google Scholar]
- 6.Granelli-Piperno A, Zhong L, Haslett P, Jacobson J, Steinman RM. 2000. Dendritic cells, infected with vesicular stomatitis virus-pseudotyped HIV-1, present viral antigens to CD4+ and CD8+ T cells from HIV-1-infected individuals. J Immunol 165:6620–6626. doi: 10.4049/jimmunol.165.11.6620. [DOI] [PubMed] [Google Scholar]
- 7.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayinde D, Casartelli N, Schwartz O. 2012. Restricting HIV the SAMHD1 way: through nucleotide starvation. Nat Rev Microbiol 10:675–680. doi: 10.1038/nrmicro2862. [DOI] [PubMed] [Google Scholar]
- 10.Kim B, Nguyen LA, Daddacha W, Hollenbaugh JA. 2012. Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J Biol Chem 287:21570–21574. doi: 10.1074/jbc.C112.374843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Gelais C, de Silva S, Amie SM, Coleman CM, Hoy H, Hollenbaugh JA, Kim B, Wu L. 2012. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4+ T-lymphocytes cannot be upregulated by interferons. Retrovirology 9:105. doi: 10.1186/1742-4690-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryoo J, Choi J, Oh C, Kim S, Seo M, Kim SY, Seo D, Kim J, White TE, Brandariz-Nunez A, Diaz-Griffero F, Yun CH, Hollenbaugh JA, Kim B, Baek D, Ahn K. 2014. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 20:936–941. doi: 10.1038/nm.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puigdomenech I, Casartelli N, Porrot F, Schwartz O. 2013. SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells. J Virol 87:2846–2856. doi: 10.1128/JVI.02514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. 2012. Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama H, Akari H, Adachi A, Takiguchi M. 2002. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8(+) T-cell cytolytic activity and cytokine production. J Virol 76:7535–7543. doi: 10.1128/JVI.76.15.7535-7543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang OO, Nguyen PT, Kalams SA, Dorfman T, Gottlinger HG, Stewart S, Chen IS, Threlkeld S, Walker BD. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J Virol 76:1626–1631. doi: 10.1128/JVI.76.4.1626-1631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 24.Marin M, Rose KM, Kozak SL, Kabat D. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med 9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casartelli N, Guivel-Benhassine F, Bouziat R, Brandler S, Schwartz O, Moris A. 2010. The antiviral factor APOBEC3G improves CTL recognition of cultured HIV-infected T cells. J Exp Med 207:39–49. doi: 10.1084/jem.20091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648–2654. doi: 10.1182/blood-2003-07-2532. [DOI] [PubMed] [Google Scholar]
- 29.Kløverpris HN, Payne RP, Sacha JB, Rasaiyaah JT, Chen F, Takiguchi M, Yang OO, Towers GJ, Goulder P, Prado JG. 2013. Early antigen presentation of protective HIV-1 KF11Gag and KK10Gag epitopes from incoming viral particles facilitates rapid recognition of infected cells by specific CD8+ T cells. J Virol 87:2628–2638. doi: 10.1128/JVI.02131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildum S, Schindler M, Munch J, Kirchhoff F. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80:8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2006. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther 13:991–994. doi: 10.1038/sj.gt.3302753. [DOI] [PubMed] [Google Scholar]
- 32.Cocchi F, DeVico AL, Lu W, Popovic M, Latinovic O, Sajadi MM, Redfield RR, Lafferty MK, Galli M, Garzino-Demo A, Gallo RC. 2012. Soluble factors from T cells inhibiting X4 strains of HIV are a mixture of beta chemokines and RNases. Proc Natl Acad Sci U S A 109:5411–5416. doi: 10.1073/pnas.1202240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killian MS, Teque F, Walker RL, Meltzer PS, Killian JK. 2013. CD8(+) lymphocytes suppress human immunodeficiency virus 1 replication by secreting type I interferons. J Interferon Cytokine Res 33:632–645. doi: 10.1089/jir.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol 71:3120–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang OO, Kalams SA, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker BD, Johnson RP. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol 70:5799–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 37.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mlcochova P, Watters SA, Towers GJ, Noursadeghi M, Gupta RK. 2014. Vpx complementation of “non-macrophage tropic” R5 viruses reveals robust entry of infectious HIV-1 cores into macrophages. Retrovirology 11:25. doi: 10.1186/1742-4690-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Gall S, Stamegna P, Walker BD. 2007. Portable flanking sequences modulate CTL epitope processing. J Clin Investig 117:3563–3575. doi: 10.1172/JCI32047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petit C, Buseyne F, Boccaccio C, Abastado JP, Heard JM, Schwartz O. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225–236. doi: 10.1006/viro.2001.0984. [DOI] [PubMed] [Google Scholar]
- 42.Maccormac LP, Jacque JM, Chain B. 2004. The functional consequences of delivery of HIV-1 Nef to dendritic cells using an adenoviral vector. Vaccine 22:528–535. doi: 10.1016/j.vaccine.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiedemann A, Depoil D, Faroudi M, Valitutti S. 2006. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci U S A 103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foley MH, Forcier T, McAndrew E, Gonzalez M, Chen H, Juelg B, Walker BD, Irvine DJ. 2014. High avidity CD8+ T cells efficiently eliminate motile HIV-infected targets and execute a locally focused program of anti-viral function. PLoS One 9:e87873. doi: 10.1371/journal.pone.0087873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, Denny TN, Crump JA, Cohen MS, McMichael AJ, Haynes BF, Tomaras GD. 2012. Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J Virol 86:6835–6846. doi: 10.1128/JVI.00437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, Weinhold KJ, Moore S, Letvin N, Haynes BF, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber BT, McMichael AJ. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, Thompson EA, Ortiz AM, Vinton CL, Ourmanov I, Lore K, Douek DC, Estes JD, Hirsch VM, Brenchley JM. 2014. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 41:493–502. doi: 10.1016/j.immuni.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter AE, Russell RA, Duncan CJ, Moore MD, Willberg CB, Pablos JL, Finzi A, Kaufmann DE, Ochsenbauer C, Kappes JC, Groot F, Sattentau QJ. 2014. Macrophage infection via selective capture of HIV-1-infected CD4(+) T cells. Cell Host Microbe 16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet JG, Gannage M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Maranon C. 2007. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Maranon C, Desoutter JF, Hoeffel G, Cohen W, Hanau D, Hosmalin A. 2004. Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc Natl Acad Sci U S A 101:6092–6097. doi: 10.1073/pnas.0304860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol 69:2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat Med 4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westmoreland SV, Converse AP, Hrecka K, Hurley M, Knight H, Piatak M, Lifson J, Mansfield KG, Skowronski J, Desrosiers RC. 2014. SIV vpx is essential for macrophage infection but not for development of AIDS. PLoS One 9:e84463. doi: 10.1371/journal.pone.0084463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med 18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Descours B, Cribier A, Chable-Bessia C, Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N, Benkirane M. 2012. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu L. 2012. SAMHD1: a new contributor to HIV-1 restriction in resting CD4+ T-cells. Retrovirology 9:88. doi: 10.1186/1742-4690-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chauveau L, Puigdomenech I, Ayinde D, Roesch F, Porrot F, Bruni D, Visseaux B, Descamps D, Schwartz O. 2015. HIV-2 infects resting CD4+ T cells but not monocyte-derived dendritic cells. Retrovirology 12:2. doi: 10.1186/s12977-014-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.