Abstract
Liver dysfunction reflects the status of heart failure, and previous studies have demonstrated that serum lactate dehydrogenase (S-LDH) levels are increased in patients exhibiting heart failure and liver dysfunction. Right heart failure is a main characteristic of idiopathic pulmonary arterial hypertension (IPAH). The aim of the present study was to assess the prognostic significance of S-LDH levels in patients with IPAH. S-LDH levels were determined in 173 patients with IPAH, and these patients were subclassified into two groups according to a defined upper reference limit of S-LDH (250 IU/l). Right heart catheterization was performed in all patients. A total of 53 patients were found to have elevated S-LDH to ≥250 IU/l. In a mean follow-up period of 31.2±17.9 months, 57 patients succumbed. In the group with lower S-LDH levels (S-LDH <250 IU/l), 16.7% of the patients succumbed, compared with 69.8% of patients in the group with higher S-LDH levels (S-LDH ≥250 IU/l). The Kaplan-Meier survival curves demonstrated that patients with higher S-LDH levels had a significantly lower survival rate than did those with lower S-LDH levels (log-rank test, P<0.001). Cox proportional hazard analyses identified reduced body mass index, reduced cardiac index, elevated World Health Organization functional class, higher S-LDH and an absence of PAH-targeted therapy as significant predictors of adverse outcomes. In conclusion, elevated S-LDH is a risk factor for mortality in patients with IPAH.
Keywords: serum lactate dehydrogenase, idiopathic pulmonary hypertension, mortality
Introduction
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme that is widely expressed in tissues. The enzyme converts pyruvate, which is the final product of glycolysis, to lactate when oxygen is in short supply, and it is detectable in the serum (1). Conditions that can cause increased S-LDH include tissue injury, necrosis, hypoxia, hemolysis or malignancies (2–5).
Idiopathic pulmonary arterial hypertension (IPAH) is a disease characterized by progressive pulmonary vascular remodeling, which results in right ventricular failure and mortality. IPAH is a rare condition with an annual incidence range of 1.1–7.6 cases per million, a prevalence range of 15–26 cases per million individuals (6,7). IPAH is a particular type of PAH, which is unexplained by any secondary cause. The prognostic markers of PAH including WHO functional class, the plasma concentration of brain natriuretic peptide (BNP) (8), 6-minute-walk distance and mean right atrial pressure. Despite use of these markers, however, prediction of survival for IPAH patients remains difficult.
Right ventricular failure is the main causative factor for the mortality of patients with IPAH (9). Right ventricular failure can lead to liver function abnormalities, which increase the level of certain liver enzymes, such as transaminase and LDH, in the serum. Liver function is associated with the prognosis of patients with heart failure (10–13). The aim of the present study was to determine whether S-LDH is a prognostic marker in patients with IPAH.
Materials and methods
Study subjects
Of the 188 consecutive patients in whom IPAH was diagnosed in our institute (Fuwai Hospital, Beijing China) between May 2007 and July 2012, 15 patients were excluded: Nine were excluded as a result of being lost to follow-up and six were excluded due to their involvement in clinical drug research. The remaining 173 patients (48 males and 125 females; mean age, 31±10 years) were enrolled in the study. All 173 patients were incident cases. Three patients (1.7%) were classified as World Health Organization (WHO) functional Class I, 73 (42.2%) as Class II, 94 (54.3%) as Class III and three (1.7%) as Class IV. The patients were subclassified into two groups, normal and abnormal, according to their serum-LDH (S-LDH) levels. S-LDH ≥250 IU/l was considered to be a sign of pathology (normal levels <250 IU/l). No patients in the study had any known malignancy or liver and renal function damage due to other disease.
The PAH diagnosis was confirmed when the following results were indicated using right heart catheterization (RHC): Mean pulmonary artery pressure (mPAP), >25 mmHg, mean pulmonary capillary wedge pressure (mPCWP), <15 mmHg; and pulmonary vascular resistance (PVR), >240 dyn/sec/cm5 (14). IPAH was defined as PAH unexplained by any secondary cause. This study was approved by the Institutional Review Board of Fuwai Hospital. Written informed consent was obtained from all patients when they were recruited to the study.
The following data were obtained through review of the patient records: Age at diagnosis; gender; date of diagnosis; height; weight; WHO functional class; biochemical tests, including N-terminal pro-brain natriuretic peptide (NT-proBNP), S-LDH, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (Cr) and uric acid (UA) levels; hemodynamics, as assessed by RHC, including mean right atrial pressure (mRAP), mPAP, cardiac index, PVR, mixed venous oxygen saturation (SVO2) and mPCWP. In order to perform the biochemical tests, venous blood was drawn following an overnight fast within a week of the first diagnostic catheterization.
Follow-up
Follow-up data were obtained by telephone interviews with the patients or their families. The primary endpoint was all-cause mortality. Survival was estimated from the date of diagnosis to October 2013 (the last date of contact) or until mortality.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation. Categorical variables are presented as frequencies and percentages. Differences between groups were assessed by the Mann-Whitney U test, and proportions were compared using the χ2 test. The correlation between S-LDH and the baseline variables was assessed through Spearman's rank correlation coefficients. The association between time to mortality and S-LDH was assessed using log-rank tests, with Kaplan-Meier plots used to describe the likelihood of the outcome during the follow-up period. For the comparison of the prognostic values of S-LDH and NT-proBNP, receiver operating characteristic (ROC) curves were generated, and the areas under the curves (c-statistics) were calculated. Simple Cox regression analyses were conducted to establish the predictors of mortality during the follow-up period. Variables with a P<0.05 were then tested in a stepwise forward Cox regression model (entered at a P<0.05, removed at a P>0.10). P<0.05 was considered to indicate significance. Statistical analysis was conducted using SPSS version 19.0 for Windows (IBM SPSS, Armonk, NY, USA).
Results
S-LDH levels and the severity of IPAH
The baseline characteristics of the 173 patients are shown in Table I. The mean age was 31±10 years (range, 14–59 years), and the female-to-male ratio was 2.76:1. The mean body mass index (BMI) was 22.1±3.7 kg/m2. Elevated S-LDH to ≥250 IU/l was observed in 53 patients. A comparison of the baseline clinical characteristics, hemodynamic parameters and serum biomarkers between patients with S-LDH levels <250 IU/l and those with S-LDH levels ≥250 IU/l is shown in Table I. No significant differences were found between the two groups in the age, gender, BMI, PCWP, serum Cr or ALT levels, or the use of targeted therapies; however, the WHO functional class, mRAP, mPAP and PVR, as well as the UA, total bilirubin, AST and NT-proBNP levels, were significantly higher in patients with S-LDH levels ≥250 IU/l than those in patients with S-LDH levels <250 IU/l, and the cardiac index and SVO2 were lower in patients with S-LDH levels ≥250 IU/l.
Table I.
Characteristics of the patients.
| Characteristic | All patients | S-LDH <250 IU/l | S-LDH ≥250 IU/l | P-value |
|---|---|---|---|---|
| Patients (n) | 173 | 120 | 53 | |
| Age (years) | 31±10 | 31±10 | 30±11 | 0.565 |
| Females (%) | 72.3 | 72.5 | 71.6 | 0.914 |
| BMI (kg/m2) | 22.1±3.7 | 22.1±3.7 | 22.0±3.7 | 0.883 |
| WHO functional class | 0.016 | |||
| Ia/II | 76 | 60 | 16 | |
| III/IVa | 97 | 60 | 37 | |
| mRAP (mm Hg) | 8.0±6.3 | 6.9±4.7 | 10.3±8.6 | 0.025 |
| mPAP (mm Hg) | 61.1±17.8 | 58.1±14.5 | 67.9±22.0 | 0.006 |
| mPCWP (mm/Hg) | 9.4±3.5 | 9.1±3.6 | 10.1±3.2 | 0.342 |
| Cardiac index (l/min/m2) | 2.42±0.88 | 2.54±0.87 | 2.14±0.86 | <0.001 |
| PVR (dyn/sec/cm5) | 1395±669 | 1238±527 | 1750±813 | <0.001 |
| SVO2 (%) | 66.7±8.5 | 68.2±7.7 | 63.5±9.4 | 0.001 |
| NT-proBNP (ng/l) | 1394±957 | 1238±527 | 1610±922 | 0.006 |
| Uric acid (µmol/l) | 388±114 | 379±117 | 409±106 | 0.020 |
| Serum Cr (µmol/l) | 71.8±15.9 | 70.8±16.1 | 74.0±15.4 | 0.071 |
| ALT (IU/l) | 30.3±21.3 | 28.8±20.4 | 33.8±22.8 | 0.050 |
| AST (IU/l) | 27.2±11.4 | 25.0±10.8 | 31.9±11.2 | <0.001 |
| Total bilirubin (µmol/l) | 24.4±14.7 | 23.2±14.8 | 27.0±14.3 | 0.045 |
| S-LDH (IU/l) | 222±55 | 193±27 | 287±45 | <0.001 |
| Targeted therapy (%) | 56.6 | 60.0 | 49.1 | 0.181 |
Only three patients in classes I and IV, respectively. BMI, body mass index; WHO, World Health Organization; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; mPCWP, mean pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; SVO2, mixed venous oxygen saturation; NT-proBNP, N-terminal pro-brain natriuretic peptide; Cr, creatinine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; S-LDH, serum lactate dehydrogenase.
Association of S-LDH with clinical, biochemical and hemodynamic parameters
The association of S-LDH with the WHO functional class (ρ=0.261, P=0.001), mRAP (ρ=0.241, P=0.001), mPAP (ρ=0.252, P=0.001), cardiac index (ρ=-0.402, P<0.001), SVO2 (ρ=-0.386, P<0.001) and PVR (ρ=0.412, P<0.001), as well as NT-proBNP (ρ=0.429, P<0.001), UA (ρ=0.288, P<0.001), serum Cr (ρ=0.169, P=0.028), total bilirubin (ρ=0.283, P<0.001), ALT (ρ=0.264, P<0.001) and AST (ρ=0.445, P<0.001) levels, is shown in Table II.
Table II.
Association between S-LDH and other parameters.
| Variables | r | P-value |
|---|---|---|
| Age (years) | -0.061 | 0.428 |
| Body mass index (kg/m2) | -0.050 | 0.514 |
| WHO functional class | 0.261 | 0.001 |
| mRAP (mmHg) | 0.241 | 0.001 |
| mPAP (mmHg) | 0.252 | 0.001 |
| Cardiac index (l/min/m2) | -0.402 | <0.001 |
| PVR (dyn/sec/cm5) | 0.412 | <0.001 |
| SVO2 (%) | -0.386 | <0.001 |
| NT-proBNP (ng/l) | 0.429 | <0.001 |
| Uric acid (µmol/l) | 0.288 | <0.001 |
| Total bilirubin (µmol/l) | 0.283 | <0.001 |
| Alanine aminotransferase (IU/l) | 0.264 | <0.001 |
| Aspartate aminotransferase (IU/l) | 0.445 | <0.001 |
| Serum creatinine (µmol/l) | 0.169 | 0.028 |
WHO, World Health Organization; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; SVO2, mixed venous oxygen saturation; NT-proBNP, N-terminal pro-brain natriuretic peptide; S-LDH, serum lactate dehydrogenase.
S-LDH levels and outcome in IPAH
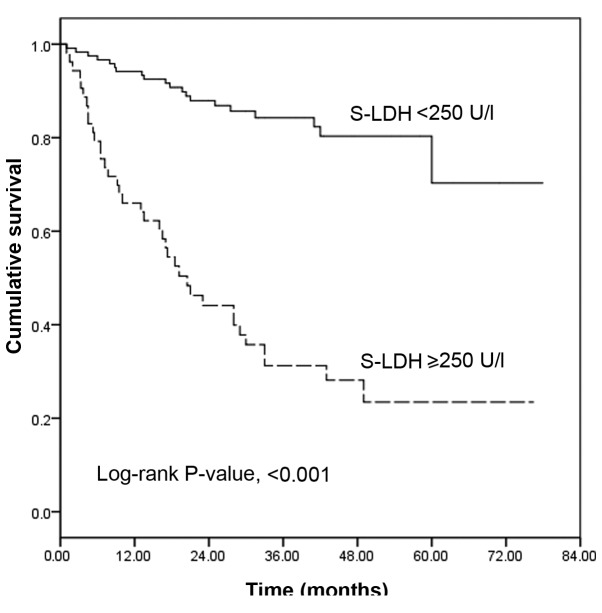
During a mean follow-up period of 31.2±17.9 months, 57 patients succumbed and one underwent transplantation during the follow-up period. Nine patients missed their appointments and survival was determined according to the last date of contact. A total of 16.7% (20/120) of the patients in the group with lower S-LDH levels succumbed, compared with 69.8% (37/53) of patients in the group with higher S-LDH levels. The Kaplan-Meier survival curves, grouped according to the S-LDH value of 250 IU/l, demonstrated that patients with higher S-LDH levels had a significantly lower survival rate than did those with lower S-LDH levels (log-rank test, P<0.001). The one-, three- and five-year survival rates of the group with lower S-LDH levels were estimated to be 94.2, 76.8 and 75.0%, respectively, and the mean survival time was 65.0±2.8 months. In comparison, the one-, three- and five-year survival rates of the group with higher S-LDH levels were 66.0, 31.1 and 23.1%, respectively, and the mean survival time was 31.2±4.0 months (Fig. 1).
Figure 1.
Survival rates of the S-LDH <250 IU/l and S-LDH ≥250 IU/l groups. Surviving patients at 0, 12, 24, 36, 48 and 60 months (n): S-LDH <250 IU/l, 120, 113, 86, 52, 29 and 8, respectively; S-LDH ≥250 IU/l, 53, 35, 21, 13, 8 and 2, respectively. S-LDH, serum lactate dehydrogenase.
S-LDH in the context of other markers of adverse outcome
In simple Cox regression analysis, the following factors were found to be associated with an increased risk of mortality: Younger age; elevated WHO functional class; increased mRAP, mPAP, mPCWP or PVR; reduced BMI, cardiac index or SVO2; elevated levels of NT-proBNP, AST and S-LDH; and absence of PAH-targeted therapy. Based on forward stepwise Cox regression analysis, only reduced BMI and cardiac index, elevated WHO functional class, higher S-LDH and an absence of PAH-targeted therapy remained as significant predictors of adverse outcomes (Table III).
Table III.
Results of Cox proportional hazard analyses.
| Single-variable model | Multivariable model | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age, per 10-year ↑ | 0.751 (0.571–0.987) | 0.040 | ||
| BMI in kg/m2, per 1 SD ↑=3.65 | 0.659 (0.497–0.874) | 0.004 | 0.530 (0.378–0.745) | <0.001 |
| WHO functional classa | 3.226 (1.735–5.997) | <0.001 | 2.043 (1.073–3.889) | 0.030 |
| mRAP, mm Hg | 1.059 (1.031–1.089) | <0.001 | ||
| mPAP, mm Hg | 1.017 (1.006–1.028) | 0.002 | ||
| Cardiac index, per 0.5 l/min/m2 ↑ | 0.669 (0.537–0.834) | <0.001 | 0.657 (0.524–0.823) | <0.001 |
| PVR, per 100 dyn/sec/cm5 ↑ | 1.088 (1.057–1.121) | <0.001 | ||
| mPCWP, mm Hg | 1.072 (1.009–1.138) | 0.024 | ||
| SVO2, per 5% ↑ | 0.814 (0.729–0.909) | <0.001 | ||
| NT-proBNP, per 100 ng/l ↑ | 1.025 (1.004–1.046) | 0.019 | ||
| UA, per 100 µmol/l ↑ | 1.183 (0.947–1.477) | 0.138 | ||
| Total bilirubin, µmol/l | 1.008 (0.992–1.024) | 0.344 | ||
| ALT, IU/l | 1.011 (1.001–1.021) | 0.346 | ||
| AST, IU/l | 1.024 (1.008–1.041) | 0.004 | ||
| Serum Cr, µmol/l | 1.012 (0.997–1.028) | 0.119 | ||
| S-LDH in IU/l, per 1 SD ↑=54 | 1.498 (1.273–1.763) | <0.001 | 1.349 (1.118–1.628) | 0.002 |
| Targeted medication | 0.259 (0.148–0.454) | <0.001 | 0.236 (0.132–0.422) | <0.001 |
Only three patients in classes I and IV, respectively. BMI, body mass index; WHO, World Health Organization; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; mPCWP, mean pulmonary capillary wedge pressure; SVO2, mixed venous oxygen saturation; NT-proBNP, N-terminal pro-brain natriuretic peptide; UA, uric acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, serum creatinine; S-LDH, serum lactate dehydrogenase; SD, standard deviation; HR, hazard ratio; CI, confidence interval.
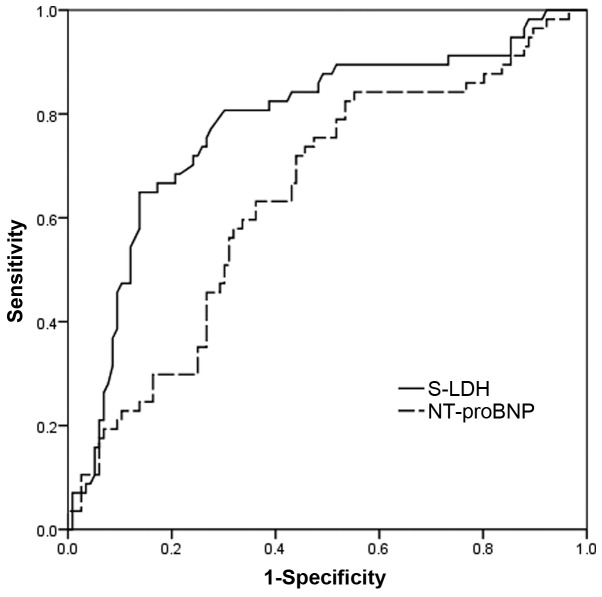
ROC curve analyses further illustrated that a high level of S-LDH is a strong indicator of adverse outcomes in IPAH. The optimal S-LDH cutoff level for predicting outcome was 218.5 IU/l (sensitivity, 80%; specificity, 70%). The c-statistic for S-LDH was 0.778 [95% confidence interval (CI), 0.701–0.855], which was superior to the c-statistic for NT-proBNP level (0.642; 95% CI, 0.555–0.730) (Fig 2).
Figure 2.
Receiver operating characteristic curve analyses investigating the association of levels of S-LDH and NT-proBNP with outcome in patients with IPAH. S-LDH, serum lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide; IPAH, idiopathic pulmonary arterial hypertension.
Discussion
The present study showed that approximately one-third of the study population presented with elevated LDH levels. These individuals had an inferior prognosis compared with those with LDH levels below the upper reference limit. S-LDH levels were significantly associated with WHO functional class, mRAP, mPAP, SVO2, UA, total bilirubin, ALT and serum Cr, and particularly with AST, NT-proBNP, cardiac index and PVR, suggesting that elevated levels of S-LDH are reflective of a range of functional, hemodynamic and biochemical indicators of more severe disease and poor prognosis in patients with IPAH, in addition to indicators of right heart failure-induced poor liver function.
It has been demonstrated that severe IPAH has a number of characteristics exhibiting certain similarities with the characteristics of cancer, such as the unexplained proliferation of pulmonary smooth muscle and endothelial cells (15) and metabolic shifts. Parallel to cancer, anaerobic glycolysis is a dominant vascular feature in IPAH (16,17), which leads to the increased activity of LDH and lactate production. The present study showed that the S-LDH level was significantly associated with the hemodynamic parameters in IPAH, which suggests that pulmonary smooth muscle and endothelial cells may be the sources of the increased S-LDH; however, this hypothesis requires further investigation for verification.
The current study also showed that the S-LDH level is a strong, independent predictor of an adverse prognosis in patients with IPAH, even following adjustment for a variety of clinical and biochemical variables. In a study by Takeda et al (18), it was found that total bilirubin was a prognostic marker in patients with PAH, while S-LDH was not (18); in the present study, however, S-LDH was found to be a more effective prognostic marker than total bilirubin. This difference may have been due to the fact that the study by Takeda et al (18) included patients with connective tissue disease-associated PAH in addition to patients with IPAH, and because fewer patients were recruited by Takeda et al (18) compared with the number of patients in the present study (37 vs. 173).
The present study also demonstrated that S-LDH was a more effective predictor of mortality than NT-proBNP in patients with IPAH. This may be due to the fact that NT-proBNP is highly specific for changes in cardiac hemodynamics and thus can be considered to be a ‘myocardial exclusive’ protein (19), while S-LDH a non-specific test that appears to reflect the general condition of patients with severe disease and to have systemic implications.
S-LDH level measurement is a simple, noninvasive, relatively low-cost and routinely available procedure. Although a number of noninvasive markers for the severity of IPAH, such as neurohormones and echocardiographic parameters, have been suggested, S-LDH levels should also be considered as a potential noninvasive indicator for the severity of IPAH.
There were several limitations to the present study. Firstly, this was a retrospective, observational, single-center study, in which S-LDH was only assessed at baseline. Further assessments should performed during the therapeutic follow-up. In addition, S-LDH is a highly sensitive but nonspecific test; levels of LDH isoenzyme, which could provide more information, were not measured.
In conclusion, S-LDH levels increase in proportion to the clinical severity of IPAH and have a strong, independent association with the long-term mortality of patients with IPAH. It may be worthwhile to assess the potential role of S-LDH as a biomarker and as a potential mediator involved in the pathogenesis of IPAH in further studies.
Acknowledgements
This study was supported by grants from the National Key Technology R&D Program, China (no. 2011BAI11B15) and the Capital Medical Scientific Development Fund, Beijing, China (no. 2009-1003).
References
- 1.Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Graeber GM, Clagett GP, Wolf RE, et al. Alterations in serum creatine kinase and lactate dehydrogenase. Association with abdominal aortic surgery, myocardial infarction and bowel necrosis. Chest. 1990;97:521–527. doi: 10.1378/chest.97.3.521. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson M, Wiberg-Itzel E, Chakkarapani E, et al. Lactate dehydrogenase predicts hypoxic ischaemic encephalopathy in newborn infants: a preliminary study. Acta Paediatr. 2010;99:1139–1144. doi: 10.1111/j.1651-2227.2010.01802.x. [DOI] [PubMed] [Google Scholar]
- 4.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eigentler TK, Figl A, Krex D, et al. Dermatologic Cooperative Oncology Group and the National Interdisciplinary Working Group on Melanoma: Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer. 2011;117:1697–1703. doi: 10.1002/cncr.25631. [DOI] [PubMed] [Google Scholar]
- 6.Escribano Subias P, Barberà Mir JA, Suberviola V. Current diagnostic and prognostic assessment of pulmonary hypertension. Rev Esp Cardiol. 2010;63:583–96. doi: 10.1016/s1885-5857(10)70120-1. [DOI] [PubMed] [Google Scholar]
- 7.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of pulmonary arterial hypertension patients in the United States: how the REVEAL registry differs from historic and non-US contemporary registries. Chest. 2011;139:128–137. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.CIR.102.8.865. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 10.Domingo E, Grignola JC, Aguilar R, et al. Impairment of pulmonary vascular reserve and right ventricular systolic reserve in pulmonary arterial hypertension. BMC Pulm Med. 2014;14:69. doi: 10.1186/1471-2466-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batin P, Wickens M, McEntegart D, et al. The importance of abnormalities of liver function tests in predicting mortality in chronic heart failure. Eur Heart J. 1995;16:1613–1618. doi: 10.1093/oxfordjournals.eurheartj.a060785. [DOI] [PubMed] [Google Scholar]
- 12.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002;90:1405–1409. doi: 10.1016/S0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 13.Poelzl G, Ess M, Mussner-Seeber C, et al. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest. 2012;42:153–163. doi: 10.1111/j.1365-2362.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 14.McGoon M, Gutterman D, Steen V, et al. American College of Chest Physicians: Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. (1 Suppl) [DOI] [PubMed] [Google Scholar]
- 15.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 16.Sakao S, Tatsumi K. Vascular remodeling in pulmonary arterial hypertension: multiple cancer-like pathways and possible treatment modalities. Int J Cardiol. 2011;147:4–12. doi: 10.1016/j.ijcard.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Invest. 2013;43:855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda Y, Takeda Y, Tomimoto S, et al. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med. 2010;10:22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.CIR.102.8.865. [DOI] [PubMed] [Google Scholar]