Abstract
Patients with congenital heart disease and pulmonary valve disease need multiple procedures over their lifetimes to replace their pulmonary valves. Chronic pulmonary stenosis, regurgitation, or both have untoward effects on ventricular function and on the clinical status of these patients. To date, all right ventricle–pulmonary artery conduits have had relatively short lifespans. Percutaneous pulmonary valve implantation, although relatively new, will probably reduce the number of operative procedures that these patients will have to undergo over a lifetime. Refinement and further development of this procedure holds promise for the extension of this technology to other patient populations.
Keywords: Bioprosthesis; chronic disease; heart defects, congenital/therapy; heart valve prosthesis implantation/methods/trends; minimally invasive surgical procedures/trends; pulmonary valve stenosis; stents; tetralogy of Fallot/surgery
The successes of congenital heart disease repair over the past few decades have enabled more children to survive well into adulthood. In fact, in the United States, it is now estimated that there are more adults than children with congenital heart defects. Most patients with congenital right-sided lesions (such as tetralogy of Fallot) need multiple pulmonary valve (PV) replacements over their lifetimes because of PV dysfunction from stenosis, regurgitation, or both. Chronic pulmonary stenosis and regurgitation have deleterious effects on the hearts of these individuals.1–14 Current thresholds for replacing the PV (in addition to symptoms) include severe right ventricular (RV) hypertension with an RV-to-left ventricular pressure ratio of >0.7 or peak and mean Doppler gradients of >50 mmHg and 30 mmHg, respectively,1–3 indexed RV end-diastolic volume of >150 to 170/mL/m2 or RV end-systolic volume of >70 to 85 mL/m2,4–7 RV ejection fraction of <0.40 to 0.45,5,8 and a QRS duration of ≥180 ms.5,11–13 Other important factors to consider when planning for PV replacement are sustained atrial or ventricular arrhythmias, substantial coexisting lesions (for example, significant aortic regurgitation, tricuspid regurgitation, and residual ventricular septal defects), and left ventricular dysfunction.1–3,5,7,12
In one large study,14 the freedom from replacement of surgically implanted PVs was 54% at 10 years. Because of the barely adequate durability of any surgically implanted PV, physicians must weigh the benefits of an operative procedure against the advantage of awaiting further signs or symptoms in these patients. We and others have been impressed by the impact of this less invasive treatment approach, which has the potential to significantly reduce the number of open cardiac surgical procedures that a patient will undergo over his or her lifetime.
Historical Perspective
Percutaneous PV implantation is arguably the most innovative procedure developed to treat congenital heart disease in the past 2 decades. In August 2000, Bonhoeffer and colleagues15 first reported percutaneous implantation of a PV in a lamb model. Although only 7 of 11 implants were successful (because of the acute angles in these small animals), the study paved the way for application in human beings. In October 2000, Bonhoeffer and colleagues16 from France reported the first human implantation of a percutaneous PV, in a 12-year-old boy who had presented with pulmonary atresia, a ventricular septal defect, and a dysfunctional RV–pulmonary artery conduit. In 2002, the first published clinical series (8 patients from Paris and London17) showed the acute success of the procedure. Clinical use of the percutaneous PV—that is, the Melody® Transcatheter Pulmonary Valve (Medtronic, Inc.; Minneapolis, Minn)—continued in Europe and Canada. Modification of the valve led to CE Marking in September 2006 and to Health Canada approval in December 2006. The first U.S. implantation occurred in January 2007 in Boston Children's Hospital, and subsequent trials led to U.S. Food and Drug Administration (FDA) approval under Humanitarian Device Exemption (HDE) status in 2010. As of March 2013, more than 4,500 (>2,000 in the U.S.) Melody valve implantations have occurred worldwide.*
Indications, Valve Design, and Delivery
The Melody valve is intended for use in children and adults who have a surgically implanted RV-to-pulmonary artery conduit manifesting at least moderate stenosis or regurgitation. It is generally intended for use in conduits that were originally >16 mm in diameter and had a waist-on-balloon sizing of between 14 and 20 mm. The device consists of an 18-mm bovine jugular-valve segment that is sutured onto a Cheatham platinum CP Stent™ (NuMED, Inc.; Hopkinton, NY) made of platinum and iridium. Although the leaflet morphology is highly variable, in general all available leaflet variations function well. The deep coaptation of the leaflets means that the valve functions well over a wide spectrum of diameters. The initial length of the valve is 28 mm, but it is shortened in accordance with the final implanted diameter. The valve can be expanded from 16 to 22 mm in diameter, and in some instances up to 24 mm.18 The valve is delivered via a 22F Ensemble® Transcatheter Delivery System (Medtronic). This consists of a balloon-in-balloon (BiB) system (available in 18-, 20-, and 22-mm sizes), which enables the valve to be repositioned, if needed, after the inner balloon has been inflated. At this time, the Melody valve is FDA approved, under HDE guidelines, for use only in previously placed surgical conduits, as stated here. However, off-label use in native, dysfunctional RV outflow tracts is possible in certain cases, provided that the size of the landing zone meets the requirements set forth above for a surgical conduit.19,20 The Melody valve should not be implanted if there are concerns about active endocarditis or about the size of central veins that might limit the ability of the delivery system to advance.
Valve Implantation Procedure
Preprocedural planning is most important for these cases. The original operative notes are vital in providing details of the original conduit and relevant anatomy. Moreover, we have found variability in the internal and external diameters of various conduits that have been surgically placed. In addition to routine cardiac testing, we obtain cardiac magnetic resonance imaging (MRI) scans in all patients before intervention, to evaluate the anatomy of the existing conduit and other structures (particularly the branch pulmonary arteries and coronary arteries). In some patients, a concurrent intervention—on a branch pulmonary artery, for example—might need to be performed. The MRI also provides an estimate of the relationship of the coronary arteries to the conduit. Because many patients with congenital heart disease have variant origins of the coronary arteries, their conduits can be found in positions that differ from those of the usual native outflow conduits. These anatomic variations can place the coronary arteries close to the conduit and thereby compromise coronary flow if the conduit is expanded by stenting or by implantation of the Melody valve.21,22 In the event that a coronary artery courses close to the conduit, we still recommend cardiac catheterization to define the spatial relationship accurately, with implantation of the valve during the same procedure if coronary compression is excluded. A cardiac computed tomographic scan can be helpful in patients who have pacemakers or other contraindications to MRI scans.
We perform all these procedures with use of general anesthesia and biplane fluoroscopy, together with on-site surgical coverage in case it is needed. Covered stents (whether commercial or “self-fabricated”) should be available in the fortunately rare event of an expanding tear, dissection, or rupture of the conduit. We obtain access through the femoral venous and arterial vessels. In some patients, we anticipate the need to change our angle of approach to the neck, so we place a small sheath in the right internal jugular vein at the beginning of the procedure (this can be upsized later if needed). Patients are administered heparin after access is obtained, and throughout the procedure, to maintain activated clotting times of more than 200 s. A right-sided heart catheterization is first performed. It is important to use a “flow-directed” balloon catheter when performing these procedures. An inflated balloon ensures that the catheter crosses the largest effective orifice of the tricuspid valve. This is important because the sheaths and delivery systems used in this procedure are relatively large, and if they are advanced through small chordal spaces, damage to the tricuspid valve can occur.
Angiograms are performed in the RV outflow tract (Fig. 1), where measurements are made of the minimal diameter, the largest diameter, and the length of the conduit that needs to be covered. A test dilation is performed at relatively low pressure in the conduit with use of a balloon that is the same diameter as the intended final diameter of the Melody valve but not more than 110% of the nominal diameter of the originally implanted conduit. This not only provides another way to judge the diameters within the conduit, but, more importantly, gauges the compliance of the conduit. If a significant waist is seen on the balloon, this will alert the operator to an increased risk of conduit tear or dissection. In such circumstances, any stent or Melody valve that needs to be placed in the conduit might need to be implanted at a smaller diameter before the conduit is postdilated to its final intended diameter. Because the Melody valve is a venous graft and therefore “covered,” it is safer to dilate further and at higher pressure. In some patients with borderline or smaller diameter conduits, a significant waist can exclude them from percutaneous PV implantation. During a balloon test dilation, one can perform aortic root injection or selective coronary angiography (or both) in multiple projections to show the relationship of the expanded conduit to the coronary arteries (Fig. 2). If there is no evidence of coronary artery compression, percutaneous PV implantation is performed. If there is any question of potential coronary artery compression, either from study of the angiograms or electrocardiographic changes, percutaneous PV implantation is aborted. We prefer, if possible, to first attempt delivery of the valve from the femoral vein. For placement of a stiff guidewire in a distal pulmonary artery branch (Fig. 3A), we choose either the right or the left pulmonary artery for access, in accordance with the anatomy of the conduit and the pulmonary arteries. To facilitate advancement, it is important to pre-shape the guidewire to conform to the shape of the conduit and the branch pulmonary artery that is the intended target. If attempts at advancing the sheaths or the Melody valve are unsuccessful from the femoral vein, neck access should be used (Fig. 3B) to change the angle of approach. We have found neck access to be extremely helpful when dealing with tight curves and angles within the heart; in many cases, it is a surprisingly faster approach.
Fig. 1.
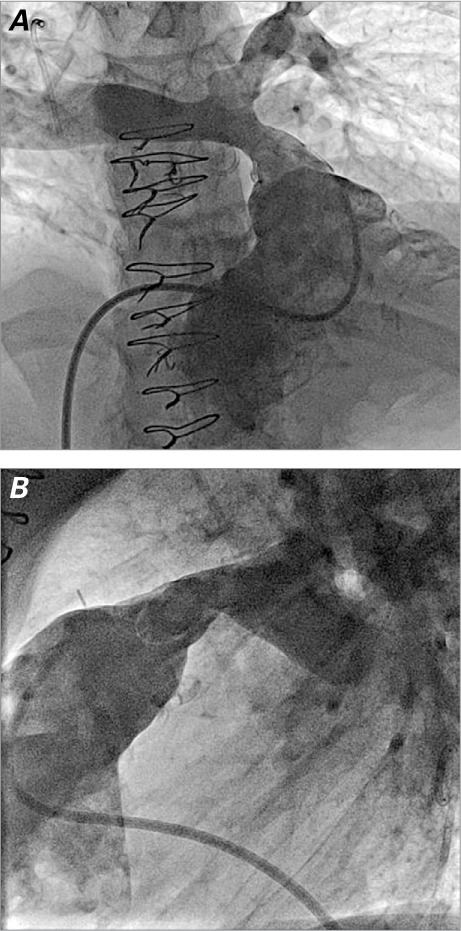
Right ventricular outflow tract angiograms in A) anteroposterior/cranial and B) lateral projections show the anatomy of a stenotic and regurgitant right ventricle-to-pulmonary artery conduit. The angiograms are used to obtain relevant measurements for planning Melody valve placement.
Fig. 2.
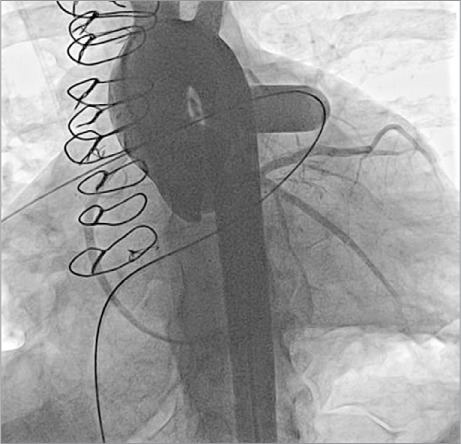
Aortic root angiogram (left anterior oblique/caudal projection) with an inflated balloon at the site of intended Melody valve implantation shows the coronary arteries to be distant from the landing zone with no visible coronary artery compression. A simultaneous angiogram (not shown) in straight lateral projection was also performed, with similar findings.
Fig. 3.
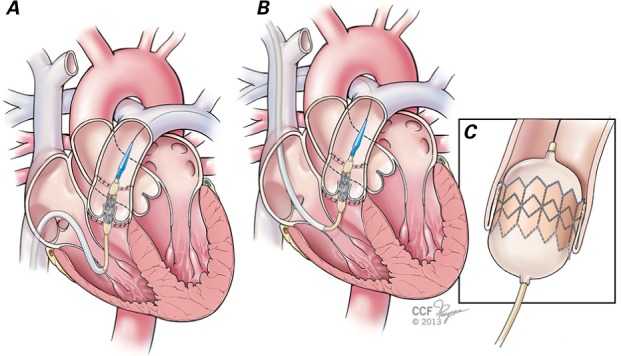
Illustration shows that percutaneous pulmonary valve implantation can be performed via 2 approaches in the catheterization laboratory. A) The femoral venous approach has the advantage of being a relatively easier position for the interventional cardiologist to work from. The Melody valve is seen uncovered with its delivery sheath withdrawn, and positioned across the dysfunctional pulmonary valve. However, in some patients, the Melody valve system cannot be advanced easily across the right ventricular outflow tract from a femoral venous approach (because of difficult angles in the heart and right ventricular outflow tract or a large right atrium). In such instances, B) the internal jugular vein approach is easier and faster. C) Once in position, the Melody valve is deployed by inflating the inner and outer balloons.
Images by Cleveland Clinic Center for Medical Art & Photography ©2013–2015. All rights reserved.
We generally prefer to place a bare-metal stent within the conduit before Melody valve implantation. This is because many of these conduits exhibit “recoil” after Melody valve implantation alone due to extrinsic forces (for example, compression from the sternum); placement of a stent first has been shown to prevent fractures of the Melody valve stent.23,24 If significant recoil is seen after implanting the first stent, more than one stent might need to be implanted to reinforce the conduit. We generally do not fully expand the stents at this point, because the narrowing or “waist” can serve as an anchor for the Melody valve, which can later be postdilated. Implanting the Melody valve and then postdilating to a larger diameter is safer, because the covering will seal any tear that might occur in this area. The Melody valve is then implanted by inflating the inner and outer balloons sequentially (Figs. 3C and 4). Once implanted, the valve is usually postdilated with a higher-pressure balloon to obtain the intended final diameter. Repeat hemodynamic measurements and angiograms are then performed to evaluate valve function (Fig. 5).
Fig. 4.
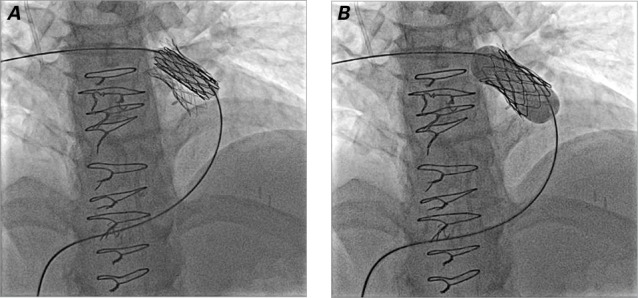
Fluoroscopy shows sequential inflation of the A) inner and B) outer balloon to deploy the Melody valve in the same patient shown in Figure 1. Note that a stent has been placed to prepare the landing zone before deployment of the Melody valve.
Fig. 5.
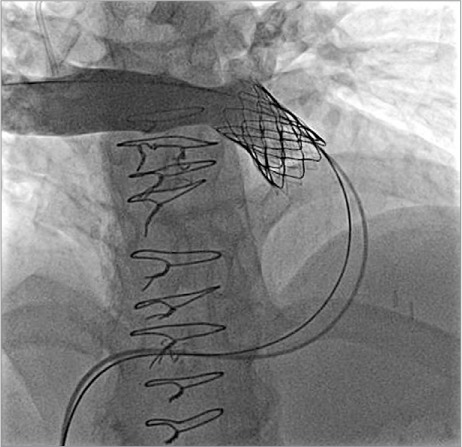
Angiogram in the main pulmonary artery after Melody valve deployment and after deployment dilation with a high-pressure balloon, in the same patient shown in Figures 1 and 4. Note the disappearance of the waist that was still present after Melody valve placement (Fig. 4B), intentionally dilated to high pressure only after deployment of the covered stent on which the Melody valve is mounted, to decrease the risk of conduit rupture. After Melody valve implantation, there is no pulmonary regurgitation.
If a waist is present, the technique of postdilating the pre-stented area and Melody valve should be performed only if the operator has confirmed that there will be no coronary compression should the waist on the balloon be abolished; this can be difficult to gauge. Another alternative is the abolition of waists before stent and Melody valve implantation, by means of serial high-pressure balloon dilations. This ensures that the final implant diameter will not result in coronary compression, provided that there was no coronary compression when predilation was performed with a balloon of the same diameter (in the absence of a residual waist).
At the end of the procedure, hemostasis is achieved by removing the sheaths and applying manual pressure alone. Despite relatively large sheath sizes and the availability of closure devices and other methods to achieve hemostasis, our experience has shown that manual pressure alone is usually sufficient to achieve hemostasis, because of relatively low-pressure venous flow.
Data from Worldwide Experience and from the Cleveland Clinic
In 2005, Khambadkone and colleagues25 reported the European results of 59 consecutive Melody valve implants. Their good hemodynamic and clinical results showed the overall safety of the procedure. During their experience, the valve was substantially revised because of a “hammock” effect that created stenosis: the valve at the outset had not been sutured along the entire length of the stent. The initial U.S Melody Feasibility trial21 was a multicenter prospective nonrandomized study (using the revised Melody valve) that focused on safety, procedural success, and short-term outcomes in 30 patients. Significant sequelae were reported in 3 patients, but no deaths. In the short term, valve function was good. This led to FDA approval of use of the Melody valve under HDE status in January 2010. The trial continued, and later in 2010 McElhinney and associates22 reported midterm results of 136 patients in whom Melody valve implantation had been intended. One hundred twenty-four patients had undergone implantation of the Melody valve, and 12 had been excluded. Importantly, 6 patients had been excluded for risk of coronary artery compression. Serious sequelae had occurred in 6% of the patients. Freedom from stent fracture of the valve was ~78% at 14 months, with a freedom from Melody valve dysfunction/reintervention of ~94% at one year. In this trial, not all patients had first undergone implantation of a bare-metal stent within the conduit—an important technical advantage that has been shown to decrease the fracture rate of the Melody valve23,24 and perhaps to improve overall valve function. Another key endpoint of procedural success, functional and clinical status as evaluated in accordance with New York Heart Association functional class and exercise testing,22,25–27 has been shown to improve in these patients after percutaneous PV placement.
Since the first Melody valve implant at the Cleveland Clinic in July 2010, 47 patients have undergone Melody valve implantation in the RV-to-pulmonary artery position at the Cleveland Clinic. There was one major procedural sequela (ventricular fibrillation requiring cardiopulmonary resuscitation and defibrillation, with no other sequelae). Two patients needed reintervention (both for recurrent stenosis, treated by balloon dilation in one patient and reimplantation of a Melody valve in the other patient). Thus, over a 4.25-year period, 96% of patients were free of reintervention. One patient developed endocarditis.
Comparison with Surgery
Surgery has traditionally been the gold standard in replacing dysfunctional RV–pulmonary artery conduits. As highlighted above, transcatheter PV replacement achieves acute and intermediate-term successes similar to those of surgically placed conduits. Patients undergoing transcatheter PV replacement are generally admitted to the regular floor, are ambulatory within 6 hours of the procedure, and are discharged from the hospital within 24 hours. This contrasts with the need for cardiopulmonary bypass when conduits are placed surgically, for a 24- to 48-hour stay in the intensive care unit, and for a hospital stay of 3 to 7 days.
Cost-analysis models have been proposed to compare the 2 methods of therapy. Although the actual Melody valve is more expensive than surgically placed conduits, the total initial procedural costs are similar.28 Despite the less invasive nature of the procedure, percutaneous PV placement resulted in slightly higher costs in the intermediate and long terms than did surgery.28,29 These estimates are, however, based on models, because long-term data for transcatheter PV placement and its impact over a patient's lifetime still remain to be seen. Newer valve technology (resulting in lower reintervention rates) and more widespread use of percutaneous PVs (resulting, one would expect, in lower production costs per valve) will probably decrease the long-term costs of percutaneous PVs. Clearly other factors, such as emotional well-being after a less invasive procedure and the ability to return to work sooner, will need to be factored into these models as well.
The Edwards Sapien Pulmonic Transcatheter Valve
In addition to the Melody valve, the Edwards Sapien transcatheter valve (Edwards Lifesciences LLC; Irvine, Calif) has been implanted in RV outflow tract conduits in a small number of patients enrolled in phase I of the Compassion trial.30 The device is the same as the Cribier-Edwards transcatheter aortic valve, for which there is extensive experience in high-risk elderly patients with severe aortic stenosis.31 This valve consists of 3 bovine pericardial leaflets sewn to a stainless-steel balloon-expandable stent. The delivery system (22F and 24F) is a little bulkier than that of the Melody valve. The valve is available in 23- and 26-mm diameters, and 14- and 16-mm lengths. Pre-stenting with a bare-metal stent to a diameter 2 to 3 mm less than the final valve diameter is always performed, given the relatively short length of this valve. The larger size (in comparison with the Melody valve) expands implantation to patients with conduit diameters up to 24 mm on balloon sizing. Early results of the phase I trial show that in 28 of the 31 patients in whom valve implantation was successful, there was a 96% freedom from reintervention or valve failure at the 6-month follow-up. 30 There was a 20.5% rate of adverse events in this early experience, including 3 valve migrations (with subsequent successful implantation of a second Edwards Sapien valve in 2 of the 3), but no deaths. The valve has received the CE Mark in Europe, whereas in the U.S. further investigation is under way, in order to receive HDE.
Future Directions and Conclusions
Percutaneous PV placement holds great promise for many children and adults with PV dysfunction. In common with other new technology, as experience has grown with Melody valve implantation, so has the overall number of clinical outcomes.32 Although the significance of this technology has already been seen over the last decade or so, its real impact is likely to be seen in the years to come. In comparison with the past, patients and their families can now be advised that the need for repetitive surgery to replace PVs will be decreased. In addition to this medical benefit, the emotional well-being of patients and families will be favorably affected. Percutaneous PV therapy, like many other interventions in the current era, highlights the collaborative efforts between interventional cardiologists and cardiac surgeons in planning procedures that impart both short- and long-term benefits. Future developments (which are already under way) will include extending this technology to native PVs, decreasing the profile of the delivery system, refining the valve design to minimize reintervention rates, and perhaps even developing a valve made of the patient's own cells.
Footnotes
From: Center for Pediatric and Congenital Heart Disease, Pediatric Institute (Drs. Prieto and Qureshi), and Department of Cardiovascular Medicine, Heart and Vascular Institute (Dr. Qureshi), Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, the Cleveland Clinic, 9500 Euclid Ave., Cleveland, Ohio 44195
*Personal communication, Medtronic, Inc., Minneapolis, Minn.
References
- 1.Warnes C. A., Williams R. G., Bashore T. M., Child J. S., Connolly H. M., Dearani J. A. et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Warnes C. A., Williams R. G., Bashore T. M., Child J. S., Connolly H. M., Dearani J. A. et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118(23):e714–833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 3.Warnes C. A., Williams R. G., Bashore T. M., Child J. S., Connolly H. M., Dearani J. A. et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease) Circulation. 2008;118(23):2395–451. doi: 10.1161/CIRCULATIONAHA.108.190811. [DOI] [PubMed] [Google Scholar]
- 4.Therrien J., Provost Y., Merchant N., Williams W., Colman J., Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95(6):779–82. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:11–22. doi: 10.1053/j.pcsu.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Oosterhof T., van Straten A., Vliegen H. W., Meijboom F. J., van Dijk A. P., Spijkerboer A. M. et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545–51. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 7.Tweddell J. S., Simpson P., Li S. H., Dunham-Ingle J., Bartz P. J., Earing M. G., Pelech A. N. Timing and technique of pulmonary valve replacement in the patient with tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15(1):27–33. doi: 10.1053/j.pcsu.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Therrien J., Siu S. C., McLaughlin P. R., Liu P. P., Williams W. G., Webb G. D. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36(5):1670–5. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 9.Vliegen H. W., van Straten A., de Roos A., Roest A. A., Schoof P. H., Zwinderman A. H. et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation. 2002;106(13):1703–7. doi: 10.1161/01.cir.0000030995.59403.f8. [DOI] [PubMed] [Google Scholar]
- 10.van Straten A., Vliegen H. W., Hazekamp M. G., Bax J. J., Schoof P. H., Ottenkamp J. et al. Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology. 2004;233(3):824–9. doi: 10.1148/radiol.2333030804. [DOI] [PubMed] [Google Scholar]
- 11.Gatzoulis M. A., Balaji S., Webber S. A., Siu S. C., Hokanson J. S., Poile C. et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356(9234):975–81. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 12.Knauth A. L., Gauvreau K., Powell A. J., Landzberg M. J., Walsh E. P., Lock J. E. et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94(2):211–6. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 13.Scherptong R. W., Hazekamp M. G., Mulder B. J., Wijers O., Swenne C. A., van der Wall E. E. Follow-up after pulmonary valve replacement in adults with tetralogy of Fallot: association between QRS duration and outcome. J Am Coll Cardiol. 2010;56(18):1486–92. doi: 10.1016/j.jacc.2010.04.058. et al. [DOI] [PubMed] [Google Scholar]
- 14.Tweddell J. S., Pelech A. N., Frommelt P. C., Mussatto K. A., Wyman J. D., Fedderly R. T. et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation. 2000;102(19 Suppl 3):III130–5. doi: 10.1161/01.cir.102.suppl_3.iii-130. [DOI] [PubMed] [Google Scholar]
- 15.Bonhoeffer P., Boudjemline Y., Saliba Z., Hausse A. O., Aggoun Y., Bonnet D. et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation. 2000;102(7):813–6. doi: 10.1161/01.cir.102.7.813. [DOI] [PubMed] [Google Scholar]
- 16.Bonhoeffer P., Boudjemline Y., Saliba Z., Merckx J., Aggoun Y., Bonnet D. et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403–5. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 17.Bonhoeffer P., Boudjemline Y., Qureshi S. A., Le Bidois J., Iserin L., Acar P. et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002;39(10):1664–9. doi: 10.1016/s0735-1097(02)01822-3. [DOI] [PubMed] [Google Scholar]
- 18.Cheatham S. L., Holzer R. J., Chisolm J. L., Cheatham J. P. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter--it works. Catheter Cardiovasc Interv. 2013;82(5):816–23. doi: 10.1002/ccd.24821. [DOI] [PubMed] [Google Scholar]
- 19.Momenah T. S., El Oakley R., Al Najashi K., Khoshhal S., Al Qethamy H., Bonhoeffer P. Extended application of percutaneous pulmonary valve implantation. J Am Coll Cardiol. 2009;53(20):1859–63. doi: 10.1016/j.jacc.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 20.Boshoff D. E., Cools B. L., Heying R., Troost E., Kefer J., Budts W., Gewillig M. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013;81(6):987–95. doi: 10.1002/ccd.24594. [DOI] [PubMed] [Google Scholar]
- 21.Zahn E. M., Hellenbrand W. E., Lock J. E., McElhinney D. B. Implantation of the Melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit: early results from the U.S. clinical trial. J Am Coll Cardiol. 2009;54(18):1722–9. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 22.McElhinney D. B., Hellenbrand W. E., Zahn E. M., Jones T. K., Cheatham J. P., Lock J. E., Vincent J. A. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US Melody valve trial. Circulation. 2010;122(5):507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmeyer J., Lurz P., Khambadkone S., Schievano S., Jones A., McElhinney D. B. et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart. 2011;97(2):118–23. doi: 10.1136/hrt.2010.198382. [DOI] [PubMed] [Google Scholar]
- 24.McElhinney D. B., Cheatham J. P., Jones T. K., Lock J. E., Vincent J. A., Zahn E. M., Hellenbrand W. E. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circ Cardiovasc Interv. 2011;4(6):602–14. doi: 10.1161/CIRCINTERVENTIONS.111.965616. [DOI] [PubMed] [Google Scholar]
- 25.Khambadkone S., Coats L., Taylor A., Boudjemline Y., Derrick G., Tsang V. et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189–97. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]
- 26.Vezmar M., Chaturvedi R., Lee K. J., Almeida C., Manlhiot C., McCrindle B. W. et al. Percutaneous pulmonary valve implantation in the young: 2-year follow-up. JACC Cardiovasc Interv. 2010;3(4):439–48. doi: 10.1016/j.jcin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Batra A. S., McElhinney D. B., Wang W., Zakheim R., Garofano R. P., Daniels C. et al. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J. 2012;163(2):280–7. doi: 10.1016/j.ahj.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Gatlin S. W., Kim D. W., Mahle W. T. Cost analysis of percutaneous pulmonary valve replacement. Am J Cardiol. 2011;108(4):572–4. doi: 10.1016/j.amjcard.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 29.Raikou M., McGuire A., Lurz P., Bonhoeffer P., Wegmueller Y. An assessment of the cost of percutaneous pulmonary valve implantation (PPVI) versus surgical pulmonary valve replacement (PVR) in patients with right ventricular outflow tract dysfunction. J Med Econ. 2011;14(1):47–52. doi: 10.3111/13696998.2010.545465. [DOI] [PubMed] [Google Scholar]
- 30.Kenny D., Hijazi Z. M., Kar S., Rhodes J., Mullen M., Makkar R. et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58(21):2248–56. doi: 10.1016/j.jacc.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Webb J. G., Pasupati S., Humphries K., Thompson C., Altwegg L., Moss R. et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116(7):755–63. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 32.Lurz P., Coats L., Khambadkone S., Nordmeyer J., Boudjemline Y., Schievano S. et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117(15):1964–72. doi: 10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]


