Abstract
Pseudoaneurysm of the mitral-aortic intervalvular fibrosa is a rare but serious sequela of endocarditis or valve replacement surgery. Because open-heart surgery is a high-risk treatment option, alternative methods are sought. We present the case of a 77-year-old man with a noninfected mechanical mitral valve whose pseudoaneurysm was repaired by introducing an occluder device into the defect by a transapical approach. Upon follow-up imaging, the defect was successfully closed. We conclude that percutaneous closure of pseudoaneurysm of the mitral-aortic intervalvular fibrosa is a viable alternative to surgery and that a transapical approach is an appropriate method of access.
Keywords: Aneurysm, false/diagnosis/etiology/closure, percutaneous; aortic root; aortic valve; cardiac catheterization; mitral valve
The mitral-aortic intervalvular fibrosa (MAIVF) is an area of fibrous tissue forming the anterior boundary of the mitral annulus and the posterior boundary of the aortic annulus. It is sometimes called the mitral-aortic curtain. This region is avascular and is generally spared from clinically recognized disease processes. However, in the presence of aortic or mitral valve abnormalities with disruption of the cardiac fibrous skeleton associated with surgical intervention or infection, this area has a potential for pseudoaneurysm formation.
Pseudoaneurysm of the MAIVF (P-MAIVF) has been described most often in patients who have a history of endocarditis or valve surgery. This condition most likely arises from fibrotic damage caused by infection or by sutures rooted in the MAIVF during valve replacement.1 Although rare, P-MAIVF can produce life-threatening sequelae. Certainly it warrants early definitive treatment.
We present the case of a 77-year-old man with P-MAIVF and a noninfected mechanical mitral valve who underwent successful transcatheter closure of the defect by the transapical approach. To our knowledge, this is only the 3rd percutaneous repair performed for this abnormality to be noted in the medical literature and the first through the transapical approach.
Case Report
In June 2013, a 77-year-old man was admitted because of shortness of breath during physical activity. The patient had a history of mitral regurgitation, for which a mechanical prosthetic mitral valve had been implanted in 1993. In more recent years, he also had undergone the implantation of 6 coronary stents, which had led to the discovery of a P-MAIVF. A transesophageal echocardiogram (TEE) confirmed the presence of a P-MAIVF (Fig. 1A), as did a cardiac computed tomographic angiogram (CTA) (Fig. 2A). Because of the patient's age, history of cardiac surgery, and severe emphysema, he was considered at high risk for repeat surgery, and a percutaneous repair was planned. The cardiac CTA enabled measurement of the opening and the subsequent selection of an occlusion device. The patient had no history of endocarditis, and his TEE revealed no evidence of present infection.
Fig. 1.
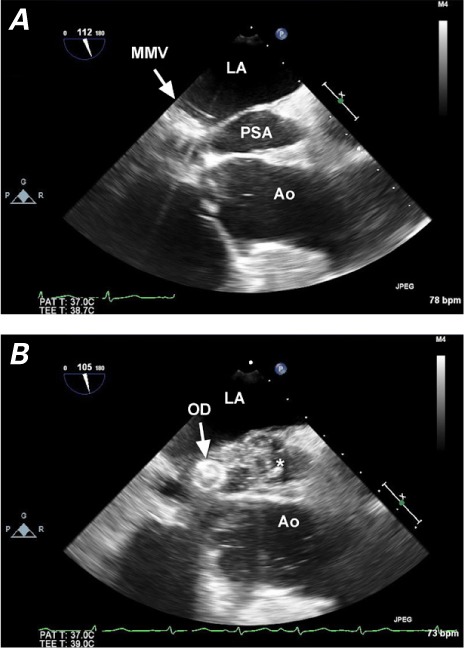
Two-dimensional transesophageal echocardiograms show A) the open defect at mid-esophageal view (112°) and B) substantial smoke in the pseudoaneurysm during the closure procedure.
* = occluded pseudoaneurysm; Ao = aorta; LA = left atrium; MMV = mechanical mitral valve; OD = occluder device; PSA = pseudoaneurysm
Fig. 2.
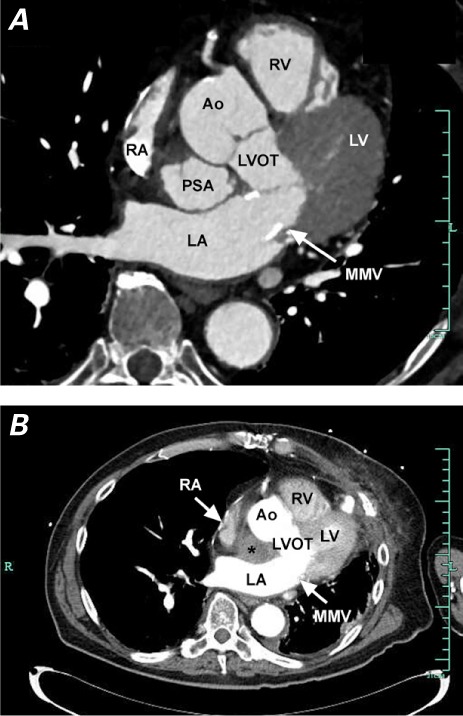
Computed tomographic angiograms show the pseudoaneurysm A) untreated, in the mitral-aortic intervalvular fibrosa; and B) with no contrast entry, indicating successful occlusion.
* = occluded pseudoaneurysm; Ao = aorta; LA = left atrium; LV = left ventricle; LVOT = left ventricular outflow tract; MMV = mechanical mitral valve; PSA = pseudoaneurysm; RA = right atrium; RV = right ventricle
Initially, we attempted to repair the pseudoaneurysm transfemorally, but this proved unsuccessful, because the angle of approach was too acute for effective placement of the occluder. During the transfemoral attempt, we were able to view the defect by contrast injection (Fig. 3).
Fig. 3.
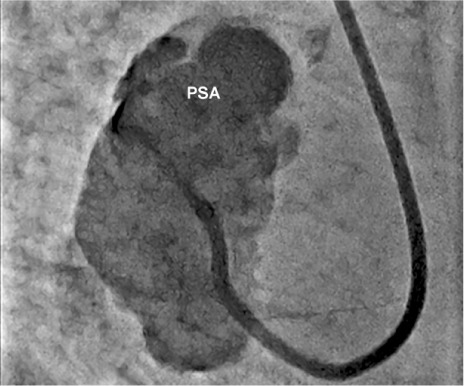
Angiogram during the transfemoral attempt shows the catheter coursing retrograde through the aortic valve into the pseudoaneurysm (PSA), with contrast injection.
When the patient was returned for a transapical approach, he underwent general anesthesia and intraprocedural TEE. A left minithoracotomy was performed to approach the apex. A 14F sheath was then inserted into the defect by transapical entry, and a 12-mm/10-mm Amplatzer® Ductal Occluder (St. Jude Medical, Inc.; St. Paul, Minn) was advanced into the defect. The occluder was placed successfully, in a manner that did not interfere with the left ventricular outflow tract or with prosthetic mitral valve function.
Upon installation of the occluder (Fig. 4), substantial smoke was noted on echocardiographic views of the pseudoaneurysm, indicating sluggish flow therein (Fig. 1B). A cardiac CTA obtained postprocedurally showed successful closure of the defect (Fig. 2B).
Fig. 4.
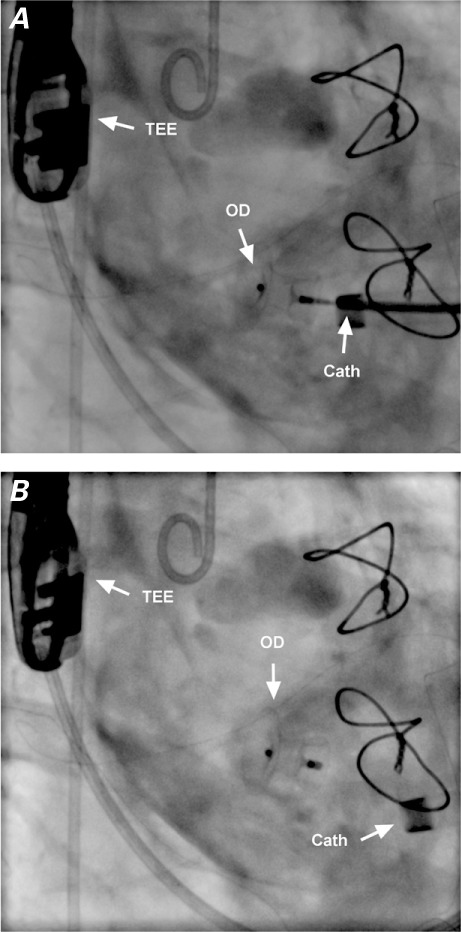
Periprocedural cineangiograms (right anterior oblique views) show the closure device A) being placed from a transapical approach through a delivery catheter and B) after deployment.
Cath = delivery catheter; OD = occluder device; TEE = transesophageal echocardiographic probe
Transesophageal echocardiography was used to view the defect, to direct placement of the device, and to document closure. The patient tolerated the procedure well. Three months after the operation, we performed follow-up left-sided heart catheterization and coronary angiography to check on the pseudoaneurysm. This procedure showed no filling of the occluded defect, which was now thrombosed. The most recent clinical follow-up at our institution took place 3 months later, at which time the patient was feeling better and had only mild shortness of breath on exertion.
Discussion
Pseudoaneurysms of the MAIVF can have a substantial impact on the cardiovascular system. The expansion of the pulsatile cavity can compress the left atrium and coronary vessels. The left circumflex coronary artery is most often involved, but the other arteries can be involved to varying degrees.1 The pseudoaneurysm can rupture into the aorta, the left atrium,2 or the pericardium (this last resulting in cardiac tamponade).3,4 These life-threatening sequelae suggest that early treatment is advisable.
In the past, P-MAIVFs were treated surgically, sometimes by aortic root replacement.5 Because of the risks of major open-heart surgery, less invasive methods of closure have been sought. The first documented percutaneous closure was performed in Spain in 2005 via the transfemoral approach.6 Percutaneous repair has also been performed through the transseptal approach, as reported more recently.7 Because our patient had a mechanical prosthesis, we could not use the transseptal approach. Our attempt at using the transfemoral approach was unsuccessful because of the acute angle, so we chose a direct transapical approach to the opening of the pseudoaneurysm (Fig. 5). To minimize obstruction to the left ventricular outflow tract, we chose the patent ductus arteriosus occluder.
Fig. 5.
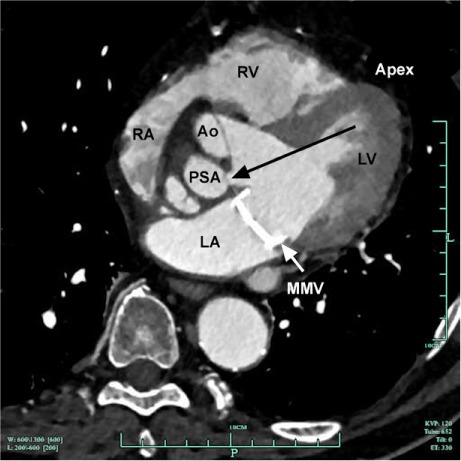
Cardiac computed tomogram shows the pseudoaneurysm; black arrow indicates that the transapical approach is the most direct and least problematic.
Ao = aorta; Apex = apex of the heart; LA = left atrium; LV = left ventricle; MMV = mechanical mitral valve; PSA = pseudoaneurysm; RA = right atrium; RV = right ventricle
We conclude that transcatheter closure is a viable and effective treatment of P-MAIVF. To our knowledge, this is the 3rd published report of this treatment option and the first of a transapical approach to the closure of P-MAIVF.
Acknowledgments
We would like to thank the following individuals: Dr. Robert A. Leonardi, for his contributions to the formation of the manuscript, to image selection and retrieval, and to substantial editing of the case report; Dr. B. Robinson Williams III, for his guidance in the drafting of the manuscript and his substantial editing of the text; and Mr. Patrick T. Strickland, for his assistance with writing and data-gathering.
Footnotes
From: Department of Medicine, Division of Cardiology (Drs. Babaliaros, Clements, Hartlage, Lerakis, and Shirazi) and Department of Surgery, Division of Cardiothoracic Surgery (Drs. Iturra and Thourani), Emory University School of Medicine, Atlanta, Georgia 30322
References
- 1.Sudhakar S., Sewani A., Agrawal M., Uretsky B. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): a comprehensive review. J Am Soc Echocardiogr. 2010;23(10):1009–18. doi: 10.1016/j.echo.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Spampinato R. A., Borger M. A., Strotdrees E., Mohr F. W. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa as a complication after minimally invasive mitral valve repair. Interact Cardiovasc Thorac Surg. 2013;16(3):396–8. doi: 10.1093/icvts/ivs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesler E., Korns M. E., Porter G. E., Reyes C. N., Edwards J. E. False aneurysm of the left ventricle secondary to bacterial endocarditis with perforation of the mitral-aortic intervalvular fibrosa. Circulation. 1968;37(4):518–23. doi: 10.1161/01.cir.37.4.518. [DOI] [PubMed] [Google Scholar]
- 4.Qizilbash A. H., Schwartz C. J. False aneurysm of left ventricle due to perforation of mitral-aortic intervalvular fibrosa with rupture and cardiac tamponade. Rare complication of infective endocarditis. Am J Cardiol. 1973;32(1):110–3. doi: 10.1016/s0002-9149(73)80095-5. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama Y., Tamaki S., Kato N., Yokote J., Mutsuga M. Pseudoaneurysm from the mitral-aortic intervalvular fibrosa following endocarditis. Jpn J Thorac Cardiovasc Surg. 2003;51(8):374–7. doi: 10.1007/BF02719470. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez Valero S., Garcia E., Gonzalez Pinto A., Delcan J. L. Percutaneous closure of pseudoaneurysm of the mitral-aortic intervalvular fibrosa [in Spanish] Rev Esp Cardiol. 2005;58(12):1473–5. [PubMed] [Google Scholar]
- 7.Romaguera R., Slack M. C., Waksman R., Ben-Dor I., Satler L. F., Kent K. M. et al. Image cardio med: percutaneous closure of a left ventricular outflow tract pseudoaneurysm causing extrinsic left coronary artery compression by transseptal approach. Circulation. 2010;121(4):e20–2. doi: 10.1161/CIR.0b013e3181cf2fe2. [DOI] [PubMed] [Google Scholar]


