Abstract
Endostar, a modified recombinant human endostatin, inhibits the growth of a variety of tumors by suppressing neovascularization. Vascular endothelial growth factor (VEGF) has an important role in malignant ascites formation. In order to determine whether Endostar can suppress the formation of ascites and prolong survival times, mouse models of malignant ascites were established using S180 and H22 tumor cells. The experimental mice were randomly divided into four groups: The three treatment groups received different doses of Endostar (4, 8 and 16 mg/kg), and the control group received 0.9% w/v NaCl. The volume of ascites, and the tumor cell, red blood cell (RBC), VEGF protein and mRNA content of the ascites was measured alongside the peritoneal permeability and the mouse survival time. In vitro analysis of cultured Endostar-treated S180 and H22 cells was also performed in order to examine cellular proliferation and the level of VEGF secreted protein and mRNA. The results revealed that Endostar suppressed the ascites volume, decreased the level of tumor cells, RBCs and VEGF in the ascites fluid, and lowered the permeability of the peritoneum. The tumor cells collected from the ascites in the Endostar-treated mice demonstrated a decrease in the expression of VEGF mRNA. The survival rates of the 8 and 16 mg/kg Endostar-treated mice were longer than those of the controls. The in vitro experiments revealed a significant inhibition of VEGF protein secretion and VEGF mRNA by Endostar, but no effect on cellular proliferation. In conclusion, Endostar lowers ascites production by downregulating VEGF expression, and may therefore be effective for the treatment of malignant ascites.
Keywords: Endostar, malignant ascites model, peritoneum permeability, antiangiogenesis, vascular endothelial growth factor
Introduction
Malignant ascites is caused by the accumulation of fluid in the peritoneal cavity following the intraperitoneal (i.p.) spread of tumor cells. Ascites can occur in a variety of cancers, including ovarian (37%), pancreaticobiliary (21%), gastric (18%), esophageal (4%), colorectal (4%) and breast (3%) cancer (1–3). Ascites may result in a poor quality of life due to symptoms of abdominal distention, pain, nausea, vomiting, anorexia, dyspnea, limb edema, insomnia and fatigue (1–3).
Chemotherapy is a common first-line treatment for patients with ascites. However, limited evidence exists concerning its efficacy in patients with recurrent ascites. The i.p. administration of radioisotopes, or chemotherapy and peritoneovenous shunting procedures, has also been used (3,4). However, data regarding these approaches is also lacking. These methods are able to relieve the symptoms of recurrent ascites, but do not prolong survival (4). Repeated paracentesis is associated with regular hospital admissions and can lead to a number of problems, including pain, protein loss, hypovolemia, infection, peritonitis and bowel perforation (3,4). The preclinical and clinical evidence concerning anti-angiogenic agents, such as vascular endothelial growth factor (VEGF) antagonists and matrix metalloproteinase inhibitors, suggest that these agents may have a role in the treatment of malignant ascites (5–8). A requirement exists to identify an effective clinical therapy for the treatment of malignant ascites.
Endostatin is a carboxyl-terminal proteolytic fragment of collagen XVIII, and an endogenous inhibitor of angiogenesis (9,10). Furthermore, it is a broad-spectrum and low toxicity multitarget angiogenesis inhibitor, which may inhibit up to 65 types of tumor (10). However, the antitumor effects of endostatin are yet to be demonstrated in phase II clinical trials. This apparent lack of efficacy may be due to problems in recombinant techniques and treatment strategies (11,12).
Certain problems associated with endostatin have been addressed by the development of Endostar. Endostar is a modified endostatin expressed and purified from Escherichia coli (13). Although Endostar has achieved clinical efficacy in treating a variety of advanced malignant tumors (14–16), evidence concerning its effectiveness in treating malignant ascites is limited and requires systematic investigation and standardization of the studies. The aim of the present study was to identify novel indications for Endostar in order to provide a theoretical basis and practical experience for its use in the treatment of advanced cancers that are associated with malignant hydrothorax and ascites.
Materials and methods
Construction of mouse models
Female imprinting control region (ICR) mice, aged 5–7 weeks old and weighing 18–20 g, were obtained from the Shanghai Laboratory Animal Center (Shanghai, China). The mice were kept under specific pathogen-free conditions, fed autoclaved pellets and had access to water ad libitum. Animal quality was controlled (certification, SCXK Shanghai 20070005). The general health status of the mice was monitored daily. All procedures and animal experiments were approved by the Animal Ethical Committee of the 81st Hospital of the People's Liberation Army (The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China) and conducted in accordance with all state regulations.
The present study used mouse sarcoma S180 and mouse hepatocellular carcinoma H22 cell lines, which were obtained from the Chinese Academy of Science (Shanghai, China). The cells had a good capacity to grow in female ICR mice. Following the i.p. injection of these cells for 6–8 days, the mice developed ascites similar to that observed in advanced clinical cancers.
Floating tumor cells were collected and washed with RPMI-1640 media (without fetal calf serum; Gibco Life Technologies, Carlsbad, CA, USA), and then centrifuged at 100 × g and 4°C for 5 min. Next, the cells were suspended in RPMI-1640 medium and immediately used to inoculate the experimental mice (n=120). Each mouse was injected i.p. with 2×106 viable tumor cells suspended in 0.2 ml medium.
Grouping and treatment of the mice
Subsequent to inoculation with the tumor cells, the mice were randomly distributed into four groups, with each group consisting of 30 mice. The three treatment groups received different doses of Endostar (4, 8 and 16 mg/kg), and the control group received 0.9% w/v NaCl. Following tumor cell inoculation, Endostar (9.7 g/l; batch number, YY2009005; Simcere Pharmaceutical Research Co., Ltd., Nanjing, Jiangsu, China), and normal saline as a vehicle, were immediately administered by i.p. injection once daily for a total of 10 days. Subsequent to treatment, 12 mice from each group were sacrificed by 200 mg/kg pentobarbital sodium i.p. injection and samples, including tumor cells and cell-free ascites fluid, were stored at −80°C for subsequent analysis. Each assay was performed three times to ensure satisfactory reproducibility.
The general health status of the experimental mice was monitored daily. The body weights were measured on alternate days and used to plot body-weight growth curves.
Measurement of ascites volume, tumor cell count and red blood cell (RBC) count
At the end of the experimental period, six mice from each group were sacrificed by 200 mg/kg pentobarbital sodium i.p. injection. The ascites volume, and the number of tumor cells and RBCs were measured. A laparotomy was performed, and sterile pipettes were used to collect ascites into centrifuge tubes in order to calculate the average volume of ascites. In total, 1 ml ascites per nude mouse was added into a test tube with heparin and diluted 100 times. The number of tumor cells and RBCs was then calculated by an Automated Cell Counter (C10227; Invitrogen Life Technologies, Carlsbad, CA, USA). The number of tumor cells and RBCs in each sample was counted three times to obtain an average.
Assay of peritoneal permeability
In total, six mice from each group underwent peritoneal capillary permeability analysis. The mice were slowly injected with 0.2 ml of 5 mg/ml Evans' blue dye (total dose 1 mg; Sigma-Aldrich, St. Louis, MO, USA) into the vena caudalis. After 2 h, ascites fluid was collected and the samples were centrifuged at 250 × g for 5 min. The optical density of the Evans' blue dye in the supernatants was measured at 540 nm using a spectrophotometer (UV-2450; Shimadsu Scientific Instruments, Columbia, MD, USA) in order to indirectly reflect the concentration of the dye. This value was then used as a measure of membrane permeability.
Survival analysis
The four groups of mice (n=18 per group) received Endostar or 0.9% w/v NaCl for 10 days. Following tumor cell inoculation, the mice were observed until they succumbed to the disease. The survival time of each mouse was recorded.
Quantitative assay for VEGF in vivo and in vitro by ELISA
In order to measure the level of VEGF in the ascites, cell-free ascites fluid was collected from the peritoneal cavity of the vehicle- and Endostar-treated mice. The level of VEGF in the ascites fluid was measured using an ELISA kit according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN, USA).
For the in vitro analysis of secreted VEGF protein, S180 and H22 cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum (Gibco Life Technologies), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 g/l NaHCO3 and 2.4 g/l Hepes (Invitrogen Life Technologies) in a 37°C incubator with 95% air and 5% CO2. Next, 1×104 cells/well were seeded into plastic culture plates and treated with culture medium containing various concentrations of Endostar (0, 2.5, 10 and 40 µg/ml) for 48 h under cell culture conditions. The medium from each well was then removed and analyzed for its VEGF content using an ELISA kit. The trypan blue exclusion method was used to determine the number of viable cells at the end of the treatment period.
VEGF mRNA determination by reverse transcription-polymerase chain reaction (RT-RCR) in vivo and in vitro
For the in vivo analysis, tumor cells collected from the ascites fluid of the experimental mice were examined for the expression of VEGF mRNA by RT-PCR. For the in vitro analysis, tumor cells treated with culture medium containing various concentrations of Endostar (0, 2.5, 10 and 40 µg/ml) for 48 h under cell culture conditions were individually collected and analyzed for the expression of VEGF mRNA using RT-PCR. The total RNA was extracted from the tumor cells using the High Pure RNA Isolation kit (Invitrogen Life Technologies) according to the manufacturer's instructions. The primers used for the amplification of VEGF were constructed based upon the following sequences: Forward, 5′-TGAAGCCCTGGAGTGCGT-3′; and reverse, 5′-ATGATGGCGTGGTGGTGA-3′. RT-PCR was performed according to the manufacturer's instructions (Qiagen GmbH, Hilden, Germany). All samples were run for 32 cycles in triplicate (denaturation at 94°C for 1 min, primer annealing at 60°C for 1 min, and primer extension at 72°C for 35 sec) and a final extension of 72°C for 10 min. The RT-PCR products were visualized by electrophoresis (DYY-6B,China) (25 min at 120 V) on 2% agarose gel in 1X TAE buffer containing ethidium bromide. To quantify the size of the products, a 100-bp DNA ladder was run with the samples. β-actin was used as the reference. The specific primers were designed using Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA).
Cell proliferation assay
Cellular proliferation was assessed using the MTT staining assay (Sigma-Aldrich). The S180 and H22 cells were plated onto 96-well plates at a density of 5×103 cells/well and left to grow overnight. Next, Endostar was added at concentrations of 0.1, 0.5, 2.5, 12.5 and 62.5 µg/ml. A negative (RPMI-1640 medium) and positive (10 µg/ml Taxol) control were also established. The cells were cultivated for 72 h. In total, 20 µl MTT solution (5 mg/ml in phosphate-buffered saline) was added to each well and incubated for an additional 4 h at 37°C. The cell medium was then removed and 150 µl DMSO was added to the 96-well plates in order to dissolve the MTT crystals. Cellular growth and viability was evaluated by measuring the optical density at 490 nm on a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard deviation. The Kaplan-Meier method was used for the survival analysis, and the results are presented as the survival time and 95% confidence interval. All statistical analyses were performed using SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was evaluated using a one-way analysis of variance. P<0.05 was used to indicate a statistically significant difference.
Results
Appearance and behavior of the experimental mice
In the early stages, there were no observable differences in the behavior (degree of activity and eating) of the mice treated with 8 and 16 mg/kg Endostar, and the normal, non-tumor-inoculated mice. Up until the fifth day, there was an extensive increase in the abdominal girth and body-weight of the non-treated, tumor-inoculated mice and the 4 mg/kg Endostar-treated mice. At the advanced stages, cachexia was reflected in the emergence of symptoms, including malaise of spirit, little movement, poor response, dry feces and abdominal ectasia. The 8 and 16 mg/kg Endostar-treated mice presented with these symptoms and gained weight more slowly than the control group mice (Fig. 1A and B).
Figure 1.
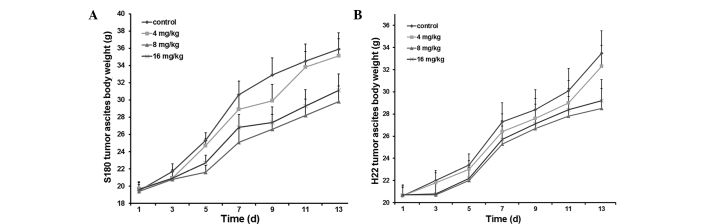
Body-weight growth curves of the experimental mice inoculated with (A) S180 and (B) H22 tumor cells. Three groups of mice received different doses of Endostar (4, 8 and 16 mg/kg) by intraperitoneal injection for 10 consecutive days. The control group received 0.9% normal saline. The results indicate that the 8 and 16 mg/kg Endostar-treated mice gained body weight more slowly than the control group.
Endostar treatment inhibits ascites production and decreases the levels of tumor cells and RBCs
The average volume of ascites, and the number of tumor cells and RBCs in the ascites are shown in Tables I and II. The 8 and 16 mg/kg Endostar doses significantly suppressed ascites production and led to a significant reduction in the number of viable floating tumor cells and RBCs within the peritoneal cavity.
Table I.
Effect of different doses of Endostar on the volume of ascites and the number of red blood cells and tumor cells in a mouse S180 tumor-bearing model of malignant ascites (means ± standard deviation; n=6).
| Group | Volume of ascites, ml | Red blood cell count, 107/ml | Tumor cell count, 107/ml |
|---|---|---|---|
| Control | 12.5±2.6 | 36.4±6.7 | 23.9±3.9 |
| 4 mg/kg Endostar | 11.8±1.5a | 32.6±4.6a | 19.0±4.3a |
| 8 mg/kg Endostar | 9.0±2.4b,c | 12.7±4.8b,c | 12.3±2.8b,c |
| 16 mg/kg Endostar | 10.2±1.8b | 12.3±5.1b | 12.6±3.7b |
P>0.05
P<0.05 vs. control group
P>0.05 vs. 16 mg/kg Endostar group.
Table II.
Effect of different doses of Endostar on the volume of ascites and the number of red blood cells and tumor cells in a mouse H22 tumor-bearing model of malignant ascites (mean ± standard deviation; n=6).
| Group | Volume of ascites, ml | Red blood cell count, 107/ml | Tumor cell count, 107/ml |
|---|---|---|---|
| Control | 10.75±1.0 | 26.1±4.5 | 15.7±1.9 |
| 4 mg/kg Endostar | 9.75±1.5a | 21.5±3.6a | 13.1±2.3a |
| 8 mg/kg Endostar | 8.75±1.2b,c | 7.3±4.8b,c | 8.0±2.8b,c |
| 16 mg/kg Endostar | 8.5±1.1b | 7.7±3.3b | 8.4±2.1b |
P>0.05
P<0.05 vs. control group
P>0.05 vs. 16 mg/kg Endostar group.
Endostar reduces peritoneal permeability
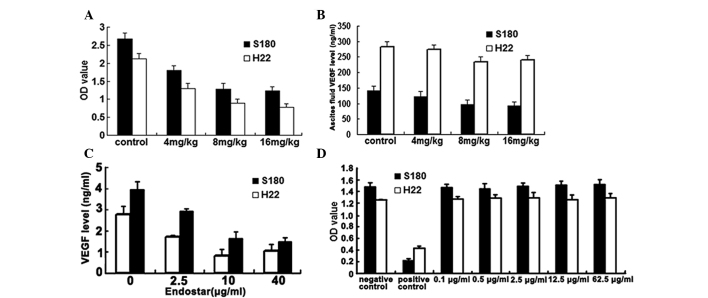
Angiogenesis and vessel hyperpermeability are two important factors involved in ascites formation (1–5,17). The peritoneal permeability assay is used to determine vessel permeability in animal models (18,19). The present study analyzed the membrane permeability according to the optical density of Evans' blue dye. The results are shown in Fig. 2A. The different doses of Endostar (4, 8 and 16 mg/kg) significantly decreased the permeability of the peritoneum (all P<0.05) in the S180 and H22 malignant ascites.
Figure 2.
(A) Results of the peritoneal permeability assay in the S180 and H22 mouse models. Endostar (4, 8 and 16 mg/kg) significantly decreased the permeability of the peritoneum (all P<0.05 vs. control group). (B) Results of the ELISA for VEGF levels in ascites. The cell-free ascites fluid was collected from the peritoneal cavity of the experimental mice. The 8 and 16 mg/kg Endostar-treated groups exhibited marked differences in the level of VEGF (P<0.05 vs. control group). (C) Results of the ELISA for in vitro VEGF secretion in S180 and H22 cells. In total, 1×104 cells/well were treated with various concentrations of Endostar (0, 2.5, 10 and 40 µg/ml) for 48 h. The media from the wells was then individually collected and analyzed. Endostar reduced VEGF secretion (all P<0.05 vs. control group). (D) Results of the in vitro MTT proliferation assay for S180 and H22 cells. The S180 and H22 cells were treated with Endostar at various doses (0.1, 0.5, 2.5, 12.5 and 62.5 µg/ml). Endostar did not reduce the proliferation of S180 and H22 cells (all P>0.05 vs. control group). The data are expressed as the mean ± standard deviation. VEGF, vascular endothelial growth factor; OD, optical density.
Survival time
The results revealed that the survival times of the mice treated with 8 and 16 mg/kg Endostar were longer than those of the control group. The differences between the 8 and 16 mg/kg Endostar groups and the control group were statistically significant (P<0.05; Table III and Fig. 3).
Table III.
Effect of different doses of Endostar on the survival time of S180 and H22 tumor-bearing mouse models of malignant ascites (means ± standard deviation; n=18).
| Group | S180 | H22 |
|---|---|---|
| Control | 12.7±1.2 | 11.6±0.8 |
| 4 mg/kg Endostar | 13.8±1.4a | 12.8±1.4a |
| 8 mg/kg Endostar | 17.2±1.5b,c | 15.9±1.9b,c |
| 16 mg/kg Endostar | 16.3±2.0b | 15.0±2.0b |
P>0.05
P<0.05 vs. control group
P>0.05 vs. 16 mg/kg Endostar group.
Figure 3.
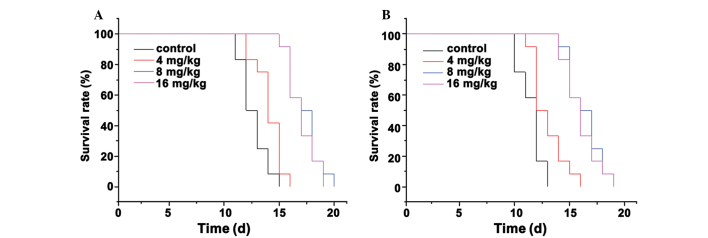
Survival analysis curves for the Endostar-treated mouse models. (A) S180 mice and (B) H22 mice. In total, 18 mice from each of the four groups received 0, 4, 8 and 16 mg/kg Endostar or 0.9% w/v NaCl solution for 10 consecutive days. The mice were then monitored until they succumbed to the disease.
Endostar reduces the level of secreted VEGF in ascites fluid in vitro
Using a standard ELISA kit, the concentration of VEGF in the ascites fluid was determined. The results demonstrated that mice treated with 8 and 16 mg/kg Endostar had significantly lower VEGF levels in the ascites fluid compared with the controls (P<0.05; Fig. 2B).
To further validate the effect of Endostar on the level of VEGF, the S180 and H22 cells were treated with various concentrations of Endostar (0, 2.5, 10 and 40 µg/ml). The results are presented in Fig. 2C. The results indicated that Endostar reduced the VEGF concentration. The 2.5, 10 and 40 µg/ml Endostar concentrations were all effective (all P<0.05) in decreasing VEGF. These results further verified the inhibitory effect of Endostar on VEGF secretion.
Endostar downregulates VEGF mRNA levels in vivo and in vitro
In order to investigate the mechanism by which Endostar lowered the VEGF levels, VEGF mRNA was examined using RT-PCR. The results revealed a downregulation of VEGF mRNA in in vivo Endostar-treated mice (Fig. 4A and B) and in vitro cell lines (Fig. 5A and B).
Figure 4.
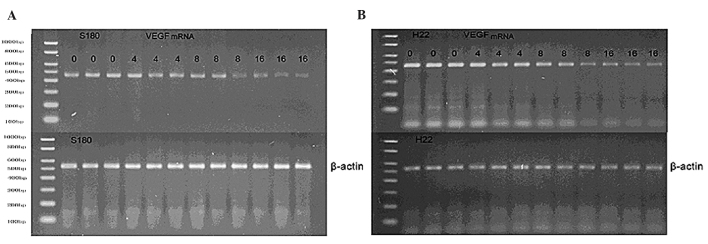
Agarose gel of the reverse transcription polymerase chain reaction revealing the levels of VEGF mRNA from tumor cells collected from the control- and Endostar-treated (4, 8 and 16 mg/kg) mice. (A) S180 and (B) H22 mice. The β-actin gene was used as an internal control. The results indicate a decrease in the level of VEGF mRNA in the Endostar-treated (8 and 16 mg/kg) mice. VEGF, vascular endothelial growth factor.
Figure 5.
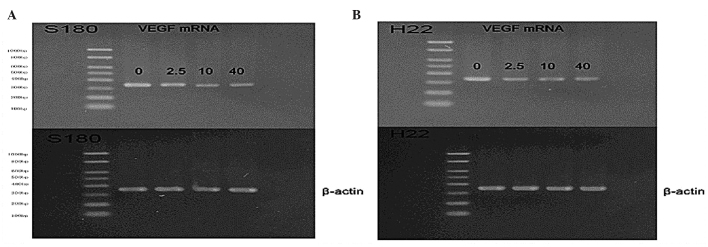
Agarose gel of the reverse transcription polymerase chain reaction of VEGF mRNA from control- and Endostar-treated (2.5, 10 and 40 mg/kg) cultured tumor cells. The β-actin gene was used as an internal control. The results indicate a decrease in the level of VEGF mRNA in Endostar-treated (2.5, 10 and 40 mg/kg) cells.
Effect of Endostar on the growth of S180 and H22 cells in vitro
The MTT assay is commonly used as a simple non-radioactive colorimetric assay to measure cell cytotoxicity, proliferation or viability. In the present study, the MTT assay was used to evaluate the proliferation of S180 and H22 cells in the absence or presence of Endostar. Treatment with various doses of Endostar (0.1, 0.5, 2.5, 12.5 and 62.5 µg/ml) did not reduce the proliferation of the S180 and H22 cells (all P>0.05 vs. control; Fig. 2D).
Discussion
The aim of the present study was to investigate whether Endostar could affect the production of malignant ascites and prolong survival. The experimental models proved to be effective as the mice developed ascites similar to those observed in advanced cancers. The results revealed that Endostar suppressed the volume of ascites produced in the mice, decreased the number of tumor cells and RBCs in the ascites, reduced the permeability of the peritoneum and downregulated VEGF expression. These beneficial results all increased the survival time of the mice treated with Endostar.
An improved understanding of the molecular mechanisms that underlie the formation of malignant effusions may lead to the identification of novel and more effective therapies for the treatment of malignant ascites. It has been suggested that tumor-induced angiogenesis and vessel hyperpermeability are two factors that result in the formation of ascites (1–5,17). The results of the present study demonstrated that Endostar treatment significantly lowered peritoneal permeability in the mouse models, and reduced the leakage of erythrocytes into the ascites. The results from the analysis of VEGF protein levels suggest that this may be connected. VEGF has an important role in increasing vascular permeability during malignant ascites formation (1–5). Previous studies have revealed that high concentrations of VEGF are detected in the hydrothorax and ascites fluid of cancer patients (20–22). The vascular permeabilizing activity of VEGF has been reported to be 50,000 times more potent than histamine. Furthermore, it has been suggested that the secretion of VEGF by tumor cells is essential and sufficient to promote the formation and accumulation of ascites. In experimental animal models, an association between ascites volume and VEGF levels has been demonstrated. In addition a VEGF blockade has been identified to lead to marked reductions in ascites formation (1,5,8,19). Cancer-associated effusions contain significantly more VEGF than those resulting from inflammatory diseases, which suggests that VEGF levels are an independent and statistically significant prognostic indicator of survival (20,21). The results of a previous study revealed that hemorrhagic malignant effusions (RBC count, >1×104/µl) exhibit significantly higher VEGF levels (1,942 pg/ml) compared with non-hemorrhagic effusions (202 pg/ml) (P=0.016) in malignant patients (21). In addition, malignant patients with large volumes of effusion (>1 liter) demonstrate higher concentrations of VEGF in the fluid than malignant patients with smaller volumes of effusion (<1 liter) (21). Previous studies have also demonstrated that a high level of VEGF is detected in ascites, that the number of microvessels is increased in the peritoneal wall of mice with ascitic tumors, and that anti-VEGF treatment reduces the volume of ascites fluid (5,8,19).
In order to determine how a reduction in the level of VEGF protein was established, the VEGF mRNA status of the tumor cells was examined in the present study. The results revealed a downregulation of VEGF mRNA in the tumor cells obtained from the peritoneal cavity of the Endostar-treated mice. Certain studies have indicated that the effects of endostatin are associated with VEGF, a crucial regulator in angiogenesis (23,24). Previous data have revealed that Endostar may exert anti-angiogenic effects via a similar mechanism to endostatin. However, Endostar differs from endostatin by the addition of nine amino acids (MGGSHHHHH), which improves the half-life of the protein and its ability to combine with zinc (25). In the present study, the results of the MTT assays did not demonstrate an in vitro inhibition of S180 and H22 cellular proliferation with various concentrations of Endostar. This may be due to the fact that the primary function of Endostar in controlling malignant ascites was in suppressing VEGF expression and vessel permeability, rather than impacting on cell growth. However, the mice that were treated with Endostar demonstrated longer survival times than the controls as a consequence of their improved situation.
To the best of our knowledge, the present study is the first to demonstrate that the modified human endostatin, Endostar, inhibits ascites production and causes a marked reduction in VEGF in ascites fluid. The study also establishes the importance of VEGF in inhibiting ascites production. The results revealed the potential of Endostar in treating malignant ascites and possibly other VEGF-related conditions, and the potential for prolonging the survival time of affected individuals. It was determined that 8 mg/kg Endostar was the optimum dose used in the present study.
The results have provided important information regarding the potential therapeutic roles of Endostar, but are limited by the use of animal models. The results should provide the theoretical basis and practical experience for developing multicentric clinical studies for Endostar in the treatment of advanced cancer associated with malignant effusion. Endostar will therefore be a promising and novel therapy for the treatment of malignant ascites.
Acknowledgements
The study was supported, in part, by a grant from the China Postdoctoral Science Foundation (no. 20090451574) and Jiangsu Simcere Pharmaceutical Research Co., Ltd (no. SIM-67). This study has previously been published as an abstract in the 2011 ASCO Annual Meeting.
References
- 1.Saâda E, Follana P, Peyrade F, Mari V, François E. Pathogenesis and management of refractory malignant ascites. Bull Cancer. 2011;98:679–687. doi: 10.1684/bdc.2011.1373. (In French) [DOI] [PubMed] [Google Scholar]
- 2.Smith EM, Jayson GC. The current and future management of malignant ascites. Clin Oncol (R Coll Radiol) 2003;15:59–72. doi: 10.1053/clon.2002.0135. [DOI] [PubMed] [Google Scholar]
- 3.Chung M, Kozuch P. Treatment of malignant ascites. Curr Treat Options Oncol. 2008;9:215–233. doi: 10.1007/s11864-008-0068-y. [DOI] [PubMed] [Google Scholar]
- 4.Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Pourgholami MH, Yan Cai Z, Lu Y, Wang L, Morris DL. Albendazole: a potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin Cancer Res. 2006;12:1928–1935. doi: 10.1158/1078-0432.CCR-05-1181. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Covic L, Sevigny LM, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites and progression of ovarian cancer. Mol Cancer Ther. 2008;7:2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beattie GJ, Smyth JF. Phase I study of intraperitoneal metalloproteinase inhibitor BB94 in patients with malignant ascites. Clin Cancer Res. 1998;4:1899–1902. [PubMed] [Google Scholar]
- 8.Kobold S, Hegewisch-Becker S, Oechsle K, Jordan K, Bokemeyer C, Atanackovic D. Intraperitoneal VEGF inhibition using bevacizumab: a potential approach for the symptomatic treatment of malignant ascites? Oncologist. 2009;14:1242–1251. doi: 10.1634/theoncologist.2009-0109. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/S0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Antiangiogenesis in cancer therapy - endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JP, Arzoomanian RZ, Alberti D, et al. Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2003;21:223–231. doi: 10.1200/JCO.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Hess KR, Tran HT, et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792–3803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Wong MK, Zhao Q, et al. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res. 2001;61:478–481. [PubMed] [Google Scholar]
- 14.Han B, Xiu Q, Wang H, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with Endostar for advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:1104–1109. doi: 10.1097/JTO.0b013e3182166b6b. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Ye HY, Zhang YH, Guan YS, Wu H. Epidermal growth factor receptor antibody plus recombinant human endostatin in treatment of hepatic metastases after remnant gastric cancer resection. World J Gastroenterol. 2007;13:6115–6118. doi: 10.3748/wjg.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmenkühler A. Spreading depression - cortical reactions: disorders of the extracellular microenvironment. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1990;21:1–6. (In German) [PubMed] [Google Scholar]
- 17.Ayhan A, Gultekin M, Taskiran C, et al. Ascites and epithelial ovarian cancers: a reappraisal with respect to different aspects. Int J Gynecol Cancer. 2007;17:68–75. doi: 10.1111/j.1525-1438.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 18.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai YJ, Zheng DY, Luo RC, et al. Avastin combined with cisplan inhibits malignant ascites production in nude mice bearing transplanted ovary carcinoma with high VEGF expression. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:647–649. (In Chinese) [PubMed] [Google Scholar]
- 20.Hirayama N, Tabata C, Tabata R, et al. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir Med. 2011;105:137–142. doi: 10.1016/j.rmed.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Ishimoto O, Saijo Y, Narumi K, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology. 2002;63:70–75. doi: 10.1159/000065723. [DOI] [PubMed] [Google Scholar]
- 22.Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist. 2000;5:45–50. doi: 10.1634/theoncologist.5-suppl_1-45. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 23.Tepper D. Frontiers in congestive heart failure: consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Congest Heart Fail. 2000;6:168–169. doi: 10.1111/j.1527-5299.2000.80156.x. [DOI] [PubMed] [Google Scholar]
- 24.Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 25.Han Q, Fu Y, Zhou H, He Y, Luo Y. Contributions of Zn(II)-binding to the structural stability of endostatin. FEBS Lett. 2007;581:3027–3032. doi: 10.1016/j.febslet.2007.05.058. [DOI] [PubMed] [Google Scholar]