Abstract
The aim of the present study was to investigate the in vitro effect of osteopontin (OPN) on the expression of hypoxia-inducible factor-2α (HIF-2α) in chondrocytes and the role of OPN in osteoarthritis (OA). Cartilage was purified from the tibial surfaces of patients with OA of the knee and cultured in vitro to obtain chondrocytes. Recombinant human OPN (rhOPN) and OPN small interfering RNA (siRNA) were used to treat the chondrocytes, and the changes in the expression levels of the HIF-2α gene were measured. An anti-CD44 blocking monoclonal antibody (mAb) was used to determine the probable ligand-receptor interactions. Reverse transcription-quantitative polymerase chain reaction assays were designed and validated with SYBR® Green dyes for the simultaneous quantification of the mRNA expression levels of OPN and HIF-2α. The mRNA expression level of HIF-2α was markedly decreased in the rhOPN-treated group compared with that in the control group; by contrast, OPN siRNA increased HIF-2α gene expression. CD44 blocking mAb suppressed the inhibitory effect of OPN on HIF-2α mRNA expression. The results of the present study suggest that OPN may play a protective role in OA by inhibiting HIF-2α gene expression in osteoarthritic chondrocytes through CD44 interaction.
Keywords: chondrocyte, CD44, HIF-2α, osteopontin, osteoarthritis
Introduction
Osteoarthritis (OA) is a metabolically active, dynamic process that affects all joint tissues. The major clinical signs of the disease include destruction of the articular cartilage and changes to the underlying subchondral bone. However, the etiology of the disease remains poorly understood. Several biochemical and biomechanical factors are considered to play a role in the pathogenesis. Osteopontin (OPN), also known as early T cell activation gene-1 (Eta-1) is abundant in bone tissue and may be secreted by a number of different cell types including chondrocytes and synoviocytes (1–4). OPN is upregulated in human chondrocytes (5). A previous study found that the level of OPN mRNA isolated from human OA cartilage was increased as compared with that in normal cartilage (5). Furthermore, the expression level of OPN in the plasma, synovial fluid and articular cartilage is associated with progressive joint damage and may be a useful biomarker for determining the severity and progression of disease in knee OA (6,7). OPN interacts with a variety of cell surface receptors, including integrin and CD44 (8). The receptor CD44 has been implicated in the development and progression of OA, and CD44 present in articular cartilage has been associated with progressive knee OA joint damage (9,10). However, the role of OPN in the pathological changes in knee OA remains unknown.
Articular cartilage is an avascular tissue that derives its nutritional and oxygen supply by a diffusion process from the synovial fluid and subchondral bone. Thus, articular cartilage is maintained in a low oxygen environment in the body (11). Chondrocytes are therefore adapted to these hypoxic conditions. A number of previous studies have shown that hypoxia triggers essential positive signals for the chondrocyte phenotype (12–14). Adaptation to this avascular environment is mediated by hypoxia-inducible factor (HIF)-1 and HIF-2 (12). The HIF protein family consists of α and β subunit members that function by forming heterodimers (12). Two HIF isoforms (HIF-1α and HIF-2α) mediate the response of cells to hypoxia (13,14). In a previous study, HIF-2α was demonstrated to be essential for the endochondral ossification of cultured chondrocytes and embryonic skeletal growth in mice (15). Furthermore, HIF-2α expression has been found to be higher in osteoarthritic cartilage than in non-diseased cartilage in mice and humans (15). Another study observed that HIF-2α increased the expression levels of genes encoding catabolic factors, including matrix metalloproteinases (MMPs) −1, 3, 9, 12 and 13, aggrecanase-1, nitric oxide synthase-2 and prostaglandin-endoperoxide synthase-2 in chondrocytes (16). Thus, HIF-2α is an important catabolic transcription factor in the process of OA development and may be considered as a therapeutic target for OA.
The association between HIF-2α and OPN in chondrocytes remains unclear. The aim of the current in vitro study was to investigate the effect of OPN on HIF-2α mRNA expression in chondrocytes from patients with OA of the knee in order to reveal the role of OPN in OA.
Materials and methods
Chondrocyte culture
The present study protocol was approved by the Institutional Review Board of the Xiangya Hospital Central South University (Changsha, China). The articular hyaline cartilage was removed from the tibial surfaces of 6 patients with OA of the knee who had undergone a total knee replacement. Written informed consent was obtained from the patients. After washing twice with phosphate-buffered saline (PBS), the cartilage was ground with a scalpel blade into 1–5-mm3 sections. The cartilage sections were subsequently digested with 5–8 ml 0.2% collagenase II (Sigma-Aldrich, St. Louis, MO, USA) for 12–16 h at 37°C with 5% CO2. The digestion was terminated with 8–10 ml Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Hyclone, Logan, UT, USA). The released chondrocyte pellets at the bottom of the centrifuge tube were suctioned and transferred to a culture flask following centrifugation at 150 × g for 6 min. The cells were subsequently counted using a hemocytometer (Beckman Coulter, Brea, CA, USA) and cell viability was determined using trypan blue exclusion. Cell pellets were resuspended in 5 ml DMEM/F12 containing 15% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin solution (Gibco), and incubated for 24 h at 37°C with 5% CO2 in a plastic culture flask. The non-adherent cells were subsequently washed out. The remaining adherent cells were cultured for an additional 2 weeks in a flask, while the growth medium was changed every 3 days prior to trypsinization, and then transferred to new culture flasks.
Transfection of OPN small interfering RNA (siRNA) into chondrocytes
The siRNAs specific to OPN were designed and synthesized by Invitrogen Trading (Shanghai) Co., Ltd (Shanghai, China) with reference to the coding sequence for human OPN. The siRNA sequences were as follows: sense, 5′-CCU GUG CCA UAC CAG UUA ATT-3′ and antisense, 5′-UUA ACU GGU AUG GCA CAG GTT-3′. Transfection of siRNAs to the cells was performed with Lipofectamine™ 2000 reagent (Invitrogen Life Technologies, San Diego, CA, USA) according to the manufacturer's instructions. Briefly, on the day prior to transfection, exponentially growing cells were seeded onto six-well plates at a density of 1.5×105 cells/well in the DMEM without antibiotics. Upon reaching 70% confluence, the cells were transfected with 50 nmol siRNA using Lipofectamine™ 2000. After 24 h, total RNA was isolated and the expression levels of the relative transcripts were detected by reverse transcription (RT)-quantitative polymerase chain reaction (qPCR).
Cell treatment
The chondrocytes were plated in 6-well culture plates and serum starved for 24 h in DMEM/F12 medium containing 1% FBS to synchronize cells in a non-activating and non-proliferating phase. The chondrocytes were subsequently cultured in DMEM/F12 containing 15% FBS. Three groups were established. The control group comprised chondrocytes that were unstimulated and untreated. The recombinant human OPN (rhOPN) group comprised chondrocytes that were treated with 1 µg/ml rhOPN (1433-OP; R&D Systems, Minneapolis, MN, USA) for 24 h, and the third group was the OPN siRNA group comprising chondrocytes transfected with OPN siRNA. In blocking experiments carried out to determine the possible involvement of CD44, chondrocytes were incubated with a mouse anti-CD44 monoclonal antibody (20 µg/ml; LS-C87848; LifeSpan Biosciences, Seattle, WA, USA) or isotype control IgG1 (10 µg/ml; Abcam, Cambridge, UK) 1 h prior to rhOPN treatment for 24 h.
Cell viability assay
Cell viability following treatment with rhOPN or siRNA for 24 h was determined using a colourimetric MTT assay. One day prior to the rhOPN or siRNA treatment, the cells were seeded into 96-well plates. After 24 h of rhOPN or siRNA treatment, culture medium was removed and 20 µl MTT solution (5 mg/ml in PBS) was added into each well and incubated at 37°C with 5% CO2 for 4 h. The supernatant was then aspirated and the formazan reaction products were dissolved using dimethyl sulphoxide (Sigma-Aldrich) solution and agitated for 15 min. Spectrophotometric absorbance was measured at 570 nm using a Multiskan MK3 ELISA plate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
RNA isolation, quantification and RT
Following treatment, the chondrocytes were lysed and total RNA was extracted with TRIzol® reagent (Invitrogen Life Technologies, Rockville MD, USA) according to the manufacturer's instructions. Total RNA was quantified using a Biochrom Libra S60 spectrophotometer (Biochrom Ltd, Cambridge, UK). A total of 1 µg RNA was converted to cDNA using a RevertAid™ First Strand cDNA Synthesis kit (Fermentas, Thermo Fisher Scientific, Inc.). First, all components were mixed, including the template RNA (1 µg), oligo (dT)18 primer (1 µl) and RNase-free water (to a total volume of 12 µl). The mixture was produced by gentle mixing, brief centrifugation at 8,000 × g for 15 sec and incubation at 65°C for 5 min. It was subsequently chilled on ice, centrifuged at 1,000 × g for 5 sec and the vial was placed back on ice. The following components were added: 5X Reaction buffer (4 µl), RiboLock™ RNase inhibitor (20 u/µl) (1 µl), 10 mM dNTP mix (2 µl) and RevertAid™ M-MuLV Reverse transcriptase (200 U/µl; 2 µl) to a final volume of 20 µl. This reaction mixture was incubated for 60 min at 42°C and terminated by heating at 70°C for 5 min. The cDNA products were stored in aliquots at −80°C until required.
qPCR assays
Primers were synthesized by Shanghai Genechem Co., Ltd (Shanghai, China). The sequences of the primers are as follows: OPN forward: 5′-GTGGGA AGG ACA GTT ATG AA-3′ and reverse: 5′-CTG ACT TTG GAA AGT TCC TG-3′; HIF-2α forward: 5′-GTG ACA TGA TCT TTC TGT CGG AA-3′ and reverse: 5′-CGC AAG GAT GAG TGA AGT CAAA-3′; GAPDH forward: 5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse: 5′-CAC CCT GTT GCT GTA GCC AAA-3′. The components used for qPCR were as follows: 12.5 µl Maxima® SYBR Green/ROX qPCR Master mix (2X; Thermo Fisher Scientific, Inc.), 2.5 µl forward primer (0.3 µM), 2.5 µl reverse primer (0.3 µM), 2 µl template cDNA and 5.5 µl RNase free water at a volume of 25 µl. The TP800 Thermal Cycler Dice Real Time system (Takara Bio, Inc., Otsu, Japan) was used for all qPCR. The PCR thermal conditions were as follows: 50°C uracil-DNA glycosylase (UDG; Roche Diagnostics, Basel, Switzerland) pretreatment for 2 min, 1 cycle at 95°C for 10 min for initial denaturation, 40 repeats of a 15 sec 95°C denaturation step, a 30 sec 30°C annealing step and a 30 sec extension step at 72°C. A melting curve was constructed following the final amplification period via a temperature gradient from 95°C for 15 sec, 55°C for 30 sec and 95°C for 15 sec. The GAPDH gene was used as an endogenous control. Relative expression levels of the genes of interest were calculated and expressed as 2−ΔΔCt. All quantities were expressed as n-fold relative to a calibrator.
Statistical analysis
Data were analyzed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA) for statistical evaluation. Data are expressed as the mean ± standard error of the mean. The statistical analysis of the differences between experimental groups was performed by the Student's t-test. One-way analysis of variance followed by the Student-Newman-Keuls test were used to analyze the differences among the three experimental groups. P<0.05 was considered to indicate a statistically significant difference.
Results
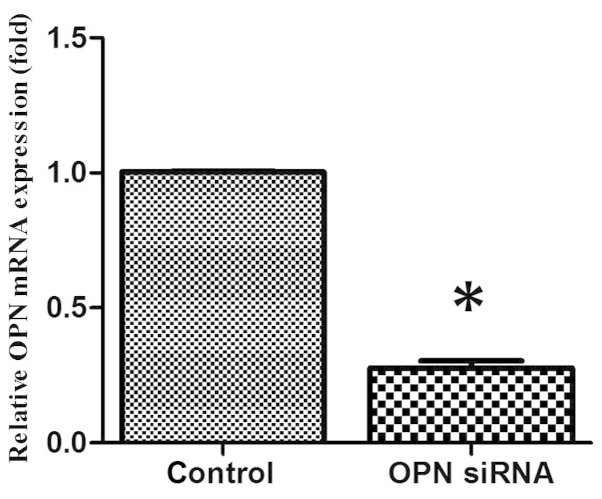
OPN siRNA effectively inhibits endogenous chondrocyte OPN expression in vitro
Chondrocytes were transfected with OPN siRNA oligonucleotides using Lipofectamine™ 2000. After 24 h of culture in vitro, OPN gene expression was analyzed by RT-qPCR. The OPN siRNA oligonucleotide was able to effectively suppress OPN gene expression in vitro when compared with the control group (Fig. 1). The OPN-specific siRNAs did not affect the expression of the housekeeping gene GAPDH (data not shown).
Figure 1.
Inhibition of endogenous osteopontin (OPN) expression by OPN small interfering RNA (siRNA). *P<0.05 compared with the control group.
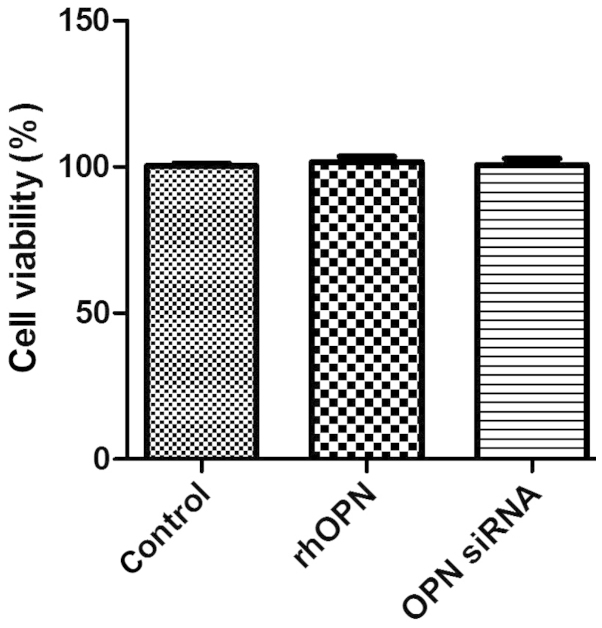
Cell viability
Fig. 2 shows the MTT data as the percentage of cell viability compared with that of the control. The results revealed that rhOPN and OPN siRNA, respectively, did not suppress human chondrocyte survival in vitro following incubation for 24 h (P>0.05).
Figure 2.
Cell viability detected by an MTT assay following treatment with recombinant human osteopontin (rhOPN) and osteopontin (OPN) small interfering RNA (siRNA). There were no significant differences (P>0.05) among the groups.
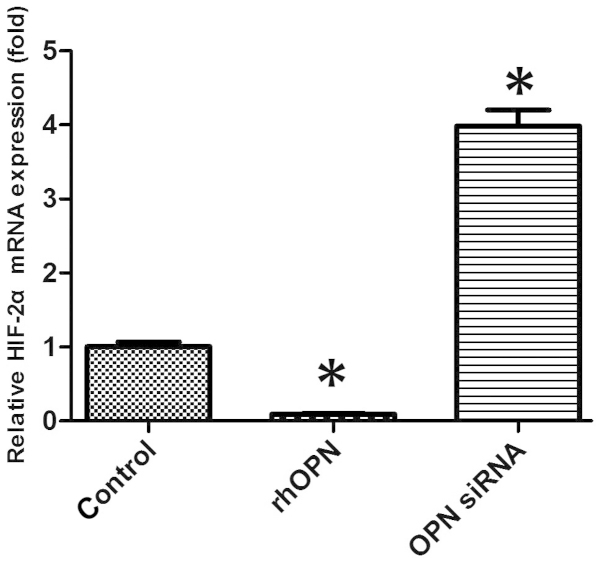
HIF-2α mRNA expression of chondrocytes in vitro
The mRNA expression level of HIF-2α was markedly decreased in the rhOPN group compared with the control group following 24 h of treatment. OPN siRNA, however, increased the HIF-2α mRNA expression level compared with that in the other groups (P<0.05; Fig. 3).
Figure 3.
Relative hypoxia-inducible factor (HIF)-2α mRNA expression was altered following treatment with recombinant human OPN (rhOPN) and osteopontin (OPN) small interfering RNA (siRNA). *P<0.05 vs. control group.
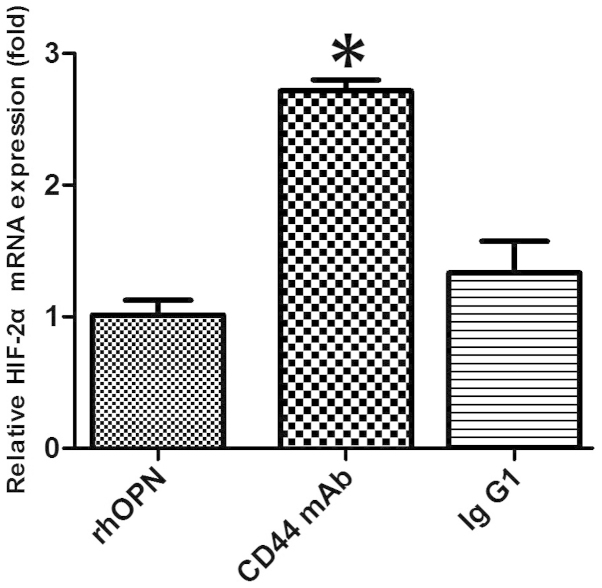
CD44-blocking mAb attenuates the inhibitory effect of OPN on HIF-2α mRNA expression
In the chondrocytes obtained from patients with OA, pretreatment with anti-CD44 blocking mAb caused the level of OPN-induced HIF-2α mRNA expression to increase when compared with that in the rhOPN group (P<0.05; Fig. 4). However, pretreatment with an isotype-matched control IgG1 had no significant effect on the HIF-2α mRNA expression level when compared with that in the rhOPN group (P>0.05; Fig. 4).
Figure 4.
CD44-blocking monoclonal antibody (mAb) suppressed the inhibitory effect of osteopontin (OPN) on the expression of hypoxia-inducible factor (HIF)-2α mRNA when compared with that in the recombinant human OPN (rhOPN) group (*P<0.05). Pretreatment with isotype control IgG1 had no significant effect on (HIF)-2α mRNA expression (P>0.05) when compared with that in the rhOPN group. HIF-2α, hypoxia-inducible factor-2α.
Discussion
Cartilage damage is one of the main pathological changes in OA. In a study of human OA cartilage samples conducted by Attur et al (17), it was found that the expression levels of OPN were increased in OA cartilage, with a significant upregulation of the expression of OPN mRNA compared with that in normal cartilage. The study also observed that the addition of recombinant OPN to the OA cartilage under ex vivo conditions inhibited the production of nitric oxide and prostaglandin E2. This suggests that OPN is overexpressed in OA cartilage and may function as an endogenous inhibitor of inflammatory mediators in cartilage (17). In another study, OPN deficiency was demonstrated to exacerbate aging-associated and instability-induced OA; the structural changes and loss of proteoglycan from cartilage tissue were shown to be greater in OPN-deficient mice than in wild-type mice (18). The study also found that OPN deficiency led to the induction of MMP-13. This indicates that OPN is involved in the progression of OA; however, the role of OPN in arthritis and joint diseases remains incompletely understood (18). Previous studies have shown that the level of OPN is elevated in OA plasma, cartilage and synovial fluid (6,7). RT-PCR is the preferred technique for the analysis of gene expression due to its high sensitivity. In particular, RT-qPCR has the advantages of a wide dynamic detection range and a higher reliability of results than conventional PCR (19). In RT-qPCR, DNA fragment amplification may be quantified using Taqman probes or SYBR Green fluorescence with equivalent accuracy, however SYBR Green is less expensive compared with the Taqman probes (20). In the current study, RT-qPCR assays were conducted with SYBR Green dye for quantification of the changes in the expression of different genes.
The present study showed that OPN is able to inhibit the expression of HIF-2α at the mRNA level in chondrocytes obtained from patients with OA of the knee. To the best of our knowledge, the current study is the first to report this association. Articular cartilage is an avascular connective tissue in which the availability of oxygen and glucose is significantly lower compared with that in the synovial fluid and plasma (11). Oxygen and nutrient maintenance is critical to cell fate, senescence and apoptosis (21). Previous studies have suggested that chondrocyte death plays a key role in cartilage degeneration (22–26). Chondrocyte cell death through apoptosis, necrosis, chondroptosis or a combination of these processes has been implicated in the pathogenesis of OA (26). The level of HIF-2α in human and mouse OA chondrocytes has been observed to be markedly elevated compared with normal chondrocytes, and to be associated with the increased apoptosis of articular chondrocytes (27). HIF-2α increases Fas-mediated chondrocyte apoptosis, which is associated with OA cartilage destruction (28). In addition, a previous study reported an enhanced expression of OPN under hypoxia; OPN is known to confer cytoprotection against hypoxia/reoxygenation-induced apoptosis (29). In the current study, HIF-2α mRNA expression was decreased with OPN upregulation, whereas it was increased with OPN downregulation. Since HIF-2α is a catabolic regulator of osteoarthritic cartilage destruction (16), the downregulation of HIF-2α by an elevated level of OPN may be a mechanism for the affected chondrocytes to return to a state of homeostasis. Thus, the present results indicate that OPN may play a protective role in OA, which has also been speculated in certain previous studies (17,18).
OPN interacts with integrin receptors and CD44 to initiate chemotaxis, promote cell adhesion and modulate cell function. CD44 is noted to be an important mediator in chondrocyte cell-matrix interactions that involve proteoglycan, hyaluronan or link protein aggregates (29–31). A previous study indicated that it is not necessary for each CD44 present on the chondrocyte cell to be occupied with hyaluronan in order to maintain a cell-associated matrix (21). A previous study demonstrated the re-expression of bovine chondrocyte cell surface CD44 following trypsin-treatment and indicated that only 25% of normal cell surface CD44 expression on bovine chondrocytes is required for the assembly of a hyaluronan-anchored, cell-associated matrix (32). In the present study, pretreatment of chondrocytes with anti-CD44 blocking mAb suppressed the inhibitory effect of OPN on the mRNA expression of HIF-2α. The results suggest that the inhibitory effects of OPN on HIF-2α in chondrocytes are mediated through the interaction of CD44 with OPN. Further studies are required to elucidate the OPN-CD44 downstream signaling pathway in OA chondrocytes.
In conclusion, the results of the present study indicate that OPN may play a protective role in OA by inhibiting HIF-2α gene expression in osteoarthritic chondrocytes via CD44 interaction.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 81272034), the Hunan Provincial Innovation Foundation for Postgraduate (no. CX2012B086) and the Fundamental Research Funds for the Central Universities of Central South University (no. 2013zzts081).
References
- 1.Gravallese EM. Osteopontin: A bridge between bone and the immune system. J Clin Invest. 2003;112:147–149. doi: 10.1172/JCI200319190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang FJ, Gao SG, Cheng L, et al. The effect of hyaluronic acid on osteopontin and CD44 mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int. 2013;33:79–83. doi: 10.1007/s00296-011-2339-3. [DOI] [PubMed] [Google Scholar]
- 3.Denhardt DT, Noda M. Osteopontin expression and function: Role in bone remodeling. J Cell Biochem Suppl. 1998;30–31:92–102. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<92::AID-JCB13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Lampe MA, Patarca R, Iregui MV, Cantor H. Polyclonal B cell activation by the Eta-1 cytokine and the development of systemic autoimmune disease. J Immunol. 1991;147:2902–2906. [PubMed] [Google Scholar]
- 5.Pullig O, Weseloh G, Gauer S, Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: Protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol. 2000;19:245–255. doi: 10.1016/S0945-053X(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 6.Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem. 2009;42:808–812. doi: 10.1016/j.clinbiochem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage. 2010;18:82–87. doi: 10.1016/j.joca.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 9.Dunn S, Kolomytkin OV, Waddell DD, Marino AA. Hyaluronan-binding receptors: Possible involvement in osteoarthritis. Mod Rheumatol. 2009;19:151–155. doi: 10.3109/s10165-008-0136-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang FJ, Luo W, Gao SG, et al. Expression of CD44 in articular cartilage is associated with disease severity in knee osteoarthritis. Mod Rheumatol. 2013;23:1186–1191. doi: 10.3109/s10165-012-0818-3. [DOI] [PubMed] [Google Scholar]
- 11.Milner PI, Fairfax TP, Browning JA, Wilkins RJ, Gibson JS. The effect of O2 tension on pH homeostasis in equine articular chondrocytes. Arthritis Rheum. 2006;54:3523–3532. doi: 10.1002/art.22209. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 13.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 17.Attur MG, Dave MN, Stuchin S, et al. Osteopontin: An intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum. 2001;44:578–584. doi: 10.1002/1529-0131(200103)44:3<578::AID-ANR106>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Iwasaki N, Kon S, et al. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009;60:2362–2371. doi: 10.1002/art.24705. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm J, Pingoud A. Real-time polymerase chain reaction. ChemBioChem. 2003;4:1120–1128. doi: 10.1002/cbic.200300662. [DOI] [PubMed] [Google Scholar]
- 20.Ponchel F, Toomes C, Bransfield K, et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martens G, Cai Y, Hinke S, Stangé G, Van de Casteele M, Pipeleers D. Nutrient sensing in pancreatic beta cells suppresses mitochondrial superoxide generation and its contribution to apoptosis. Biochem Soc Trans. 2005;33:300–301. doi: 10.1042/BST0330300. [DOI] [PubMed] [Google Scholar]
- 22.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001;3:107–13. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 24.Kühn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Zamli Z, Sharif M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int J Rheum Dis. 2011;14:159–166. doi: 10.1111/j.1756-185X.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JH, Shin Y, Huh YH, Yang S, Chun CH, Chun JS. Hypoxia-inducible factor-2 α regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ. 2012;19:440–450. doi: 10.1038/cdd.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Denhardt DT, Cao H, et al. Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene. 2005;24:6555–6563. doi: 10.1038/sj.onc.1208800. [DOI] [PubMed] [Google Scholar]
- 29.Underhill C. CD44: The hyaluronan receptor. J Cell Sci. 1992;103:293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- 30.Ishii S, Ford R, Thomas P, Nachman A, Steele G, Jr, Jessup JM. CD44 participates in the adhesion of human colorectal carcinoma cells to laminin and type IV collagen. Surg Oncol. 1993;2:255–264. doi: 10.1016/0960-7404(93)90015-Q. [DOI] [PubMed] [Google Scholar]
- 31.Isacke CM. The role of the cytoplasmic domain in regulating CD44 function. J Cell Sci. 1994;107:2353–2359. doi: 10.1242/jcs.107.9.2353. [DOI] [PubMed] [Google Scholar]
- 32.Knudson CB, Nofal GA, Pamintuan L, Aguiar DJ. The chondrocyte pericellular matrix: A model for hyaluronan-mediated cell-matrix interactions. Biochem Soc Trans. 1999;27:142–147. doi: 10.1042/bst0270142. [DOI] [PubMed] [Google Scholar]