Abstract
Esophageal squamous cell carcinoma (SCC) possesses one of the worst prognoses out of the digestive carcinomas. Several studies have suggested that transforming growth factor β receptor type II (TGF-βRII), Smad family member 4 (Smad4) and p21 wild-type p53-activated factor 1 (p21waf1) are associated with esophageal SCC. The aim of the present study was to evaluate the effect of Smad4, TGF-βRII and p21waf1 in esophageal squamous cancer tissue and the pathological significance of the effect. An immunohistochemical method was used to evaluate the expression levels of Smad4, TGF-βRII and p21waf1 in specimens of esophageal SCC lesions obtained from 80 patients. It was found that the expression of Smad4, TGF-βRII and p21waf1 in histologically-classified grade I esophageal SCC, without invasion or lymph node metastasis, was markedly higher compared with grade III esophageal SCC that had invaded into the deep muscular or serous layer and metastasized to the lymph nodes (P<0.05). Analysis of the expression level of Smad4, TGF-βRII and p21waf1, as well as the clinical and pathological characteristics of esophageal SCC, revealed that the three proteins may be associated with the carcinogenesis, biological behavior and prognosis of esophageal SCC, parallel to the pathological stage and cell grade.
Keywords: transforming growth factor β receptor type II, p21waf1, esophageal squamous cell neoplasms, Smad family member 4
Introduction
Esophageal squamous cell carcinoma (SCC) possesses one of the worst prognoses out of the digestive carcinomas. At present, early detection is the best and only opportunity to improve the prognosis of esophageal SCC (1) Transforming growth factor β (TGF-β) plays an important role in numerous critical cellular processes, including regulation of the cell cycle, cell differentiation and extracellular matrix synthesis. One of the most prominent activities of TGF-β is the inhibition of cell proliferation. TGF-β receptor type II (TGF-βRII) is required for the growth-inhibitory effects of TGF-β on proliferating epithelial cells. TGF-βRII mutations have been identified in a broad spectrum of human epithelial malignancies, including colon and gastric cancers, and are strongly associated with the development of TGF-β resistance in the cell lines derived from these tumors (2). Altered immunohistochemical expression of TGF-βRII in bladder carcinoma and pancreatic cancer (3) has been associated with an advanced pathological stage, but Fukai et al suggested that the reduced expression of TGF-β receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma (4). Additionally, the present study focuses on the association between TGF-βRII expression and the pathological characteristics of patients with esophageal SCC.
The Smad protein family is a family of intracellular signal transducers that act downstream of receptors for TGF-β family members (2). Smad family member 4 (Smad4), which is considered to be a tumor suppressor gene, is a common mediator of TGF-β superfamily signaling and plays a role in TGF-β induced growth inhibition (5). In pancreatic and colorectal cancer, the inactivation of the Smad4 gene through mutation occurs frequently in association with malignant progression (6). Lv et al (7) suggested that Smad4 was associated with the prognosis of esophageal SCC. The present study aims to clarify the association between the expression of the Smad4 protein and the clinicopathological features of SCC, which remains unclear at present (2).
The protein product of the p21 wild-type p53-activated factor 1 (p21waf1) gene is a negative regulatory element of the cell cycle. The function of p21waf1 is mediated by the inhibition of G1 cyclin-dependent kinase (CKD) complexes, thereby inhibiting cell cycle progression and cell growth (8). In previous years, the expression of p21waf1 has been investigated in esophageal SCC and in tumors of other tissues, including the larynx, stomach and head and neck (9,10). Several studies have revealed the prognostic significance of p21 expression in patients with esophageal squamous cell carcinoma (11–13). p21waf1 overexpression has been reported to be associated with a worse prognosis in bladder (14), ovarian (15), breast (16) and esophageal carcinomas (17,18), and also in oral SCC (19).
In the present study, the changes in the expression of Smad4, TGF-βRII and p21waf1 were investigated in esophageal SCC by immunohistochemical staining, and the association between the expression of these proteins and the clinicopathological features of the patient was also examined.
Materials and methods
Tumor samples
In total, 80 esophageal SCC samples were obtained from patients that were consecutively recruited at Renmin Hospital of Wuhan University (Wuhan, China) between 1 January 2008 and 30 December 2012. The patients consisted of 49 men and 31 women, with a mean age of 63.0 years (range, 45–72 years). The histopathology of the lesions was graded according to the World Health Organization (WHO) classification system (20), and the esophageal SCC tissue samples were obtained from 21 patients with lesions that were classified as stage I, 24 as stage II and 35 as stage III. Cancer cells had infiltrated to the deep muscularis or serosa in 65 patients and regional lymph node metastasis was observed in 47 patients. The study was approved by the ethics committee of Renmin Hospital of Wuhan University and written informed consent was obtained from all patients.
Immunohistochemical SP assay
Paraffin-embedded esophageal SCC tumor tissues and normal esophageal tissues were used to identify the expression of Smad4, TGF-βRII and p21waf1. Sections of tissue, 4–6 µm thick, were deparaffinized in xylene, followed by treatment with a graded series of 100, 95 and 80% ethanol, dilution with double-distilled H2O (v/v), and rehydrated in phosphate-buffered saline (PBS; pH 7.5). The sections analyzed for p21waf1 and Smad4 expression were microwaved for 5 min for antigen retrieval. For the detection of TGF-βRII, sections of paraffin-embedded tissues were treated with pepsin (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at 37°C and then washed with PBS. All samples were incubated with 3% hydrogen peroxide in methanol (v/v) for 12 min to block endogenous peroxidase, washed with PBS (pH 7.5), and incubated in protein blocking solution, comprising 5% normal human serum and 0.5% normal goat serum in PBS (v/v), for 30 min. Sections were analyzed for the expression of Smad4, TGF-βRII and p21waf1 using the following primary antibodies, respectively: Smad4 antibody, mouse monoclonal IgG (catalog no. sc-7966, Santa Cruz Biotechnology, Dallas, TX, USA); TGF βRII antibody, rabbit polyclonal IgG (catalog no. sc-400, Zhongshan Jingqiao Biotechnology Company, Beijing, China); and p21waf1 antibody, mouse monoclonal IgG (catalog no. sc-6246, Zhongshan Jingqiao Biotechnology Company). Sections were incubated with the primary antibody (dilution, 1:100) in a humidified chamber for 2 h at 37°C and then rinsed three times with PBS.
The sections were subsequently incubated for 1 h with the corresponding peroxidase-conjugated secondary antibody [goat anti-mouse IgG for Smad4 and P21waf1 (catalog no. sc-2005), and goat anti-rabbit IgG for TGF-βRII (catalog no. sc-2004); dilution, 1:200; Santa Cruz Biotechnology] for 1 h at room temperature. Positive reactions were visualized by incubating the slides with stable 3,3′-diaminobenzidine (DAB) for 5–10 min (Zhongshan Jinqiao Biotechnology Co., Ltd.). The sections were rinsed with distilled water, counterstained with Gill's hematoxylin for 1 min (Sigma-Aldrich, St. Louis, MO, USA), and images were captured using a BX53 Olympus upright microscope (Olympus, Tokyo, Japan). The mean density of the sections was calculated using Image-Pro Plus 4.5 software automatic counting (Media Cybernetics, Inc., Rockville, MD, USA).
An immunohistochemical Substance P assay kit was purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. and performed according to the manufacturer's instructions. The results were observed under a light microscope. The cells exhibiting granular brown substances in the cytoplasm or membrane were defined as Smad4- and TGF-βRII-positive. Staining in the nucleus was defined as p21waf1-positive. Normal esophageal tissues were stained as the controls. Immunoreactivity was evaluated by two observers using a blind procedure. Compared with the control, the slides with the same or higher staining of Smad4 were defined as Smad4-positive and those with >25% stained cells in a slide were considered TGF-βRII- and p21waf1-positive.
Statistical analyses
Exact probability tests and χ2 tests were used for intergroup comparisons, and P<0.05 was considered to indicate a statistically significant difference.
Results
Immunohistochemical analysis Smad4, TGFβRII and p21waf1 expression decreased with increasing malignancy of the esophageal SCC tumor tissues
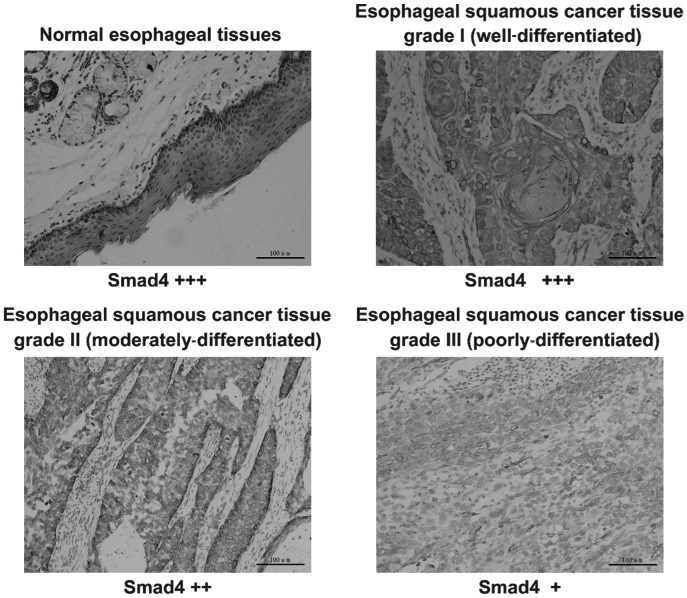
In total, 80 tissue samples obtained from patients diagnosed with esophageal SCC were classified as grades I–III, according to the WHO classification system. In all samples, Smad4 and TGF-βRII staining was restricted to the tumor epithelial cells and no staining was observed in the fibrocytes associated with the desmoplastic reaction. Smad4 and TGF-βRII were predominantly expressed in the cytoplasm (Figs. 1 and 2), while p21waf1 was predominantly expressed in the nucleus (Fig. 3).
Figure 1.
Immunohistochemical detection of Smad4 in in esophageal squamous cell carcinoma tissue samples, classified as grades I–III according to the World Health Organization classification, and normal esophageal tissues. The expression of Smad4 demonstrates grade dependency. The staining intensity varied between specimens, with the staining in normal esophageal tissues and well-differentiated tissues being strong (+++), the staining in moderately-differentiated tissues being moderate (++) and the staining of poorly-differentiated tissues being weak (+). Magnification, x200. Smad 4, Smad family member 4.
Figure 2.
Representative immunohistochemical staining of the TGF-βRII protein in esophageal squamous cell carcinoma tumor tissues and normal esophageal tissues. Normal esophagela tissues exhibited strong (+++) TGF-βRII expression. Strong expression (+++) of TGF-βRII was also present in well-differentiated esophageal squamous cell carcinoma tissues. Moderate expression (++) of TGF-βRII was observed in moderately-differentiated tissues, and weak expression (+) was identified in poorly-differentiated esophageal squamous tumor tissues. Magnification, x200. TGF-βRII, transforming growth factor β receptor type II.
Figure 3.
Immunohistochemical analysis of p21waf1 expression in esophageal squamous cell carcinoma tissues of increasing malignancy, revealing that p21waf1 expression decreased with increasing malignancy of esophageal squamous cell carcinoma. p21waf1 is strongly expressed in the nuclei. Magnification, x200. p21waf1, p21 wild-type p53-activated factor 1; +++, strong expression; ++, moderate expression; +, weak expression; ±, non-positive expression.
In the normal tissue surrounding malignant esophageal SCC tumors and well-differentiated esophageal squamous grade I tumor regions, there was a distinct staining of Smad4 and TGF-βRII within the epithelial cells. In moderately-differentiated esophageal SCC grade I tumor regions, the cells demonstrated a less prominent expression of Smad4 and TGF-βRII. The poorly-differentiated esophageal SCC grade III tumor tissues also exhibited weak expression of Smad4 and TGF-βRII (Figs. 1 and 2). Additionally, immunostaining for p21waf1 revealed strong expression in well-differentiated tumor tissue, whereas weak expression was demonstrated in poorly-differentiated tumor regions (Fig. 3). Immunohistochemical analysis of esophageal SCC tissue samples of increasing malignancy revealed that the expression of Smad4, TGF-βRII and p21waf1 decreased with the increasing malignancy of the esophageal SCC tumor tissues (Figs. 1–3).
The relative cell counts of the immunopositive Smad4, TGF-βRII and p21waf1 cells were obtained as a percentage of the total number of cells within the same areas, as exhibited in Fig. 4. Significant differences were identified between the calculated relative cell counts of Smad4-positive cells in grade I (70.75%), grade II (41.25%) and grade III (19.25%) esophageal SCC tissue samples. The proportion of TGF-βRII-positive cells was highest in tissue samples classified as grade I (68.25%), followed by grade II (34.25%) and grade III (17.75%) tissues. The proportion of p21waf1-positive cells was also highest in grade I tissue samples (40.75%), followed by samples classified as grade II (16.50%) and grade III (7.25%) (Fig. 4).
Figure 4.
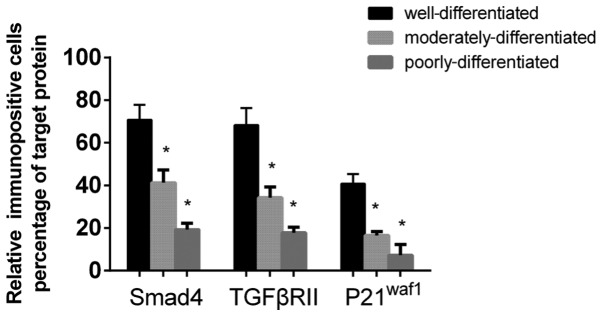
Bar graph revealing the percentage of Smad4-, TGF-βRII- and p21waf1-positive immunohistochemically stained cells in 80 esophageal squamous cell carcinoma tissue samples at various stages of differentiation. Smad4, Smad family member 4; TGFβRII, transforming growth factor β receptor type II; p21waf1, p21 wild-type p53-activated factor 1. *P<0.05.
Correlation between the expression of Smad4, TGF-βRII and p21waf1 and the clinicopathological characteristics of esophageal SCC
Evaluation of the clinical significance of the expression of Smad4, TGF-βRII and p21waf1 revealed that the expression of the three proteins was significantly associated with the degree of differentiation of the cells (P<0.05), the histopathological stage of the lesions (P<0.05), the occurrence of lymph node metastasis (P<0.05) and the depth of tumor invasion (P<0.05). It was also found that the rate of detection of positive expression was significantly higher (P<0.05) in samples that were histopathologically classified as grade I, exhibited invasion of less than two-thirds of the depth of the muscularis and did not metastasize to the lymph nodes, compared with grade III tissue samples that demonstrated invasion of over two-thirds of the depth of the muscularis and resulted in lymph node metastasis (Table I). Overall, the present results revealed that the expression of Smad4, TGF-βRII and p21waf1 was significantly associated with malignancy of the esophageal SCC tumor tissues (Table I).
Table I.
Correlation between Smad4, TGF-βRII and p21waf1 expression and the clinicopathological characteristics of esophageal squamous cell carcinoma patients.
| Smad4 expression, n | TGF-βRII expression, n | p21waf1 expression, n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| clinicopathological characteristics | n | Yes | No | P-value | Yes | No | P-value | Yes | No | P-value | |
| Histopathology grade | |||||||||||
| I–II | 45 | 25 | 20 | 20 | 25 | 27 | 18 | ||||
| III | 35 | 10a | 25 | <0.05 | 5a | 30 | <0.05 | 8a | 27 | <0.05 | |
| Differentiation | |||||||||||
| Well-differentiated | 20 | 15 | 5 | 11 | 9 | 14 | 6 | ||||
| Moderately-differentiated | 40 | 16a | 24 | <0.05 | 10a | 30 | <0.05 | 16a | 24 | <0.05 | |
| Poorly-differentiated | 20 | 4 | 16 | 4 | 16 | 5 | 15 | ||||
| Invasion into muscularis, fraction of total depth | |||||||||||
| ≤2/3 | 65 | 32 | 33 | 28 | 37 | 30 | 35 | ||||
| >2/3 | 15 | 3a | 12 | <0.05 | 5a | 10 | <0.05 | 5a | 10 | <0.05 | |
| Lymph node metastasis | |||||||||||
| No | 33 | 19 | 14 | <0.05 | 17 | 16 | <0.05 | 20 | 13 | <0.05 | |
| Yes | 47 | 16a | 31 | <0.05 | 8 | 39 | <0.05 | 15a | 32 | <0.05 | |
P<0.05 vs. well-differentiated group. Smad4, Smad family member 4; TGF-βRII, transforming growth factor β receptor type II; p21waf1, p21 wild-type p53-activated factor 1.
Discussion
The present study demonstrated that the change in the expression of Smad4, TGF-βRII and p21waf1 is a common phenomenon in esophageal SCC lesions, and also that this change is strongly associated with tumor cell differentiation. In addition, the association between the expression of Smad4, TGF-βRII and p21waf1 and the pathological tumor variables, the clinicopathological features, was immunohistologically examined in patients with esophageal SCC.
In the current study, Smad4 expression was observed in the cytoplasm in esophageal SCC cells. This was consistent with numerous previous studies performed using various carcinomas, and was similar to the results that have been reported in colon and gastric carcinoma (21,22). In the present study, Smad4 expression was inversely associated with the depth of tumor invasion. Smad4 expression was low when the proliferative activity of esophageal SCC was high, particularly in lesions that were classed as poorly-differentiated esophageal SCC grade III. The loss or reduction in the expression of Smad4 was also significantly correlated with the grade of differentiation exhibited by the carcinoma. This finding is consistent with the results reported by Miyaki et al (23). This previous study found that the frequency of Smad4 mutations was also associated with the stage of the colorectal cancer lesions (23). Inactivating mutations in TGF-βRII and its downstream effector Smad4 have been found in numerous gastrointestinal cancers (24). Smad4 acts as a central mediator of TGF-β signaling (25,26) and simultaneously acts as a tumor suppressor gene (27,28). Therefore, it is hypothesized that the loss of Smad4 expression is at least partially responsible for the resistance of esophageal SCC to the antiproliferative effects of TGF-β (5).
Macroscopic structural mutations of TGF-βRII have been observed in numerous cancer types (29) and it has previously been reported that mutation of the TGF-βRII gene is rarely found in patients with esophageal SCC (30). By contrast, the present study immunohistochemically demonstrated that the expression of TGF-βRII was correlated with the depth of invasion, lymph node metastasis and pathological stage in esophageal SCC. It was also identified that the degree of receptor expression appeared to be inversely correlated with the degree of differentiation of the tumor and with the depth of the invasion. Certain studies have found that during tumor initiation and early progression, TGF-β is often a tumor suppressor (31,32). However, late in tumor progression TGF-β signaling promotes tumorigenesis and demonstrates the potential to positively or negatively regulate the metastasis of the transformed epithelial cells (31,32), a finding that was not confirmed in the present study.
The tumor suppressor p21waf1 is among the most important known gene products involved in cell growth arrest, differentiation and senescence. p21waf1 is able to inhibit the activity of CDKs, resulting in cellular growth suspension at the G1/S phase, thus suppressing tumor proliferation. In the present study, undifferentiated carcinomas exhibited a marked loss of p21waf1 expression, whereas well- to moderately-differentiated tumors demonstrated higher levels of p21waf1 expression. Similarly, a correlation between p21waf1 expression and tumor differentiation has been identified in human cutaneous SCCs and head and neck SCCs (9,33,34). In addition, in the present study, p21waf1 expression was significantly associated with invasion depth (P<0.05), lymph node metastasis (P<0.05) and clinical stage (P<0.05) of esophageal SCC. el-Deiry et al (35) demonstrated that p21 is involved in the control of proliferation in normal digestive mucosa and that such control is lost in tumor cells, which is consistent with the present finding of an association between p21waf1 expression and the progression of esophageal carcinoma. It has been reported that p21waf1 mediates the growth response to TGF-β in human epithelial cells and that the absence of p21waf1 may be one method used by cancer cells to escape the growth inhibitory effects of TGF-β (36). The fact that high p21waf1 expression significantly correlates with poorer clinical outcome is in agreement with previous studies conducted on carcinomas, such as prostate carcinoma (37), superficial bladder tumors (38) and gastric carcinoma (39). The opposite results have also been reported from studies of several other types of tumor, including breast (40), lung (41) and laryngeal (42) carcinoma. Additional studies are therefore required to clarify the association between p21waf1 expression and the clinical outcome in esophageal SCC.
In conclusion, the overexpression of Smad4, TGF-βRII and p21waf1 was found to be associated with the stage of tumor progression, including the depth of invasion and presence of lymph node metastasis. However, additional studies are required to clarify the role of the expression of Smad4, TGF-βRII and p21waf1 in the prognosis of esophageal SCC. To conclude, the present study indicates that the expression of Smad4, TGF-βRII and p21waf1 may be a useful indicator for the treatment of esophageal SCC.
Acknowledgements
The authors would like to thank the Director of the Department of Pathology, Zesheng Wang, for the advice and stimulating discussion, Cheng Cheng for the critical reading of the original manuscript and Benhui Li for excellent technical assistance.
References
- 1.Shirakawa Y, Naomoto Y, Kimura M, et al. Topological analysis of p21WAF1/CIP1 expression in esophageal squamous dysplasia. Clin Cancer Res. 2000;6:541–550. [PubMed] [Google Scholar]
- 2.Natsugoe S, Xiangming C, Matsumoto M, et al. Smad4 and transforming growth factor β1 expression in patients with squamous cell carcinoma of the esophagus. Clin Cancer Res. 2002;8:1838–1842. [PubMed] [Google Scholar]
- 3.Friess H, Yamanaka Y, Büchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 4.Fukai Y, Fukuchi M, Masuda N, et al. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer. 2003;104:161–166. doi: 10.1002/ijc.10929. [DOI] [PubMed] [Google Scholar]
- 5.Fukuchi M, Masuda N, Miyazaki T, et al. Decreased Smad4 expression in the transforming growth factor-beta signaling pathway during progression of esophageal squamous cell carcinoma. Cancer. 2002;95:737–743. doi: 10.1002/cncr.10727. [DOI] [PubMed] [Google Scholar]
- 6.Wang LH, Kim SH, Lee JH, et al. Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res. 2007;13:102–110. doi: 10.1158/1078-0432.CCR-06-1467. [DOI] [PubMed] [Google Scholar]
- 7.Lv J, Cao XF, Ji L, et al. Association of β-catenin, Wnt1, Smad4, Hoxa9 and Bmi-1 with the prognosis of esophageal squamous cell carcinoma. Med Oncol. 2012;29:151–160. doi: 10.1007/s12032-010-9816-5. [DOI] [PubMed] [Google Scholar]
- 8.Patiño-García A, Sotillo-Piñeiro E, Sierrasesúmaga-Ariznabarreta L. p21WAF1 mutation is not a predominant alteration in pediatric bone tumors. Pediatr Res. 1998;43:393–395. doi: 10.1203/00006450-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Nadal A, Jares P, Cazorla M, et al. p21WAF1/Cip1 expression is associated with cell differentiation but not with p53 mutations in squamous cell carcinomas of the larynx. J Pathol. 1997;183:156–163. doi: 10.1002/(SICI)1096-9896(199710)183:2<156::AID-PATH908>3.3.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Seta T, Imazeki F, Yokosuka O, et al. Expression of p53 and p21WAF1/CIP1 proteins in gastric and esophageal cancers: comparison with mutations of the p53 gene. Dig Dis Sci. 1998;43:279–289. doi: 10.1023/A:1018889818855. [DOI] [PubMed] [Google Scholar]
- 11.Shiozaki A, Nakashima S, Ichikawa D, et al. Prognostic significance of p21 expression in patients with esophageal squamous cell carcinoma. Anticancer Res. 2013;33:4329–4335. [PubMed] [Google Scholar]
- 12.Yang G, Zhang Z, Liao J, et al. Immunohistochemical studies on Waf1p21, p16, pRb and p53 in human esophageal carcinomas and neighboring epithelia from a high-risk area in northern China. Int J Cancer. 1997;72:746–751. doi: 10.1002/(SICI)1097-0215(19970904)72:5<746::AID-IJC7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Nemoto T, Eishi Y, et al. Expression of the cyclin dependent kinase inhibitor p21WAF1/CIP1 in oesophageal squamous cell carcinomas. Virchows Arch. 1997;430:389–395. doi: 10.1007/s004280050048. [DOI] [PubMed] [Google Scholar]
- 14.Garcia del Muro X, Condom E, Vigues F, et al. p53 and p21 expression levels predict organ preservation and survival in invasive bladder carcinoma treated with a combined-modality approach. Cancer. 2004;100:1859–1867. doi: 10.1002/cncr.20200. [DOI] [PubMed] [Google Scholar]
- 15.Rose SL, Goodheart MJ, DeYoung BR, Smith BJ, Buller RE. p21 expression predicts outcome in p53-null ovarian carcinoma. Clin Cancer Res. 2003;9:1028–1032. [PubMed] [Google Scholar]
- 16.Caffo O, Doglioni C, Veronese S, et al. Prognostic value of p21(WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res. 1996;2:1591–1599. [PubMed] [Google Scholar]
- 17.Lam KY, Law S, Tin L, Tung PH, Wong J. The clinicopathological significance of p21 and p53 expression in esophageal squamous cell carcinoma: an analysis of 153 patients. Am J Gastroenterol. 1999;94:2060–2068. doi: 10.1111/j.1572-0241.1999.01278.x. [DOI] [PubMed] [Google Scholar]
- 18.Sarbia M, Stahl M, zur Hausen A, et al. Expression of p21WAF1 predicts outcome of esophageal cancer patients treated by surgery alone or by combined therapy modalities. Clin Cancer Res. 1998;4:2615–2623. [PubMed] [Google Scholar]
- 19.Erber R, Klein W, Andl T, et al. Aberrant p21(CIP1/WAF1) protein accumulation in head-and-neck cancer. Int J Cancer. 1997;74:383–389. doi: 10.1002/(SICI)1097-0215(19970822)74:4<383::AID-IJC4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Gabbert HE, Nakamura Y, Shimoda T, Field JK, Hainaut P, Inoue H. Squamous cell carcinoma of the oesophagus. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Digestive System. IARCPress; Lyon, France: 2000. pp. 16–32. [Google Scholar]
- 21.Korchynskyi O, Landström M, Stoika R, et al. Expression of Smad proteins in human colorectal cancer. Int J Cancer. 1999;82:197–202. doi: 10.1002/(SICI)1097-0215(19990719)82:2<197::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Xiangming C, Natsugoe S, Takao S, Hokita S, Ishigami S, et al. Preserved Smad4 expression in the transforming growth factor beta signaling pathway is a favorable prognostic factor in patients with advanced gastric cancer. Clin Cancer Res. 2001;7:277–282. [PubMed] [Google Scholar]
- 23.Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18:3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- 24.Seoane J. p21(WAF1/CIP1) at the switch between the anti-oncogenic and oncogenic faces of TGFbeta. Cancer Biol Ther. 2004;3:226–227. doi: 10.4161/cbt.3.2.717. [DOI] [PubMed] [Google Scholar]
- 25.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 26.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 27.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 28.Schutte M, Hruban RH, Hedrick L, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 29.Wang J, Sun L, Myeroff L, et al. Demonstration that mutation of the type II transforming growth factor beta receptor inactivates its tumor suppressor activity in replication error-positive colon carcinoma cells. J Biol Chem. 1995;270:22044–22049. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- 30.Osawa H, Shitara Y, Shoji H, et al. Mutation analysis of transforming growth factor beta type II receptor, Smad2, Smad3 and Smad4 in esophageal squamous cell carcinoma. Int J Oncol. 2000;17:723–728. doi: 10.3892/ijo.17.4.723. [DOI] [PubMed] [Google Scholar]
- 31.Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 33.Bullock R, Maxwell WL, Graham DI, Teasdale GM, Adams JH. Glial swelling following human cerebral contusion: an ultrastructural study. J Neurol Neurosurg Psychiatry. 1991;54:427–434. doi: 10.1136/jnnp.54.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Violette F, Senelar R. Pulmonary vascular lesions caused by explosive decompression in the dog. J Physiol (Paris) 1958;50:556–557. (In French) [PubMed] [Google Scholar]
- 35.el-Deiry WS, Tokino T, Waldman T, et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–2919. [PubMed] [Google Scholar]
- 36.Bachman KE, Blair BG, Brenner K, et al. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3:221–225. doi: 10.4161/cbt.3.2.666. [DOI] [PubMed] [Google Scholar]
- 37.Omar EA, Behlouli H, Chevalier S, Aprikian AG. Relationship of p21(WAF-I) protein expression with prognosis in advanced prostate cancer treated by androgen ablation. Prostate. 2001;49:191–199. doi: 10.1002/pros.1134. [DOI] [PubMed] [Google Scholar]
- 38.Zlotta AR, Noel JC, Fayt I, et al. Correlation and prognostic significance of p53, p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumors treated with bacillus Calmette-Guerin intravesical therapy. J Urol. 1999;161:792–798. doi: 10.1097/00005392-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Noda H, Maehara Y, Irie K, Kakeji Y, Yonemura T, Sugimachi K. Growth pattern and expressions of cell cycle regulator proteins p53 and p21WAF1/CIP1 in early gastric carcinoma. Cancer. 2001;92:1828–1835. doi: 10.1002/1097-0142(20011001)92:7<1828::AID-CNCR1699>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M, Shao ZM, Wu J, et al. p21/waf1/cip1 and mdm-2 expression in breast carcinoma patients as related to prognosis. Int J Cancer. 1997;74:529–534. doi: 10.1002/(SICI)1097-0215(19971021)74:5<529::AID-IJC9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Komiya T, Hosono Y, Hirashima T, et al. p21 expression as a predictor for favorable prognosis in squamous cell carcinoma of the lung. Clin Cancer Res. 1997;3:1831–1835. [PubMed] [Google Scholar]
- 42.Jeannon JP, Soames J, Lunec J, Awwad S, Ashton V, Wilson JA. Expression of cyclin-dependent kinase inhibitor p21(WAF1) and p53 tumour suppressor gene in laryngeal cancer. Clin Otolaryngol Allied Sci. 2000;25:23–27. doi: 10.1046/j.1365-2273.2000.00318.x. [DOI] [PubMed] [Google Scholar]