Abstract
The aim of the present study was to determine the relative quantitative expression of hypoxia-inducible factor (HIF)-1α, −2α and −3α, and VEGF-A in laryngeal carcinoma. A total of 63 patients with carcinoma of the larynx were enrolled in the study. Total RNA was isolated from fresh, frozen normal and tumor tissues of each patient, and quantitative polymerase chain reaction was performed. HIF-1α was upregulated in the majority of patients (44 patients; 69.84%). By contrast, only 7 (11.11%) patients from the whole group displayed HIF-2α overexpression, while the HIF-3α isoform was silenced in the majority of patients (48 patients, 76.19%). A small group of 5 (7.94%) patients exhibited significant overexpression of the HIF-3α isoform. VEGF-A expression was significantly higher (P<0.05) in patients with upregulated HIF-1α (2.72±1.41 RQ) compared with patients without upregulated HIF-1α (1.86±1.46 RQ). There was a moderate positive correlation between mRNA expression levels of HIF-1α and VEGF-A (rs=0.392; P<0.005). To the best of our knowledge, this study is first to report quantitative data with regard to the expression of all three HIF isoforms in malignant neoplasms. The findings suggest the existence of specific phenotypes of HIF expression in laryngeal carcinoma, where the HIF switch is absent.
Keywords: laryngeal carcinoma, hypoxia-inducible factor-1α, hypoxia-inducible factor-2α, hypoxia-inducible factor-3α, vascular endothelial growth factor A
Introduction
It is a well-established fact that rapid cell division in solid tumors leads to depletion of the oxygen levels and variable levels of hypoxia across the tumor. The latter is a major driving force for the process of endothelial proliferation and tumor angiogenesis. Squamous cell carcinoma of the larynx is the most common neoplasm of the head and neck. It is well-known that the rapid proliferation of malignant cells and the irregular local vasculature jointly favor the formation of hypoxic areas within human solid tumors including laryngeal cancer. Despite the vast amount of papers, there is a lack of quantitative studies reporting the levels of expression of hypoxia-inducible factors in this neoplasm. Hypoxia-inducible factors (HIFs) are essential in the primary transcriptional responses to hypoxic stress in normal and neoplastic cells. These molecules are heterodimeric transcription factors that activate a large number of target genes, including phosphoglycerate kinase and vascular endothelial growth factor (VEGF) A. This leads to increased glycolysis, endothelial proliferation and angiogenesis, which facilitates the adaptation of the tumor to hypoxia. HIFs are composed of α and β subunits; three α isoforms exist, which are normally rapidly degraded in an oxygen-dependent manner (1–5), while the β subunit is expressed at constant level under normoxic conditions. HIF-1α and HIF-2α (also known as EPAS1) are structurally similar, and activate the transcription of target genes by binding to hypoxia response elements (HREs) or similar sequence elements. The presence of HREs has been demonstrated in a number of angiogenic genes, including VEGFA (6,7), VEGF receptor 1/fms-related tyrosine kinase 1 (8–10), erythropoietin (2,11–14) and endothelial nitric oxide synthase (15–18). Little is known about the third HIFα isoform. It has been demonstrated that a number of splice variants of HIF-3α may act as dominant-negative regulators of the other two α isoforms; however, its primary function, and the regulatory mechanism through which HIF-3α and its variants exert their effects, remains unclear based on currently available evidence (19).
VEGF-A is a key regulator of angiogenesis, but has also been identified to be a multi-functional factor involved in tumor progression, immunosuppression and immune tolerance (20). Endothelial cells are the primary targets of VEGF-A, which acts as a survival factor for these cells, and prevents endothelial apoptosis induced by serum starvation (21–25). In addition, VEGF induces expression of Bcl-2, an anti-apoptotic protein (22).
Materials and methods
Patient recruitment and assessment
The study was conducted in the Ear, Nose and Throat Department of University Hospital ‘Queen Jovanna’ (Sofia, Bulgaria), in cooperation with the Molecular Medicine Center at the Medical University of Sofia (Sofia, Bulgaria), over the period between 2010 and 2013. A total of 63 patients with histopathologically verified carcinoma of the larynx were enrolled in the study. Informed consent was obtained from each patient, and the protocol of the study was approved by the Ethics Committee of the Medical University of Sofia. A standardized history was obtained for each patient. Detailed descriptions of the endoscopic/microscopic direct laryngoscopy findings were recorded, in addition to the computed tomography examination results. All patients underwent surgical intervention consisting of total laryngectomy or organ saving surgery, depending on the extent of the disease. Tumor and normal laryngeal tissue samples were obtained from each patient during the surgery and immediately frozen in liquid nitrogen. The tissue samples were stored at −80°C until use.
Genetic testing
Total RNA extraction and cDNA synthesis
Total RNA was isolated from normal and tumor fresh frozen tissue samples of each patient using an RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's protocol. The quality of RNA was assessed by denaturing electrophoresis on a formaldehyde gel. The amount of RNA was determined spectrophotometrically using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
From each sample, 1 µg RNA underwent reverse transcription using a High-Capacity cDNA Reverse Transcription (RT) kit (Applied Biosystems Life Technologies, Foster City, CA, USA) according to manufacturer's recommendations. In brief, 2X RT master mix, prepared according to the manufacturer's instructions, was added to RNA in a total volume of 20 µl. Reverse transcription was performed in three steps: 25°С for 10 min, 37°С for 120 min and 85°С for 5 min.
Quantitative polymerase chain reaction (PCR)
In the present study, the expression of four genes, HIF-1α, HIF-2α, HIF-3α and VEGF-A, was analyzed. Quantitative PCR was performed in a 25-µl total volume of 1X RotorGene SYBR Green PCR Mix (Qiagen), 1X QuantiTect Primer Assay (Qiagen) for the respective gene (Hs_HIF1A_1_SG, Hs_EPAS1_1_SG, Hs_HIF3A_1_SG, Hs_VEGFA_1_SG) and 100 ng cDNA. The conditions were as follows: Initial denaturation at 95°С for 5 min, followed by 45 cycles of denaturation at 95°С for 15 sec, primer annealing at 55°С for 30 sec, and synthesis with data acquisition at 72°С for 30 sec. Each sample was examined in triplicate, and the mean threshold cycle (Ct) values from the three repeats were used for the data analysis. Negative and no template controls were also evaluated. β-actin (Hs_ACTB_1_SG; QuantiTect Primer Assay) was used as a reference gene for normalization. To determine the relative expression of each gene in the tumor, the 2-ΔΔCt method was applied (26). Briefly, mean Ct values for the gene of interest (GOI) and a reference gene in tumor (CtT,GOI and CtT,Ref, respectively) and normal (CtN,GOI and CtN,Ref, respectively) tissues were used to calculate ΔCt (CtGOI - CtRef) for each tissue, and then to derive the relative quantity (RQ) of the gene in the tumor compared with the normal tissue: RQ = 2ΔΔCt, where ΔΔCt = ΔCtT - ΔCtN. An RQ of >2 was defined as overexpression, and an RQ of <0.5 was defined as underexpression of the gene, in agreement with previous studies (27,28).
Statistical analysis
IBM SPSS Statistics 21 (IBM SPSS, Armonk, NY, USA) was used for all statistical analyses. A two-sided t-test was used to calculate the statistical significance of the results. The χ2 test was used to evaluate differences in mRNA expression levels of HIF-1α and VEGF-A. Spearman analysis was used to determine correlations. P<0.05 was considered to indicate a statistically significant difference.
Results
The mean age of the study group was 60.5 years, with a standard deviation of 7.8 years (range, 41–84 years). The cohort comprised 2 female and 61 male patients, all of whom had histologically verified squamous cell carcinoma of the larynx. Distribution according to tumor-node-metastasis classification was as follows: Stage T1, 2 patients (3.17%); T2, 7 patients (11.11%); T3, 23 patients (36.51%); and T4, 31 patients (49.21%) (29). Histologically verified lymph node metastases were present in 14 patients (22.22%) at the time of surgery.
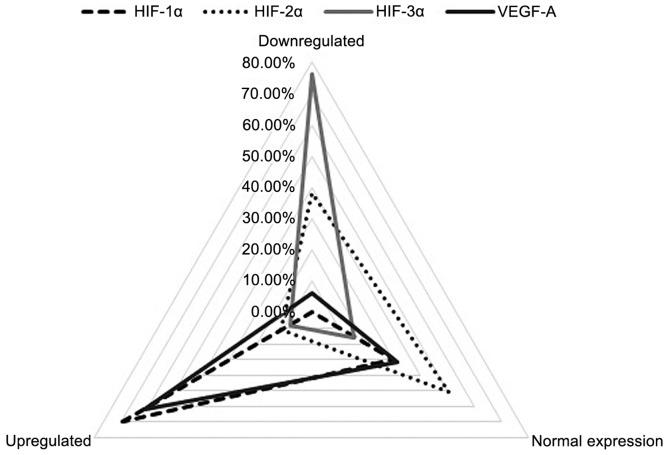
HIF-1α was upregulated (RQ>2) in the majority of patients (44 patients, 69.84%) and normally expressed (0.5<RQ<2) in the remaining 19 (30.16%) patients. By contrast, HIF-2α overexpression (RQ>2) was only identified in 7 patients (11.11%); of the remaining 56 (88.89%) patients, 24 patients exhibited almost silenced HIF-2α expression (RQ<0.5), and the other 32 patients exhibited expression similar to that of the matched normal laryngeal samples (0.5<RQ<2). The HIF-3α isoform was markedly downregulated (RQ<0.5) in the majority of patients (48 patients, 76.19%). Normal levels of HIF-3α mRNA expression (0.5<RQ<2) were registered in 10 (15.87%) patients and a small group of 5 (7.94%) patients exhibited significant overexpression of the HIF-3α isoform (RQ>2). For VEGF-A, 61.90% (39 patients) showed overexpression (RQ>2), 6.35% (4 patients) displayed low expression (RQ<0.5) and 31.75% (20 patients) exhibited normal expression levels (0.5<RQ<2) (Fig. 1).
Figure 1.
mRNA expression of hypoxia-inducible factors (HIFs) and vascular endothelial growth factor A (VEGF-A): Qualitative group distribution.
Quantitative analysis of the study group revealed mean values of HIF-1α mRNA expression that were 2.71 times higher than the corresponding normal laryngeal epithelium, while the expression levels of HIF-2α, HIF-3α and VEGF-A were 0.92, 0.50 and 2.98 times that of the normal epithelium, respectively. One patient was excluded as an outlier after the mRNA expression level of VEGF-A was measured to be 955 times higher in the tumor tissue compared with the corresponding normal laryngeal tissue (testing was repeated five times).
A χ2 test for association was conducted between patients with upregulated and without upregulated mRNA expression of HIF-1α and VEGF-A. There was a statistically significant association between the overexpression of HIF-1α and VEGF-A (χ21=7.246, P=0.008).
Spearman's rank correlation coefficient was used to assess the correlation between mRNA expression levels of HIF-1α and VEGF-A. Preliminary analyses revealed the association to be monotonic, as assessed by visual inspection of a scatterplot. Pearson's correlation could not be used, as the variables were not normally distributed, as assessed by a Shapiro-Wilk test (P>0.05). Six outliers were recognized and removed from the analyzed group. There was a moderate positive correlation between mRNA expression levels of HIF-1α and VEGF-A (rs=0.392, P<0.005).
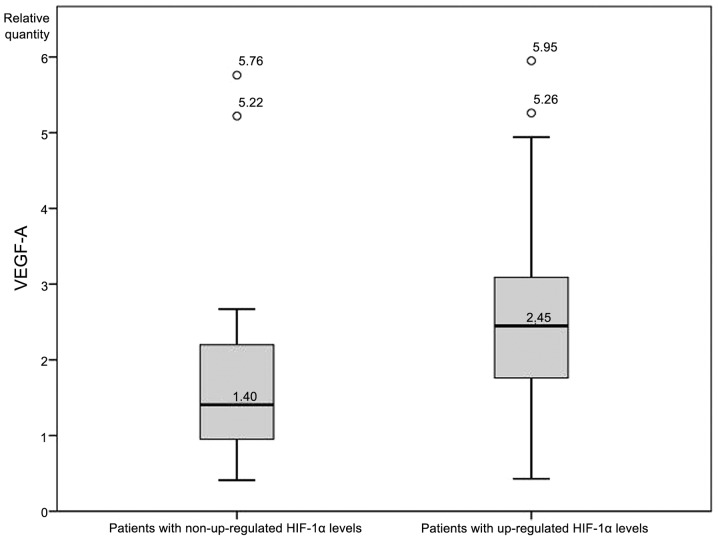
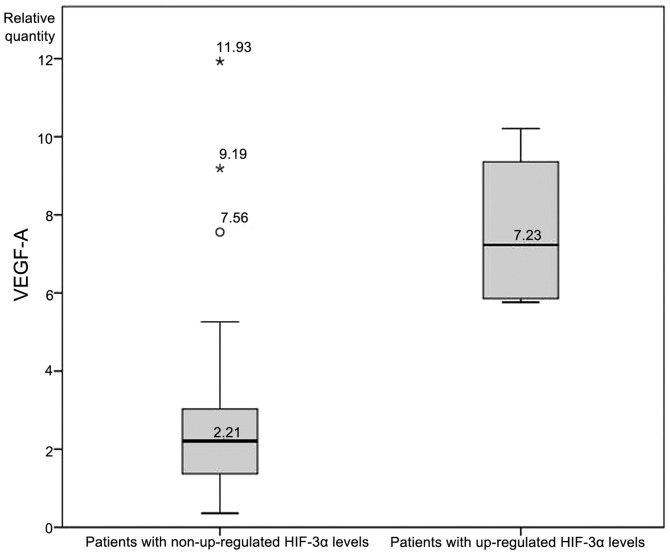
An independent-samples t-test was conducted to determine whether expression levels of VEGF-A differed significantly between patients with and without upregulated HIF-1α. VEGF-A expression was significantly higher (P<0.05; Fig. 2) in patients with upregulated HIF-1α (2.72±1.41 RQ) compared with patients without upregulated HIF-1α (1.86±1.46 RQ). There was homogeneity of variances, as assessed by Levene's test for equality of variances (P=0.813), while no significant differences in VEGF-A levels between patients with and without upregulated HIF-2α were identified. Finally, an analysis of VEGF-A levels between patients with and without upregulated HIF-3α revealed a statistically significant difference (7.61±2.14 vs. 2.66±2.13, respectively; Fig. 3).
Figure 2.
Vascular endothelial growth factor A (VEGF-A) expression levels in patients with and without upregulated hypoxia inducible factor 1α (HIF-1α).
Figure 3.
Vascular endothelial growth factor A (VEGF-A) expression levels in patients with and without upregulated hypoxia inducible factor 3α (HIF-3α) expression.
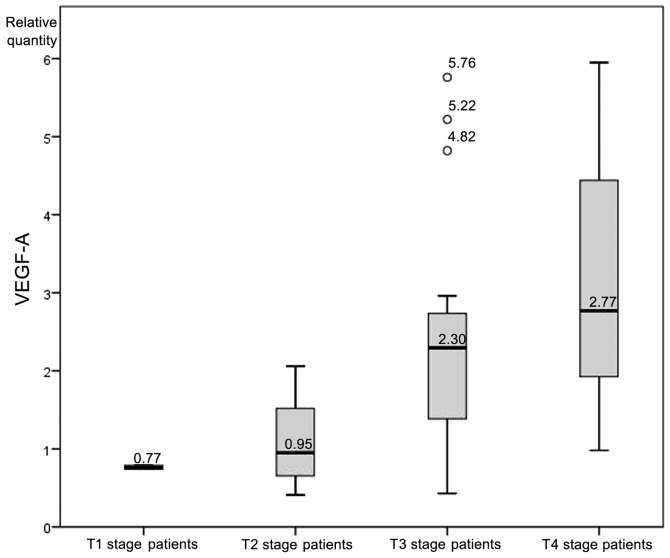
A one-way analysis of variance was conducted to determine whether the levels of VEGF-A mRNA expression differed between patients of different T stages. Participants were classified into four groups: T1 stage (n=2), T2 stage (n=7), T3 stage (n=22) and T4 stage (n=31). Six outliers were recognized and removed from the analyzed group. There was homogeneity of variances, as assessed by Levene's test of homogeneity of variances (P=0.120). Data is presented as the mean ± standard deviation. The VEGF-A expression rate was significantly different between the groups (F=4.79, P=0.005). VEGF-A expression increased from T1 stage patients (0.77±0.03), to T2 stage patients (1.11±0.61), to T3 stage patients (2.37±1.46) to T4 stage patients (2.98±1.40) (Fig. 4).
Figure 4.
Vascular endothelial growth factor A (VEGF-A) expression levels according to tumor stage.
Discussion
To the best of our knowledge, the presents study is the first to investigate the mRNA expression levels of all three isoforms of the HIF family in carcinoma tissue samples. Following a review of the literature, only 13 studies on the topic of laryngeal carcinoma and HIF were identified (Table I). These studies all examined the expression of HIF-1α only, and none investigated HIF-1α mRNA expression levels from in vivo samples (30–42). The current study is the first to present full quantitative data regarding the mRNA expression levels of HIF-1α and HIF-2α in laryngeal carcinoma samples, and is the first to report on the mRNA expression levels of HIF-3α in an in vivo study of a malignant neoplasm.
Table I.
Summary of previous studies regarding HIF expression and laryngeal carcinoma.
| First author (ref.) | Year | n | Type of studied specimen | Isoforms studied | Method |
|---|---|---|---|---|---|
| Moreno-Galindo et al (30) | 2014 | 41 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Wachters et al (31) | 2013 | 60 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Li et al (32) | 2013 | - | Laryngeal cancer cell line culture | HIF-1α | RT-PCR and western blotting |
| Xie et al (33) | 2013 | 86 | Paraffin-embedded surgical tissue specimens and laryngeal cancer cell line culture | HIF-1α | IHC and RT-qPCR |
| Wu et al (34) | 2013 | 49 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Li et al (35) | 2013 | 86 | Paraffin-embedded surgical tissue specimens and laryngeal cancer cell line culture | HIF-1α | IHC, RT-qPCR and western blotting |
| Douglas et al (36) | 2013 | 286 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Wu et al (37) | 2010 | 40 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Moon et al (38) | 2009 | - | Laryngeal cancer cell line culture | HIF-1α | Western blotting and immunofluorescence |
| Cabanillas et al (39) | 2009 | 106 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Wildeman et al (40) | 2009 | 26 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Kyzas et al (41) | 2005 | 81 | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
| Yu et al (42) | 2004 | N/A | Paraffin-embedded surgical tissue specimens | HIF-1α | IHC |
HIF, hypoxia inducible factor; IHC, immunohistochemistry; RT, reverse transcription; PCR, polymerase chain reaction; q, quantitative; N/A, not available.
Analysis of the results revealed a distinctive expression pattern among the majority of the patients: Overexpression of HIF-1α (69.84%), normal or downregulated levels of HIF-2α, and normal or downregulated levels of HIF-3α (92.06%). HIF-2α was demonstrated to be stabilized at moderate oxygen levels (2–5% O2), whereas HIF-1α is upregulated only at lower oxygen levels (0-2% O2) (reviewed in 43). Additionally, Holmquist-Mengelbier et al (30) demonstrated that HIF-1α is most active during short periods (2–24 h) of intense hypoxia or anoxia (<0.1% O2), whereas HIF-2α may be active for a longer period of mild hypoxia (<5% O2). This phenomenon is described in the literature as the HIF switch: HIF-1α drives the initial response to hypoxia, and HIF-2 subsequently takes over the major role during chronic hypoxic exposure (44–46). The HIF switch is particularly evident during the development of renal cell carcinoma, where there is a gradual shift from HIF-1α to HIF-2α expression with increasing tumor grade (46–48). In contrast to these findings, the results of the present study display a lack of such a HIF switch in laryngeal carcinoma: Of the 7 patients with upregulated HIF-2α, 4 also exhibited an upregulation in HIF-1α levels. Thus only three patients demonstrated a distinct HIF switch, despite 85.7% of the cohort having advanced-stage disease (T3 or T4 stage).
The statistically significant association between overexpression of HIF-1α and VEGF-A is expected, as this is consistent with the canonical HIF pathway: Overexpression of HIF-1α triggers the expression of VEGF-A (49). This is also supported by the fact that VEGF-A levels in the current study were significantly higher in patients with upregulated HIF-1α expression compared with those without upregulated HIF-1α expression. Additionally, there was a statistically significant correlation between the levels of expression of the two molecules, i.e., there was a quantitative association between the level of mRNA expression of HIF-1α and VEGF-A.
Another notable result from the present study was with regard to the mRNA expression of HIF-3α. HIF-3α is the most poorly studied isoform of the three, and in the review of the literature, no other studies were found that investigated in vivo expression in any malignant neoplasm. In the present study, the majority of the patients display silenced expression of HIF-3α, with the exception of a small group of five patients who exhibited upregulated mRNA expression levels. Compared with the remaining patients, significantly higher levels of VEGF-A expression were detected in this group (mean RQ, 7.61±2.14 vs. 2.66±2.13; Fig. 3). Various possible effects of HIF-3α have been reported due to its multiple splice variants. Of major significance is the downregulatory function of HIF-3α on HIF-1α and HIF-2α activity (reviewed in 43). This indicates that the overexpression of VEGF-A may be a driving force for the upregulation of HIF-3α, as the latter would act a negative feedback regulator of the canonical HIF pathway. Despite this, there were also a few cases in the present cohort of patients in which a significant upregulation of VEGF-A plus silenced HIF-3α was detected; other regulatory factors must play a role in these cases.
Finally, the clinical correlation between VEGF-A expression and the stage of the tumor may be explained by the growing size of the lesion and the expansion of the process of neoangiogenesis, in which VEGF-A is essential (49).
The present study reports, for the first time, full quantitative data on the expression of all three isoforms of the HIFs in malignant neoplasms. The findings indicate a specific phenotype of HIF expression in laryngeal carcinoma, where the HIF switch is absent. Further investigations are required to uncover the obscure nature of HIF-3α, and the factors that determine which isoform, HIF-1α or HIF-2α, would be the major driving force of the canonical HIF pathway in neoplasms.
Acknowledgements
The present study was partially funded by a grant from the Medical University of Sofia (grant no. 2-D/2012).
References
- 1.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesener MS, Turley H, Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: Characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 3.Heidbreder M, Fröhlich F, Jöhren O, et al. Hypoxia rapidly activates HIF-3alpha mRNA expression. FASEB J. 2003;17:1541–1543. doi: 10.1096/fj.02-0963fje. [DOI] [PubMed] [Google Scholar]
- 4.Li QF, Wang XR, Yang YW, Lin H. Hypoxia upregulates hypoxia-inducible factor (HIF)-3alpha expression in lung epithelial cells: Characterization and comparison with HIF-1alpha. Cell Res. 2006;16:548–558. doi: 10.1038/sj.cr.7310072. [DOI] [PubMed] [Google Scholar]
- 5.Pugh CW, O'Rourke JF, Nagao M, et al. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- 7.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda N, Maemura K, Imai Y, et al. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95:146–153. doi: 10.1161/01.RES.0000134920.10128.b4. [DOI] [PubMed] [Google Scholar]
- 9.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 10.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kertesz N, Wu J, Chen TH, et al. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 14.Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, de Muinck ED, Zhuang Z, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulet F, Nadaud S, Agrapart M, Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem. 2003;278:46230–46240. doi: 10.1074/jbc.M305420200. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Lu X, Feng Q. Deficiency in endothelial nitric oxide synthase impairs myocardial angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H2371–H2378. doi: 10.1152/ajpheart.00383.2002. [DOI] [PubMed] [Google Scholar]
- 19.Maynard MA, Qi H, Chung J, et al. Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- 20.Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): Possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11:BR280–BR292. [PubMed] [Google Scholar]
- 21.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 22.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 23.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Scrideli CA, Carlotti CG, Jr, Okamoto OK, et al. Gene expression profile analysis of primary glioblastomas and non-neoplastic brain tissue: Identification of potential target genes by oligonucleotide microarray and real-time quantitative PCR. J Neurooncol. 2008;88:281–291. doi: 10.1007/s11060-008-9579-4. [DOI] [PubMed] [Google Scholar]
- 28.Borel F, Han R, Visser A, Petry H, van Deventer SJ, Jansen PL, Konstantinova P. Réseau Centre de Ressources Biologiques Foie (French Liver Biobanks Network), France: Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- 29.Sobin LH, Gospodarowicz MK, Wittekind C, editors. In: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell; 2009. International Union Against Cancer: Head and Neck Tumours; pp. 39–46. [Google Scholar]
- 30.Moreno-Galindo C, Hermsen M, Garcia-Pedrero JM, Fresno MF, Suarez C, Rodrigo JP. p27 and BCL2 expression predicts response to chemotherapy in head and neck squamous cell carcinomas. Oral Oncol. 2014;50:128–134. doi: 10.1016/j.oraloncology.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Wachters JE, Schrijvers ML, Slagter-Menkema L, Mastik M, de Bock GH, Langendijk JA, et al. Prognostic significance of HIF-1a, CA-IX, and OPN in T1-T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope. 2013;123:2154–2160. doi: 10.1002/lary.23831. [DOI] [PubMed] [Google Scholar]
- 32.Li DW, Dong P, Wang F, Chen XW, Xu CZ, Zhou L. Hypoxia induced multidrug resistance of laryngeal cancer cells via hypoxia-inducible factor-1α. Asian Pac J Cancer Prev. 2013;14:4853–4858. doi: 10.7314/APJCP.2013.14.8.4853. [DOI] [PubMed] [Google Scholar]
- 33.Xie J, Li DW, Chen XW, Wang F, Dong P. Expression and significance of hypoxia-inducible factor-1α and MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2 cells. Oncol Lett. 2013;6:232–238. doi: 10.3892/ol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu XH, Chen SP, Mao JY, Ji XX, Yao HT, Zhou SH. Expression and significance of hypoxia-inducible factor-1α and glucose transporter-1 in laryngeal carcinoma. Oncol Lett. 2013;5:261–266. doi: 10.3892/ol.2012.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DW, Zhou L, Jin B, Xie J, Dong P. Expression and significance of hypoxia-inducible factor-1α and survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head Neck Surg. 2013;148:75–81. doi: 10.1177/0194599812464759. [DOI] [PubMed] [Google Scholar]
- 36.Douglas CM, Bernstein JM, Ormston VE, et al. Lack of prognostic effect of carbonic anhydrase-9, hypoxia inducible factor-1α and bcl-2 in 286 patients with early squamous cell carcinoma of the glottic larynx treated with radiotherapy. Clin Oncol (R Coll Radiol) 2013;25:59–65. doi: 10.1016/j.clon.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Wu XH, Lu YF, Hu XD, Mao JY, Ji XX, Yao HT, et al. Expression of hypoxia inducible factor-1α and its significance in laryngeal carcinoma. J Int Med Res. 2010;38:2040–2046. doi: 10.1177/147323001003800618. [DOI] [PubMed] [Google Scholar]
- 38.Moon SY, Chang HW, Roh JL, et al. Using YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral Oncol. 2009;45:915–919. doi: 10.1016/j.oraloncology.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Cabanillas R, Rodrigo JP, Secades P, et al. The relation between hypoxia-inducible factor (HIF)-1alpha expression with p53 expression and outcome in surgically treated supraglottic laryngeal cancer. J Surg Oncol. 2009;99:373–378. doi: 10.1002/jso.21243. [DOI] [PubMed] [Google Scholar]
- 40.Wildeman MA, Gibcus JH, Hauptmann M, et al. Radiotherapy in laryngeal carcinoma: can a panel of 13 markers predict response? Laryngoscope. 2009;119:316–322. doi: 10.1002/lary.20069. [DOI] [PubMed] [Google Scholar]
- 41.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Hypoxia-induced tumor angiogenic pathway in head and neck cancer: an in vivo study. Cancer Lett. 2005;225:297–304. doi: 10.1016/j.canlet.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 42.Yu L, Liu Y, Cui Y. Expression of hypoxia inducible factor-1alpha and its relationship to apoptosis and proliferation in human laryngeal squamous cell carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2004;24:636–638. doi: 10.1007/BF02911379. [DOI] [PubMed] [Google Scholar]
- 43.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmquist-Mengelbier L, Fredlund E, Löfstedt T, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh MY, Powis G. Passing the baton: The HIF switch. Trends Biochem Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandriota SJ, Turner KJ, Davies DR, et al. HIF activation identifies early lesions in VHL kidneys: Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/S1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 49.Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11:121–140. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]