Abstract
Purpose:
Enhanced drug exposure to the ocular surface typically relies on inclusion of viscosity-enabling agents, whereas delivery to the back of the eye generally focuses on invasive means, such as intraocular injections. Using our novel mucus-penetrating particle (MPP) technology, which rapidly and uniformly coats and penetrates mucosal barriers, we evaluated if such drug formulations could increase ocular drug exposure and improve topical drug delivery.
Methods:
Pharmacokinetic (PK) profiling of topically administered loterprednol etabonate formulated as MPP (LE-MPP) was performed in rabbits and a larger species, the mini-pig. Pharmacodynamic evaluation was done in a rabbit model of VEGF-induced retinal vascular leakage. Cellular potency and PK profile were determined for a second compound, KAL821, a novel receptor tyrosine kinase inhibitor (RTKi).
Results:
We demonstrated in animals that administration of LE-MPP increased exposure at the ocular surface and posterior compartments. Furthermore using a rabbit vascular leakage model, we demonstrated that biologically effective drug concentrations of LE were delivered to the back of the eye using the MPP technology. We also demonstrated that a novel RTKi formulated as MPPs provided drug levels to the back of the eye above its cellular inhibitory concentration.
Conclusions:
Topical dosing of MPPs of LE or KAL821 enhanced drug exposure at the front of the eye, and delivered therapeutically relevant drug concentrations to the back of the eye, in animals.
Translational Relevance:
These preclinical data support using MPP technology to engineer topical formulations to deliver therapeutic drug levels to the back of the eye and could provide major advancements in managing sight-threatening diseases.
Keywords: choroid, corticosteroids, drug delivery, retina, topical
Introduction
Rapid drug elimination from the ocular surface is a major obstacle for topical ophthalmic drug delivery. Although eye drops remain the preferred ophthalmic dosage form due to localized action and relative patient acceptance, it is well established that conventional solutions and suspensions only deliver a small portion of the administered dose to the anterior segment tissues. This is a result of static (corneal epithelium and stromal as well as the blood–aqueous barrier), dynamic (conjunctival blood and lymph flow, and tear drainage), and lastly metabolic barriers.1–3 Furthermore, blinking, eyelashes, and reflex tearing promote rapid removal of topically applied eye drops. The clearance of topically applied solutions occurs within 15 to 30 seconds primarily due to tear film turnover, which is entirely restored every 2 to 3 minutes.4 Therefore, the resulting intraocular bioavailability of topically applied drugs to the anterior chamber is less than 5%.4
The posterior segment, where additional physical and diffusional barriers exist to further reduce drug delivery, is even more challenging for topical delivery.5–7 As a result, high systemic doses and/or invasive therapies are used currently to treat a variety of conditions affecting the back of the eye, including sight-threatening diseases, such as age-related macular degeneration (AMD), diabetic retinopathy, and posterior uveitis.8–12 High systemic dosing provides limited local exposure and duration of action, and often is associated with liabilities and drug-specific toxicities.1–2 Intravitreal (IVT) injections provide the most direct method of delivery to the posterior tissues; however, IVT injections have the inherent potential for serious adverse events, such as retinal detachment, hemorrhage, endophthalmitis, and the development of cataracts.8 In addition, IVT injections must be administered by an experienced ophthalmologist and repeated injections are required frequently. This often presents significant logistic constraints to patients and caregivers, which can negatively impact compliance. There is a clear unmet medical need for noninvasive, topical drug delivery to the posterior segment.
While nanoparticles may have the potential to improve ocular tissue exposure from topical administration, this potential is undermined by the adhesive nature of the ocular mucus layer, which among other functions, serves to protect the eye from allergens, pathogens, and debris by effectively trapping and rapidly clearing foreign particles from the ocular surface.13–15 It is well-documented that most nanoparticles greater than 50 nm become readily immobilized in mucus due to steric and/or adhesive interactions with the mucin mesh.16–20 Therefore, ocular residence time of such trapped nanoparticles is limited by the turnover rate of the peripheral ocular mucus, typically on the order of seconds to minutes. To enhance topical ocular delivery, drug carriers must avoid entrapment by the tear mucins and readily penetrate into the membrane-bound mucus layer (glycocalyx) layer of the eye.
Mucus-penetrating particle (MPP) technology is a novel drug delivery platform that can be used to design drug-loaded nanoparticles to effectively penetrate human mucus secretions. This technology has been described in connection with certain engineered nanoparticles that move nearly freely through mucus, distribute evenly in the vaginal tract, lung, and gastrointestinal tracts of animals, and provide prolonged duration on these mucosal surfaces.16–21 We hypothesized that MPPs designed for topically administering certain compounds also would have a prolonged residence time on the ocular surface, which in turn may translate into enhanced drug delivery to ocular tissues, superior pharmacokinetics (PK), and improved efficacy.22 The focus of this study is to demonstrate in animal models that MPPs of two drugs can enhance delivery of those drugs to various ocular tissues beyond the mucus layer.
The MPP technology was used to create formulations of two drugs from different therapeutic classes: loteprednol etabonate (LE), a corticosteroid specifically designed for ophthalmic inflammatory indications, but known to have limited penetration into ocular tissues after topical delivery; and KAL821, a novel small molecule receptor tyrosine kinase inhibitor (RTKi) with potential application for the treatment of AMD. As a molecule with well-established ocular PK, LE served as the primary test compound, while the novel KAL821 served to further validate the hypothesis. Using rabbit and mini-pig preclinical models to decouple local and systemic contribution, we have verified that the MPPs of these two drugs can topically deliver drug to the back of the eye at therapeutically relevant concentrations, which supports the potential for topical treatment of diseases affecting the posterior segment.
Materials and Methods
Nanoparticle Formulations
The MPP formulations of LE (LE-MPP) and KAL821 (KAL821-MPP) were prepared by wet nanomilling of coarse crystalline drugs in the presence of an MPP-enabling surface-altering agent PLURONIC F127 (F127).22 This formulation process involved no chemical modifications of the drug or excipients (i.e., did not result in generation of new chemical entities), and only excipients approved by FDA for ophthalmic use were used. Resulting MPP nanoparticles were composed of a drug core with a noncovalently attached F127 coating and had an average hydrodynamic diameter of about 240 to 350 nm for LE-MPP and about 160 nm for KAL821-MPP. Nonmucus-penetrating conventional nanoparticles of LE (LE-CP) were prepared in a similar manner except that sodium dodecyl sulfate (SDS) was used as a surface-altering agent that does not impart MPP behavior. The LE-CP test article had a hydrodynamic diameter of 240 nm. In an ex vivo mucus mobility assay, LE-MPP and KAL821-MPP showed the ability to penetrate mucus and LE-CP was confirmed to be strongly mucoadhesive.
Rabbit PK Studies
A topical dose of 50 μL was administered to each eye of 4- to 6-month-old male New Zealand White (NZW) rabbits (n = 3 per group; n = 6 eyes; Covance Research Laboratories, Denver, PA) using a calibrated positive displacement pipette (Gilson M-50). Animals were restrained for approximately 2 minutes post-dose to prevent them from shaking their heads or pawing away the doses. Animals were euthanized by intravenous barbiturate overdose at various time points post-dose according to their treatment group. Both eyes from each animal were harvested, and aqueous humor and ocular tissues were collected. For the dosed versus nondosed eye studies, Dutch Belted (DB) 7-month-old male rabbits (n = 4 per time point; Covance) were dosed in a single eye and euthanized, and tissues from both eyes were collected as described above. Animals were observed for abnormalities before placement on study, after topical dosing, and at necropsy. Ocular irritation was monitored using the Draize Eye Irritation Scoring System.23 All the formulations were well-tolerated and all animals received a Draize System score of 0 pre- and post-dosing. Concentrations of drugs in the tissues were determined using liquid chromatography-tandem mass-spectrometry (LC-MS/MS) method. All rabbit PK studies, including bioanalytical analysis, were conducted at PharmOptima, LLC (Portage, MI).
VEGF-Induced Vascular Leakage Model in Rabbits
Male DB rabbits (Covance) 4 months old were randomized (n = 4 per group; n = 8 eyes) and 50 μL of 1.0% LE-MPP was administered topically (twice daily [BID] or four times daily [QID]) using a positive displacement pipet. Vehicle was dosed topically QID. On day 3, all animals were anesthetized and injected with 50 μL of recombinant human VEGF (PeproTech, Inc., Rocky Hill, NJ) into the mid-vitreous of both eyes through the pars plana, approximately 3 to 4 mm from the edge of the cornea. Additionally, one group also received an intravitreal injection of 4.0% triamcinolone acetonide on day 3. During the VEGF injection, reflux was noted in three eyes these data were removed from analysis (2 eyes from the triamcinolone acetonide group and 1 eye from the LE-MPP [QID] group). Fluorescein angiograms were conducted on day 5. Briefly, all eyes were anesthetized with proparacaine and pupils dilated with tropicamide. Animals then were anesthetized with isoflurane vapors to effect and injected with 0.3 mL of 10% ophthalmic sodium fluorescein via the ear vein at least 2 minutes before imaging. Images then were scored by a standard scoring system, and all treatment identity was blinded during the scoring of the images.24 This study was conducted at PharmOptima, LLC.
Mini-Pig PK Studies
A topical dose of LE-MPP (35 μL) was administered in the right eye of 5- to 6-month-old female Gottingen mini-pigs (Marshall Farms USA, Inc., North Rose, NY) approximately 6 hours apart (± 20 minutes), for 4 consecutive days. On the fifth day, animals were administered only two doses, approximately 6 hours apart (±20 minutes). A total of 18 doses were administered over the study duration. The dose was administered on the top of the cornea via a calibrated positive displacement micropipette and allowed to distribute across the eye. Each animal was restrained for approximately 1 minute to prevent rubbing of the eyes. Any observed irregularities or local irritation were recorded. Three animals/time point were euthanized with sodium pentobarbital and blood was collected via cardiac puncture at 0.5, 1, and 2 hours after the last dose on day 5. Blood (approximately 10 mL) was collected into tubes containing K2EDTA and samples were maintained on wet ice until centrifuged to obtain plasma. All plasma samples were stored at approximately −70°C until analyzed. At the time of sacrifice, both eyes were enucleated. Fresh tissues were collected and each eye was flash frozen in liquid nitrogen for 15 to 20 seconds, and placed on dry ice. Within 3 days, tissues were collected from both eyes. Drug concentrations were determined using LC-MS/MS. In a separate study, 6- to 8-month-old female Gottingen mini-pigs (Marshall Farms) were administered a topical dose of KAL821-MPP (35 μL) approximately 12 hours apart (±1 hour), for 4 consecutive days. On the fifth day, animals were administered one dose in the morning. A total of 9 doses were given over the study duration. Three animals/time point were sacrificed at 0.5, 1, 2, and 4 hours after the last dose on day 5. Tissues were sampled and processed as described above. Both mini-pig PK studies, including bioanalytical analysis, were conducted at Covance Research Laboratories.
All animal studies, whether conducted at PharmOptima or Covance Research Laboratories, adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cellular Assay
In the cellular VEGF-R2 phosphorylation assay, the cell line HUE, a spontaneously immortalized human umbilical vein endothelial cell clone, is used, which expresses endogenously a high level of VEGF-R2. The immortalized cell line was a gift from the group of Werner Risau (Bad Nauheim, Germany). Stimulation of these cells with human vascular endothelial growth factor A (VEGFA) results in receptor tyrosine autophosphorylation. The HUE cells were plated in endothelial cell growth medium (ECGM) supplemented with 10% fetal calf serum (FCS) in multi-well cell culture plates. After starvation in endothelial cell basal medium (ECBM) supplemented with 10% FCS overnight, cells were incubated with compounds in serum-free ECBM. Substrate phosphorylation was quantified in 96-well plates via sandwich ELISA using a substrate-specific capture antibody and an anti-phosphotyrosine detection antibody. Raw data were converted into percent substrate phosphorylation relative to High controls, which were set to 100%. The half maximal inhibitory concentration (IC50) values were determined using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA) by constraining bottom to 0 and top to 100, and using a nonlinear regression curve fit with variable hill slope. The equation is a four-parameter logistic equation. The KAL821 IC50s were determined in five independent assays performed by ProQinase GmbH (Freiburg, Germany).
Statistical Analysis
Area under the curves (AUC) values were generated using GraphPad Prism v6.0 and ratios of dosed over nondosed eyes were determined. Statistical analyses were performed using the Holm-Sidak method multiple t-test, and P values were shown on graphs. In the VEGF vascular leakage studies, 1-way analysis of variance (ANOVA) with Tukey's multiple comparison test was performed using GraphPad Prism v6.0. Cellular IC50 for KAL821 was expressed as mean ± SD.
Results
Single Dose PK Profile in Rabbits Comparing LE-MPP and Conventional Particles (LE-CP), and LE-MPP and Micronized LE Plus Mucus-Penetrating Surface Altering Agent (LE-Micro)
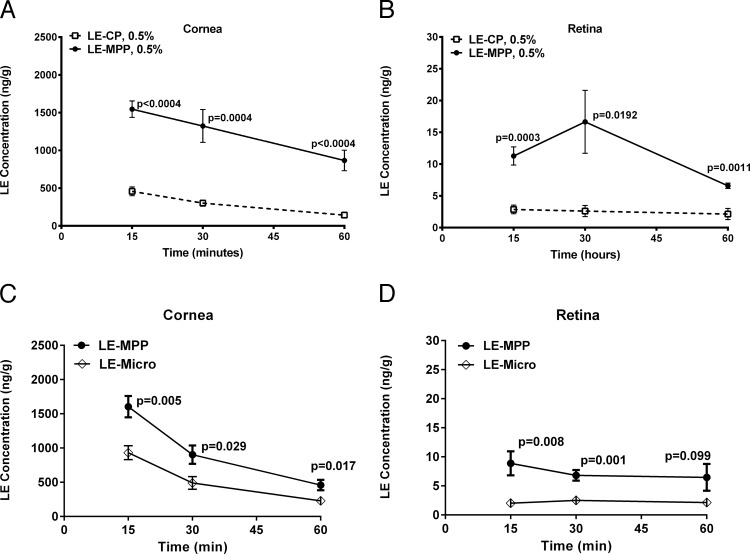
To determine whether LE-MPP has the potential to enhance topical drug delivery to ocular tissues compared to similarly sized control particles of LE without the MPP surface modification (LE-CP), we measured drug levels in cornea and retina of rabbits following instillation of an equivalent dose of LE-CP or LE-MPP. The LE-MPP was prepared by nanomilling coarse crystalline LE in the presence of an MPP-enabling surface-altering agent F127, as described previously.22 Resulting LE-MPP nanoparticles had an average hydrodynamic diameter of 240 nm. Nonmucus-penetrating conventional nanoparticles, LE-CP, were prepared in a similar manner except that a conventional surfactant, SDS, was used. The LE-CP test article also had a hydrodynamic diameter of 240 nm. Rabbits received a single dose to both eyes of LE-MPP or LE-CP. The concentration-time profiles of LE are shown in Figures 1A and 1B. Ratios of the AUC reveal a 4-fold increase in LE in the cornea when dosed as LE-MPP compared to LE-CP, and a 5-fold enhancement in the retina (Table 1).
Figure 1.
The NZW rabbit ocular levels of LE following a single topical dose. (A) Corneal LE levels post-topical administration of 0.5% LE-CP or LE-MPP. (B) Retinal LE levels post-topical administration of 0.5% LE-CP or LE-MPP. (C) Corneal LE levels post-topical administration of 0.5% LE-MPP or LE-Micro. (D) Retinal LE levels post-topical administration of 0.5% LE-MPP or LE-Micro. All data are shown as mean ± SEM (n = 6 eyes). Statistical analysis was performed using the Holm-Sidak method multiple t-test, P values were shown on graphs, and are reflective of the differences between the MPP groups and the control groups (Fig. 1) or between dosed and nondosed eyes (Figs. 2, 4, 5).
Table 1.
Area Under the Curve
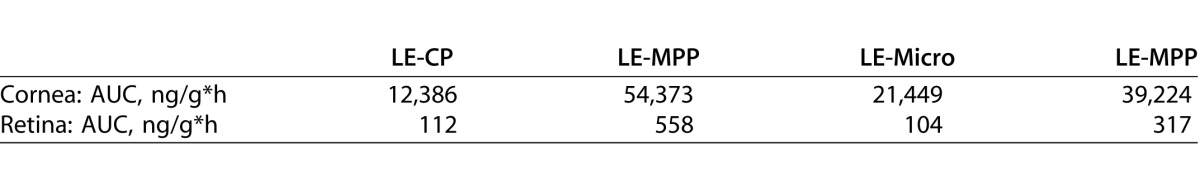
To assess the influence of MPP particle size on ocular drug exposure, LE-MPP was compared to a micronized LE suspension containing F127 (LE-Micro). The particle size of LE-Micro was >1 μm. Ratios of the AUCs reveal a 2-fold increase in LE in the cornea and a 3-fold enhancement in the retina when LE-MPP was compared to LE-Micro (Figs. 1C, 1D; Table 1).
Taken together, these data suggested that the mucus-penetrating surface modifier (F127) and the smaller particle size of LE-MPP contributed to the enhanced ocular drug levels observed in vivo.
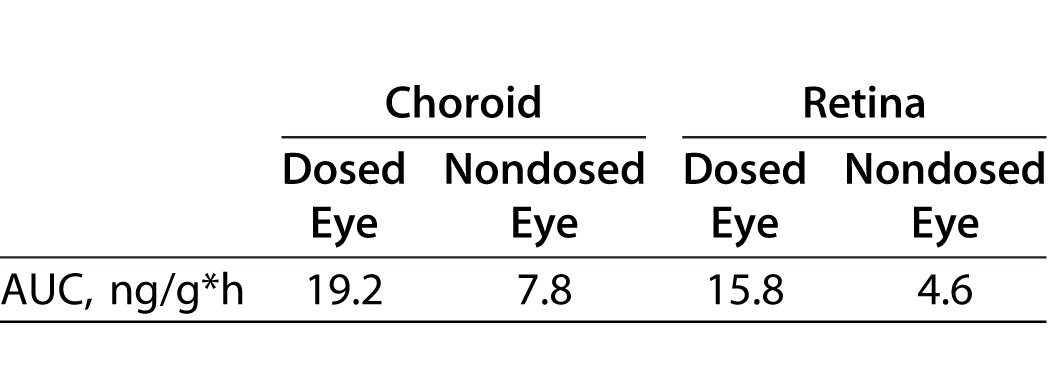
PK Profile of LE in Pigmented Rabbit Dosed Versus Nondosed Eyes After Multi-Dose Topical Administration of LE-MPP
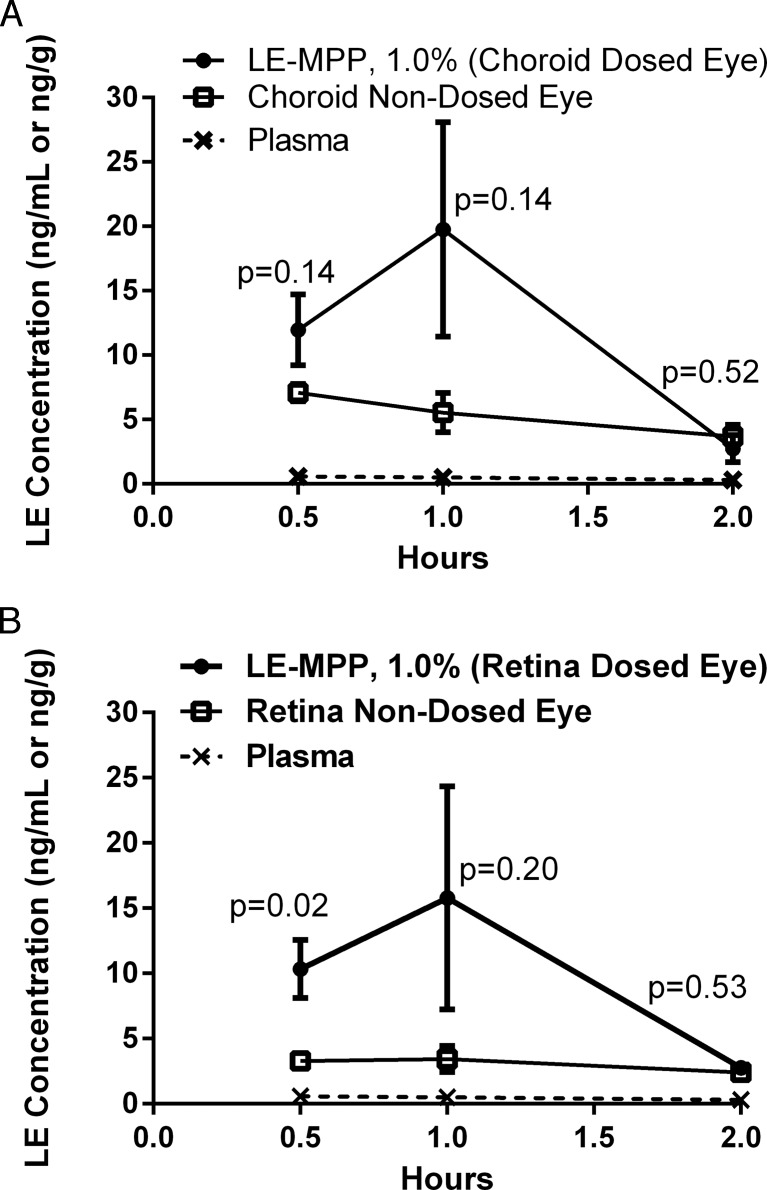
There were three distinct purposes in this rabbit PK study: first, to study the PK of LE in pigmented DB rabbits rather than the nonpigmented NZW to mimic pigmented human eyes in the event that LE binds to melanin, which occurs with many drug classes;23–25 second, to dose twice a day for 5 days to achieve steady-state drug levels in the ocular tissues; and lastly, to dose rabbits in one eye and measure drug levels in both eyes to evaluate local versus systemic delivery to the back of the eye, since it is likely that a portion of an eye drop could be absorbed systemically and, therefore, contribute to drug levels seen in the back of the eye tissues via systemic distribution.27,28 The concentration-time profiles of LE are shown in Figure 2. Drug levels in choroid were 2.5-fold higher in the dosed eye than the nondosed eye, and a 3.4-fold difference was observed in the dosed versus nondosed retina (Table 2).
Figure 2.
The PK profiles of LE in pigmented rabbits after topical administration of LE-MPP. The mean LE concentrations ± SE (ng/g, n = 4) in choroid (A) or retina (B) were shown with the following symbols: dosed eyes (closed circles), nondosed eyes (open squares), plasma (x, dashed line). Statistical analysis was performed using the Holm-Sidak method multiple t-test, P values were shown on graphs as related to differences between dosed and nondosed eyes.
Table 2.
Area Under the Curve
Effect of Topical LE-MPP in a Rabbit VEGF Vascular Leakage Model
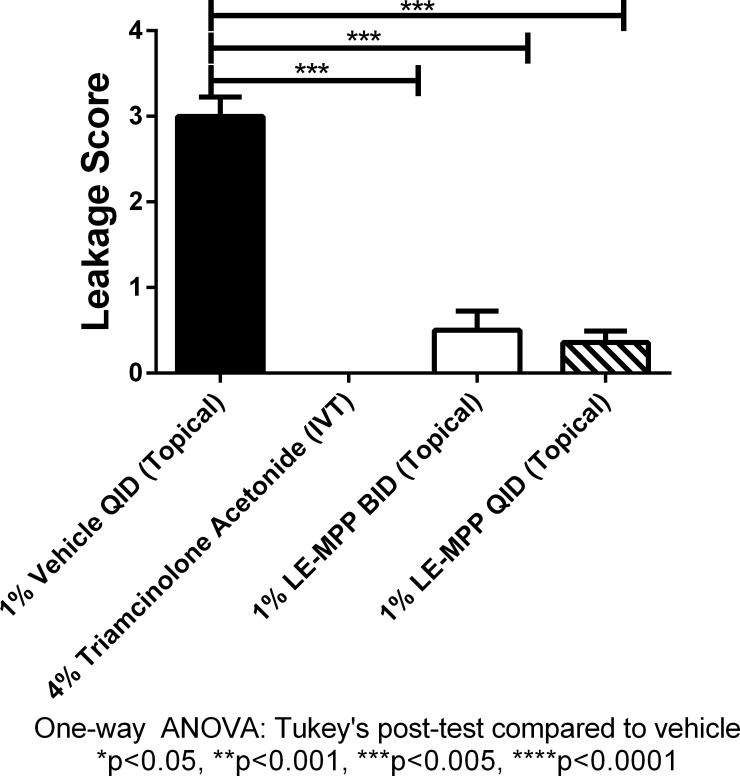
To confirm that drug levels in the retina after administration of LE-MPP would be sufficient to translate into a biological effect, we used a standard rabbit model to assess protection against vascular leakage induced by VEGF.24 Groups of rabbits received in a single eye either LE-MPP BID or QID starting on day 1. Triamcinolone acetonide given by IVT injection was used as a positive control and vehicle alone was used as a negative control. On day 3, vascular leakages at the back of the eye were induced in the animals by a VEGF challenge. At 48 hours after VEGF challenge, fluorescein angiograms were performed on both eyes following the morning dosing. For each eye, the temporal and nasal vasculature was examined and graded on a score of 0 to 4. All scoring was done blinded. As expected,24 animals treated with triamcinolone acetonide had significantly lower leakage compared to vehicle-treated animals at 48 hours after VEGF challenge. Furthermore, topical LE-MPP (QID and BID) were equally as effective as IVT triamcinolone acetonide in preventing leakage (Fig. 3).
Figure 3.
Topical administration of LE-MPP significantly prevented vascular leakage in pigmented rabbit model of VEGF-induced retinal vascular permeability. An IVT injection of VEGF on day 3 induced vascular leakage. Fluorescein angiograms were graded blinded on day 5 and plotted as means ± SEM. Vehicle was given topically QID starting on day 1 (black solid bar). An IVT administration on day 1 of 4.0% triamcinolone acetonide was used as a positive control (checked bar; all values are 0). The LE-MPP, 1.0% was given BID (white solid bar) or QID (striped bar). All groups had 4 rabbits and both eyes (n = 8) were used. Statistical significance was determined using GraphPad Prism v6.0 Tukey's multiple comparison test.
PK Profile of LE in Pigmented Mini-Pig Dosed Versus Nondosed Eyes After Multi-Dose Topical Administration of LE-MPP
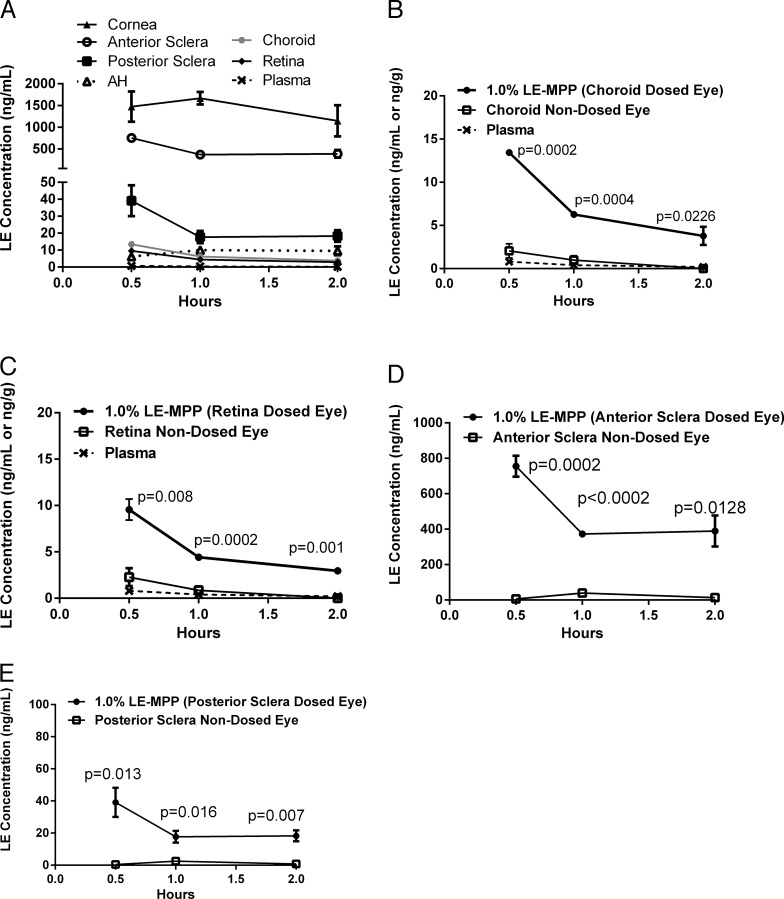
The primary objective in this study was to determine if LE could be detected at the back of the eye in significantly larger animals and to confirm the contribution of local delivery. The Gottingen mini-pig is considered an appropriate species for translation to human ocular drug distribution.29–35 The mini-pigs used here were 8-fold higher in body weight compared to the DB rabbits used in earlier studies, and also have pigmented eyes with similar melanin content to human eyes.25 The concentration-time profiles of LE in plasma, and all ocular tissues examined are shown in Figure 4A. Comparing the AUCs across the ocular compartments revealed that drug exposure had the following order, suggestive of a concentration gradient from the front of the eye to the back: cornea > anterior sclera ≫ posterior sclera > aqueous humor ∼ choroid ∼ retina. Drug levels were below detectable limits in the vitreous humor (<0.1 ng/mL). As shown in Figures 4B and 4C, the choroid in the dosed eye had 8-fold higher drug levels compared to the nondosed eye, and a 6-fold increase was observed in the retina. Anterior sclera in dosed pigs had a 17-fold higher drug level compared to the nondosed eye, and posterior sclera showed a 13-fold difference (Figs. 4D, 4E).
Figure 4.
The PK profiles of LE in Gottingen mini-pigs after topical administration of LE-MPP. The mean LE concentrations ± SE (ng/g, n = 3) were shown for the dosed eyes (A): cornea (solid triangle), anterior sclera (open circle), posterior sclera (solid square), AH (open triangle, dotted line), choroid (solid octagon), retina (solid diamond), plasma (x, dashed line). Dosed eyes (closed circles) versus nondosed eyes (open squares) versus plasma (x, dashed line) are shown for choroid (B), retina (C), anterior sclera (D), and posterior sclera (E). The AUC values were generated using GraphPad Prism v6.0 and ratios of dosed over nondosed eyes were determined. Statistical analysis was performed determined using the Holm-Sidak method multiple t-test, P values were shown on graphs (D, E).
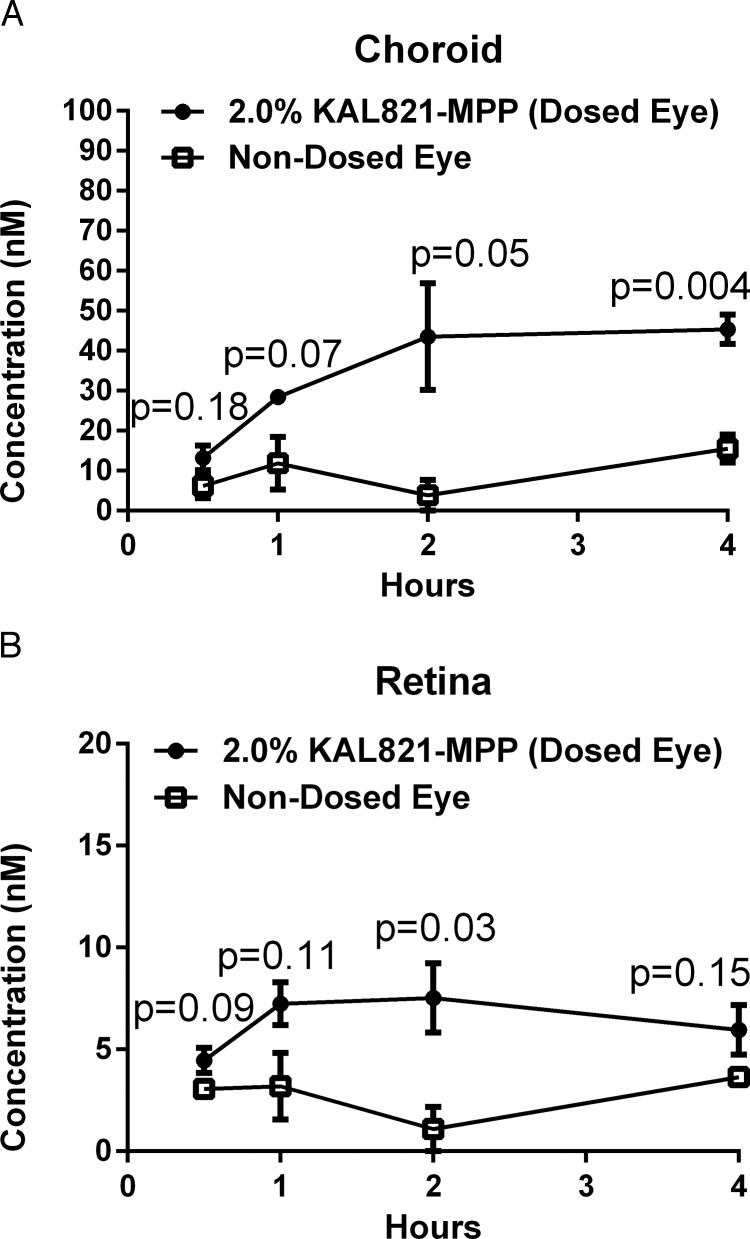
PK Profile of KAL821 (a Novel RTKi) in Pigmented Mini-Pig Dosed Versus Nondosed Eyes After Multi-Dose Topical Administration of KAL821-MPP
A new chemical entity KAL821, a potent inhibitor of VEGF-R2, was formulated as an MPP and topically administered to one eye of mini-pigs. The cellular potency, expressed as IC50, of this molecule is 1.6 ± 0.6 nM (n = 5). The concentration-time profiles of KAL821 in choroid and retina for the dosed and nondosed eyes are shown in Figure 5. Drug levels in choroid were 4-fold higher in the dosed eye than the nondosed eye, and a 3-fold difference was observed in the retina of the dosed eye versus the nondosed retina. The concentrations detected in the back of the eye were over the cellular IC50 for the target.
Figure 5.
The PK profiles of KAL821 in Gottingen mini-pigs after topical administration of KAL821-MPP. The mean KAL821 concentrations ± SE (nM, n = 3) were shown for choroid (A) and retina (B): Dosed eyes (closed circles), nondosed eyes (open squares). The AUC values were generated using GraphPad Prism v6.0 and ratios of dosed over nondosed eyes were determined. Statistical analysis was performed using the Holm-Sidak method multiple t-test, P values were shown on graphs.
Discussion
To date, means for enhancing topical drug delivery to the front of the eye often rely on viscosity-enhancing agents, which can lead to patient discomfort and lack of compliance, and generally only provide slight improvement in drug retention on the ocular surface.36–38 An even larger hurdle in ocular drug delivery is the treatment of sight-threatening diseases involving the posterior chamber, which to date has been achieved only by invasive and/or systemic delivery. Noninvasive techniques are the ultimate goal for treating retinal disease and the progress shown in nanotechnology, including the work presented here, may hold the key to success.2,3,6,39–42 If successful, topical delivery to the back of the eye would provide a major breakthrough in the treatment of sight-threatening diseases, such as AMD, diabetic retinopathy, and posterior uveitis.8
Unlike previous generations of drug-loaded particles that relied on covalent conjugation of drugs to a nanoparticle-forming polymer or encapsulation of drugs into polymeric carriers, the formulations described in this report were prepared by a novel method of making MPP via nanomilling of crystalline drugs. Contrary to the conjugation-based approaches, no chemical modifications of the drug or excipients are performed in this method and all excipients involved are those generally recognized as safe (GRAS) or, moreover, previously approved by the FDA for use in ophthalmic products.
To accurately test our hypothesis that MPPs can deliver topical drug to the back of the eye, it was critical to choose the most appropriate species to assess ocular drug distribution. Although commonly used, rodents provide little value when evaluating topical delivery due to their dramatically thinner corneal layer and smaller axial length, reduced anterior and vitreous chamber depth, and lower volume of tear film when compared to humans.43 A commonly used and more appropriate species for topical delivery evaluation is the rabbit. Features that make them an especially appropriate choice when delivering drugs topically are size and shape of their eyes, and their corneal thickness and anterior chamber depth similarities.24,44–47 The closer evolutionary distance of monkey to humans and the fact that nonhuman primates are the only other species to have a true macula have made them a favorite choice for ocular studies, particularly when using biologic interventions.44 However, there now is a body of evidence that suggests the Gottingen mini-pig may provide an excellent alternative to the use of monkeys and, in fact, has some key attributes that may even make them superior for certain applications and translation into humans. The sclera thickness in the small pigs ranges from 0.3 to 0.8 mm, which closely resembles the range in humans of 0.4 to 0.9 mm.31,48 The Gottingen mini-pig also has been found to be the closest species to humans in terms of melanin pigment content even when compared to the monkey; this may have a critical influence on drug binding within certain ocular compartments, and can affect drug partitioning and availability.25 Although pigs do not have a true macula, they do have a narrow horizontal area centralis that mimics primate macula.30 Other key porcine ocular features similar to humans include holangiotic retinal vascularization, refractive error, corneal power and thickness, choroidal blood flow, retinal pigment epithelium, the absence of a tapetum, and the presence of cone photoreceptors in the outer retina.30,33–35,49
To test our hypothesis, we first created an MPP formulation of the steroid LE and measured drug levels after topical administration to NZW rabbits. For nanoparticles to move through mucus, they must have certain defined surface properties (strong hydrophilicity and neutral charge) to circumvent adhesion and be small enough to avoid steric hindrance created by the dense fiber mesh.16 Compared to LE-CP, LE particles of the same size, but lacking these critical surface properties, the LE-MPP formulation increased the drug exposure at the corneal surface and in the retina (Figs. 1A, 1B). These results are consistent with our hypothesis that LE-MPP are able to avoid entrapment by ocular tear mucins and, thus, reduce ocular clearance. In a separate experiment, we also confirmed that the smaller particle size (e.g., 200 nm vs. >1 μm) contributes to improved ocular exposure, even if the surface modifier is identical. Together these results indicated that nanoparticles of LE combined with proper surface altering properties for LE provide the best drug delivery to tissues in the anterior and posterior segments of the eye.
To assess if the drug levels observed in the back of the eye are sufficient to elicit a biological effect, we used a standard model of vascular leakage in the rabbit to evaluate our topically administered LE-MPP formulation.24 This study was performed in the pigmented DB rabbits to account for any effect from melanin binding. The VEGF-induced vascular leakage was successfully prevented in animals receiving LE-MPP, demonstrating that the LE-MPP formulation can deliver therapeutically relevant concentrations to the back of the eye in rabbits.
Because it is possible that drug concentrations observed in the back of the eye can come from systemic circulation and local transport, we dosed drug to one eye and measured drug concentrations in both eyes. The assumption used to interpret these data is that the drug levels detected in the nondosed eye represent contribution from the systemic circulation, and the differences in drug levels between the dosed and nondosed eyes represent the local component of drug delivery from the ocular surface. Our results revealed an approximate 3-fold higher concentration of LE in back of the eye tissues in the dosed eye compared to the nondosed eye. Although some systemic contribution is likely, since detectable drug levels were seen in the nondosed back of the eye tissues, the higher concentrations in the dosed eye suggest direct local delivery of LE from the ocular surface. To further validate the observations in rabbits, we conducted a similar study in Gottingen mini-pigs. Results revealed an even greater separation of drug concentrations between the dosed and nondosed eyes, and the differences were statistically significant at every time point evaluated. These data confirmed the ability of LE-MPP to deliver drug to the back of the eye after topical delivery, and that true local delivery makes a significant contribution. Of course the ultimate test of whether LE-MPP can deliver sufficient steroid levels to exert a biological effect in humans will need to come from the clinic.
Given the positive preclinical data from LE-MPP, we decided to explore possible topical treatment options for AMD, a chronic, progressive disease of the macula. Our lead efforts are centered on small molecule inhibitors of the VEGF pathway (RTKi). KAL821, a potent inhibitor of the VEGF receptor, VEGF-R2, was formulated as an MPP and PK profiling was done in the mini-pig. Similar to LE, KAL821 was detected in the choroidal and retinal tissue after topical dosing of KAL821-MPP. Drug level in these tissues exceeded its cellular IC50 over the course of 4 hours tested. Studies are now underway to determine the best dose concentration and dose frequency to inhibit VEGF signaling with the MPP formulation.
To improve drug delivery across ocular tissues various approaches have been explored and implemented, such as pro-drugs, various viscosity-enhancing excipients and devices, and nanotechnology platforms.2,8,39–42,44,51 The posterior segment remains the highest hurdle for topical delivery. We have demonstrated in animal models that a steroid and RTKi formulated as mucus-penetrating nanoparticles provided enhanced drug penetration not only to the ocular surface, but also to the posterior segment. There are three potential pathways that a topically dosed drug can transport to the posterior segment from ocular surface: (1) the transvitreous route, where drugs diffuse through the cornea, enter the vitreous, and distribute to the back of the eye; (2) the periocular route, where diffusion occurs around the sclera and drugs are absorbed through the sclera; and (3) the uvea–scleral route where drugs diffuse through the cornea, penetrate the aqueous humor, and continue through the uvea-sclera where they can gain access to the choroid and retina.52 In our preclinical studies with LE, we observed high drug levels in cornea and aqueous humor, a concentration gradient from anterior to posterior sclera, and drug levels in the choroid and retina. However, no detectable drug levels in the vitreous humor were seen. Therefore, we propose that drug delivery with the LE-MPP is not accomplished through the transvitreous route, but via either the periocular or the uvea–scleral route. However, further distinguishing these latter two routes may prove difficult since the actual transport mechanism also may depend heavily on the physiochemical properties of any given drug.52
In summary, there is a clear unmet medical need for noninvasive, topical drug delivery to the anterior, but especially to the posterior segment of the eye. We have demonstrated here that in animal models, a steroid and an RTKi formulated as mucus-penetrating nanoparticles provided enhanced drug penetration not only to the ocular surface but also to the posterior segment.
Acknowledgments
We thank Kim Brazzell and Kristina Burgard (employees of Kala Pharmaceuticals) for their critical review of this manuscript. All rabbit PK studies were conducted at PharmOptima, LLC and mini-pig studies at Covancej Research Laboratories.
Supported by Kala Pharmaceuticals, Inc.
Disclosure: L.R. Schopf, Kala Pharmaceuticals; A.M. Popov, Kala Pharmaceuticals; E.M. Enlow, Kala Pharmaceuticals; J.L. Bourassa, Kala Pharmaceuticals; W.Z. Ong, Kala Pharmaceuticals; P. Nowak, Kala Pharmaceuticals; H. Chen, Kala Pharmaceuticals
References
- 1. Bodor N,, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J. 2005; 7: E820–E833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cholkar K,, Patel SP,, Vadlapudi AD,, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013; 29: 106–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boddu SH. Utility of transporter/receptor(s) in drug delivery to the eye. World J Pharmacol. 2013; 2: 1. [Google Scholar]
- 4. Molokhia SA,, Thomas SC,, Garff KJ,, Mandell KJ,, Wirostko BM. Anterior eye segment drug delivery systems: current treatments and future challenges. J Ocul Pharmacol Ther. 2013; 29: 92–105. [DOI] [PubMed] [Google Scholar]
- 5. Chastain JE. General considerations in ocular drug delivery. DRUGS Pharm Sci. 2003; 130: 59–108. [Google Scholar]
- 6. Wilson C. Topical drug delivery in the eye. Exp Eye Res. 2004; 78: 737–743. [DOI] [PubMed] [Google Scholar]
- 7. Thrimawithana TR,, Young S,, Bunt CR,, Green C,, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011; 16: 270–277. [DOI] [PubMed] [Google Scholar]
- 8. Geroski DH,, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000; 41: 961–964. [PubMed] [Google Scholar]
- 9. Olsen TW,, Feng X,, Wabner K,, Csaky K,, Pambuccian S,, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011; 52: 4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadam RS,, Williams J,, Tyagi P,, Edelhauser HF,, Kompella UB. Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties regional differences in delivery, and comparison with intravitreal and intracameral routes. Mol Vis. 2013; 19: 1198. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Chennamaneni SR,, Mamalis C,, Archer B,, Oakey Z,, Ambati BK. Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J Controlled Release. 2013; 167: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edelhauser HF,, Rowe-Rendleman CL,, Robinson MR,, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010; 51: 5403–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005; 57: 1595–1639. [DOI] [PubMed] [Google Scholar]
- 14. Mantelli F,, Argüeso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008; 8: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park CG,, Kim MJ,, Park M,, et al. Nanostructured mucoadhesive microparticles for enhanced preocular retention. Acta Biomater. 2014; 10: 77–86. [DOI] [PubMed] [Google Scholar]
- 16. Lai SK,, Wang Y-Y,, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009; 61: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ensign LM,, Tang BC,, Wang Y-Y,, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012; 4:139ra79. [DOI] [PMC free article] [PubMed]
- 18. Wang Y-Y,, Lai SK,, Suk JS,, Pace A,, Cone R,, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed. 2008; 47: 9726–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schuster BS,, Suk JS,, Woodworth GF,, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013; 34: 3439–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang BC,, Dawson M,, Lai SK,, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci. 2009; 106: 19268–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ensign LM,, Cone R,, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012; 64: 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schopf L,, Enlow E,, Popov A,, Bourassa J,, Chen H. Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol Ther. 2014; 3: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Draize J,, Woodward G,, Calvery H. Methods for the Study of Irritation and Toxicity of Substances Applied Topically to the Skin and Mucous Membranes. J Pharmacol Exp Ther. 1944; 82: 377–390. [Google Scholar]
- 24. Edelman J,, Lutz D,, Castro M. Corticorsteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005; 80: 249–258. [DOI] [PubMed] [Google Scholar]
- 25. Durairaj C,, Chastain JE,, Kompella UB. Intraocular distribution of melanin in human monkey, rabbit, minipig and dog eyes. Exp Eye Res. 2012; 98: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kadam RS,, Kompella UB. Influence of Lipophilicity on Drug Partitioning into Sclera Choroid-Retinal Pigment Epithelium, Retina, Trabecular Meshwork, and Optic Nerve. J Pharmacol Exp Ther. 2010; 332 (3): 1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Gaudana R,, Ananthula HK,, Parenky A,, Mitra AK. Ocular Drug Delivery. AAPS J. 2010; 12 (3): 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gray C. Systemic toxicity with topical ophthalmic medications in children. Paediatr Perinat Drug Ther. 2006; 7 (1): 23–29. [Google Scholar]
- 29. Gilger BC. Ocular Pharmacology and Toxicology. Totowa, NJ: Humana Press; 2014: 291–316. [Google Scholar]
- 30. Bode G,, Clausing P,, Gervais F,, et al. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010; 62 (3): 196–220. [DOI] [PubMed] [Google Scholar]
- 31. Olsen TW,, Sanderson S,, Feng X,, Hubbard WC. Porcine sclera: thickness and surface area. Invest Ophthalmol Vis Sci. 2002; 43 (8): 2529–2532. [PubMed] [Google Scholar]
- 32. Kadam RS,, Cheruvu NPS,, Edelhauser HF,, Kompella UB. Sclera-Choroid-RPE Transport of Eight -Blockers in Human, Bovine, Porcine, Rabbit, and Rat Models. Invest Ophthalmol Vis Sci. 2011; 52 (8): 5387–5399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Faber C,, Scherfig E,, Prause JU,, Sorensen KE. Corneal thickness in pigs measured by ultrasound pachymetry in vivo. Scand J Lab Anim Sci. 2008; 35 (1): 39. [Google Scholar]
- 34. Galdos M,, Bayón A,, Rodriguez FD,, Micó C,, Sharma SC,, Vecino E. Morphology of retinal vessels in the optic disk in a Göttingen minipig experimental glaucoma model: Glaucomatous optic disks in Göttingen minipigs. Vet Ophthalmol. 2012; 15: 36–46. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen LS,, Lind NM. Measurements of three ocular parameters in the Göttingen minipig. Scand J Lab Anim Sci. 2005; 32 (1): 9–16. [Google Scholar]
- 36. Kompella UB,, Kadam RS,, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010; 1 (3): 435–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao J. Wu, Zhou, Dahmani FZ. Enhanced and sustained topical ocular delivery of cyclosporine A in thermosensitive hyaluronic acid-based in situ forming microgels. Int J Nanomedicine. 2013; 8: 3587–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Westra, Lane S,, Coffey DeCory H. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin Ophthalmol. 2013; 7: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagarwal RC,, Kant S,, Singh PN,, Maiti P,, Pandit JK. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J Controlled Release. 2009; 136 (1): 2–13. [DOI] [PubMed] [Google Scholar]
- 40. Gan L,, Han S,, Shen J,, et al. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int J Pharm. 2010; 396 (1–2): 179–187. [DOI] [PubMed] [Google Scholar]
- 41. Okamoto N,, Ito Y,, Nagai N,, et al. Preparation of Ophthalmic Formulations Containing Cilostazol as an Anti-glaucoma Agent and Improvement in Its Permeability through the Rabbit Cornea. J Oleo Sci. 2010; 59 (8): 423–430. [DOI] [PubMed] [Google Scholar]
- 42. Jo DH,, Lee TG,, Kim JH. Nanotechnology and Nanotoxicology in Retinopathy. Int J Mol Sci. 2011; 12 (12): 8288–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams D. Rabbit and rodent ophthalmology. Eur J Companion Anim Pract. 2007; 17: 242–252. [Google Scholar]
- 44. Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol. 2008; 36 (1): 49–62. [DOI] [PubMed] [Google Scholar]
- 45. Paulsen FP,, Föge M,, Thale AB,, Tillmann BN,, Mentlein R. Animal model for the absorption of lipophilic substances from tear fluid by the epithelium of the nasolacrimal ducts. Invest Ophthalmol Vis Sci. 2002; 43 (10): 3137–3143. [PubMed] [Google Scholar]
- 46. Dong J,, Wu Q,, Wang X-G. Measurement of central corneal thickness and pre-corneal tear film thickness of rabbits using the Scheimpflug system. Int J Ophthalmol. 2013; 6 (5): 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doughty MJ,, Zaman ML. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures. Surv Ophthalmol. 44 (5): 367–408. doi:10.1016/S0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 48. Vurgese S,, Panda-Jonas S,, Jonas JB. Scleral Thickness in Human Eyes. Vavvas D. ed PLoS ONE. 2012; 7 (1): e29692 doi:10.1371/journal.pone.0029692. [DOI] [PMC free article] [PubMed]
- 49. Swindle MM,, Makin A,, Herron AJ,, Clubb FJ,, Frazier KS. Swine as Models in Biomedical Research and Toxicology Testing. Vet Pathol. 2012; 49 (2): 344–356. [DOI] [PubMed] [Google Scholar]
- 50. Gonzalez L,, Loza RJ,, Han K-Y,, et al. Nanotechnology in Corneal Neovascularization Therapy—A Review. J Ocul Pharmacol Ther. 2013; 29 (2): 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Durazo S,, Kompella U. Nanotechnology and Nanoparticles. : Kompella UB,, Edelhauser HF, Drug Product Development for the Back of the Eye. Vol 2. AAPS Advances in the Pharmaceutical Sciences Series. Springer; US; 2011: 261–290. [Google Scholar]
- 52. Gadek T,, Lee D. Topical Drug Delivery to the Back of the Eye. : Kompella UB,, Edelhauser HF, Drug Product Development for the Back of the Eye. Vol 2. AAPS Advances in the Pharmaceutical Sciences Series. Springer; US; 2011: 111–124. [Google Scholar]