Abstract
Objective. The aim of this study was to develop and validate a quantitative anti-signal recognition particle (SRP) autoantibody serum ELISA in patients with myositis and longitudinal association with myositis disease activity.
Methods. We developed a serum ELISA using recombinant purified full-length human SRP coated on ELISA plates and a secondary antibody that bound human IgG to detect anti-SRP binding. Protein immunoprecipitation was used as the gold standard for the presence of anti-SRP. Serum samples from three groups were analysed: SRP(+) myositis subjects by immunoprecipitation, SRP(−) myositis subjects by immunoprecipitation and non-myositis controls. The ELISA’s sensitivity, specificity, positive predictive value and negative predictive value were evaluated. Percentage agreement and test–retest reliability were assessed. Serial samples from seven SRP immunoprecipitation-positive subjects were also tested, along with serum muscle enzymes and manual muscle testing.
Results. Using immunoprecipitation, we identified 26 SRP(+) myositis patients and 77 SRP(−) controls (including 38 patients with necrotizing myopathy). Non-myositis control patients included SLE (n = 4) and SSc (n = 7) patients. Anti-SRP positivity by ELISA showed strong agreement (97.1%) with immunoprecipitation (κ = 0.94). The sensitivity, specificity, positive predictive value, and negative predictive value of the anti-SRP ELISA were 88, 100, 100 and 96, respectively. The area under the curve was 0.94, and test–retest reliability was strong (r = 0.91, P < 0.001). Serial samples showed that anti-SRP levels paralleled changes in muscle enzymes and manual muscle testing.
Conclusion. We developed a quantitative ELISA for detecting serum anti-SRP autoantibodies and validated the assay in myositis. Longitudinal assessment of SRP levels by ELISA may be a useful biomarker for disease activity.
Keywords: anti-signal recognition particle (SRP) autoantibody, ELISA, immunoprecipitation, idiopathic inflammatory myopathy, quantitative measure
Rheumatology key messages.
This study describes a valid ELISA-based semi-quantitative assay for anti-SRP antibody in a US cohort.
Anti-SRP antibody ELISA should improve the diagnosis of severe necrotizing myopathy.
Anti-SRP antibody levels are associated with myositis disease activity.
Introduction
The idiopathic inflammatory myopathies (IIMs) are a group of acquired, heterogeneous, systemic CTDs that include adult PM [1], adult DM, childhood myositis (predominantly JDM), myositis associated with cancer or another CTD, and IBM [2, 3]. There are several myositis-associated autoantibodies, and each autoantibody represents a unique clinical phenotype with importance in diagnosis, management and prognosis. Autoantibodies directed against signal recognition particles (SRPs) are associated with a severe form of PM with very high serum muscle enzyme levels and marked muscle weakness. Such diagnoses comprise ∼5% of IIMs and are classified as immune-mediated necrotizing myopathy (IMNM) [4]. Muscle biopsies of anti-SRP-positive subjects demonstrate a necrotizing myopathy (NM) with myofibre necrosis and little or no inflammatory infiltrates [5]. These patients are refractory to glucocorticoids and other immunosuppressive agents [4–6], and an initial aggressive approach is necessary, including the use of rituximab and IVIG [7, 8].
SRP expression is ubiquitous, and SRP facilitates the translocation of proteins across the endoplasmic reticulum during protein synthesis [9, 10]. SRP complexes consist of seven SL small RNA and six polypeptides (molecular weights 72, 68, 54, 19, 14 and 9 kDa) [9, 10]. Among all subcomponents of the SRP complex, the 54-kDa subunit (SRP54) is considered the main antigenic target of anti-SRP antibodies [11, 12]. Further studies on anti-SRP autoantibodies are limited due to the lack of a reliable quantitative measurement of serum levels of anti-SRP. The current methods for detecting anti-SRP antibodies are not quantitative and use protein immunoprecipitation techniques that are cumbersome, costly, time-consuming and generally only available in research laboratories. The development and validation of an ELISA for the quantitative measurement of anti-SRP autoantibodies would be a simpler detection technique providing earlier diagnosis of this refractory subset of myositis. Also, quantitative levels may serve as a biomarker and gauge for treatment response and flare, potentially contributing to an understanding of the aetiopathogenesis of this severe form of myopathy.
To date, there are no reported ELISA-based measurements of anti-SRP antibodies that have been validated. There is one quantitative anti-SRP antibody assay based on an addressable laser bead immunoassay, whereby investigators reported correlation of anti-SRP antibody levels with the serum creatine kinase (CK) in eight patients [13]. However, further studies are needed to understand the association of anti-SRP levels with CK and muscle strength. Our goal was to develop a semi-quantitative anti-SRP autoantibody ELISA and to validate this ELISA in a US-based, prospectively collected myositis cohort using immunoprecipitation results as the gold standard. Moreover, we also studied the association of anti-SRP autoantibody levels with serum CK levels and muscle strength on serial samples.
Patients and methods
Subjects
Serum and clinical information were obtained from a CTD registry, which contains more than three decades of prospectively collected clinical data and a matching serum repository from both inpatients and outpatients with myositis and other CTDs treated at the University of Pittsburgh Medical Center. We identified all adult myositis subjects meeting probable or definite Bohan and Peter criteria for a diagnosis of PM or DM, who were initially seen between January 1985 and December 2009, and who had a serum sample collected. Subjects fulfilling the Hoogendijk criteria for acquired IMNM were also identified [14]. All CTD subjects whose serum protein immunoprecipitation revealed characteristic 54- and 70-kDa bands (confirmed by reference serum using RNA immunoprecipitation) were considered to possess anti-SRP antibodies and were termed cases (n = 26). Myositis (PM, DM or IMNM) subjects not demonstrating anti-SRP antibodies by immunoprecipitation were randomly selected from the same database and included as non-anti-SRP antibody myositis controls (n = 67), including 38 anti-SRP-negative IMNM subjects. We also analysed non-myositis CTD control subjects with SSc (n = 7) and SLE (n = 4). We evaluated baseline (initial) visit samples from stored serum (−80°C) for all cases and controls using the anti-SRP antibody ELISA and protein immunoprecipitation techniques (described below and in Supplementary data, available at Rheumatology Online). Other myositis-specific and -associated antibodies [anti-synthetase (anti-Syn), anti-TIF1-γ, anti-Mi-2, anti-MJ, etc.], anti-3-hydroxy-3-methylglutaryl-Coenzyme A reductase antibodies (anti-HMGCR) (INOVA Diagnostics, San Diego, CA, USA), as well as other SSc- and CTD-associated autoantibodies were also measured [15–17]. In seven patients with serial samples, longitudinal serum anti-SRP levels measured by ELISA were compared with myositis disease activity parameters including: (i) serum CK, (ii) muscle strength by manual muscle testing (MMT). The University of Pittsburgh institutional review board approved this study, and subjects gave informed consent according to the Declaration of Helsinki.
Subject characteristics
We analysed serum samples from 26 subjects with anti-SRP autoantibodies identified by protein immunoprecipitation. Among the 26 anti-SRP-positive subjects, 25 had PM and 1 had undifferentiated CTD, while 24 of the 26 also met criteria for IMNM. Sixty-two per cent of subjects were female; 69% were Caucasian, 27% African American and 4% of other ethnicity. The median (IQR) age at diagnosis was 48.9 (42.8–55.4) years. None of the 26 anti-SRP-positive subjects had other myositis-associated antibodies. There were 78 serum samples from myositis (n = 67) and non-myositis control (n = 11) subjects, all with negative anti-SRP autoantibodies by protein immunoprecipitation. The 67 myositis (15 DM and 52 PM) controls included subjects with the following autoantibodies: anti-Jo-1 (n = 14), anti-TIF1-γ (n = 9), anti-Mi-2 (n = 4), anti-PL-7 (n = 1) and anti-MJ (n = 1).
Development of the anti-SRP antibody ELISA
Recombinant, purified, full-length human SRP54 (Origene Technologies, Rockville, MD, USA) was coated (100 ng/well) on a 96-well high-binding ELISA plate (Costar, Corning, NY, USA). Patient serum (dilution ≥ 1:100) was incubated with SRP54-coated ELISA plates, and a horseradish peroxidase conjugated secondary antibody that bound human IgG was used to detect anti-SRP54 binding. 3,3′,5,5′-tetramethylbenzidine was used as the horseradish peroxidase enzyme substrate, and the optical density of the resulting chromagen was measured. Matrices of different amounts of SRP54 and secondary antibody were used to determine optimum concentrations for anti-SRP antibody binding such that serum autoantibody levels were the sole limiting factor. This permitted a linear relationship between autoantibody concentration (units/ml) and optical density. Quantitative values in units/ml for anti-SRP autoantibody levels were assigned using a standard curve consisting of 4, 8, 16, 32, 64 and 128 U, where 64 was equivalent to a 1:100 dilution of a standard serum sample that was used for all ELISA runs. Serum samples with anti-SRP levels above the detection range ( > 128 U/ml) were re-run in the ELISA at a 1:1000 dilution.
Statistical analyses
Anti-SRP antibody ELISA results for anti-SRP-positive subjects and controls were compared with protein immunoprecipitation results, and an appropriate cut-off point was evaluated using a receiver-operating characteristic (ROC) curve. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and area under the curve (AUC) were determined. Mann–Whitney tests were used to compare serum levels of anti-SRP antibodies by ELISA in subjects with positive and negative anti-SRP antibody by protein immunoprecipitation. Chi-square test was used to evaluate the association between anti-SRP autoantibodies detected by ELISA and immunoprecipitation. Kappa statistics were used to measure agreement between ELISA and immunoprecipitation results. Test–retest reliability was measured using Pearson correlation of results of the same serum samples tested twice during different ELISA runs. For statistical calculations, ELISA values below the detection range (<4 U/ml) were assigned an arbitrary value of 2. The longitudinal changes of anti-SRP levels were associated with measures of myositis disease activity (CK, MMT) using linear mixed models.
Results
ELISA test characteristics
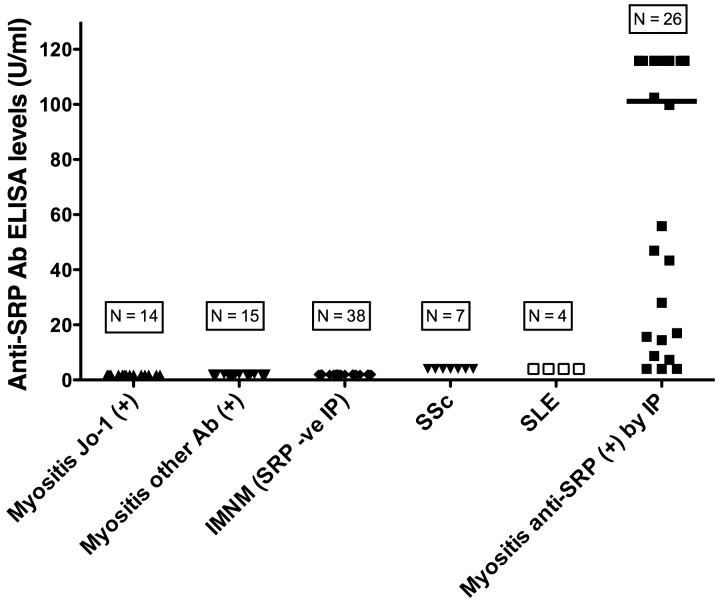
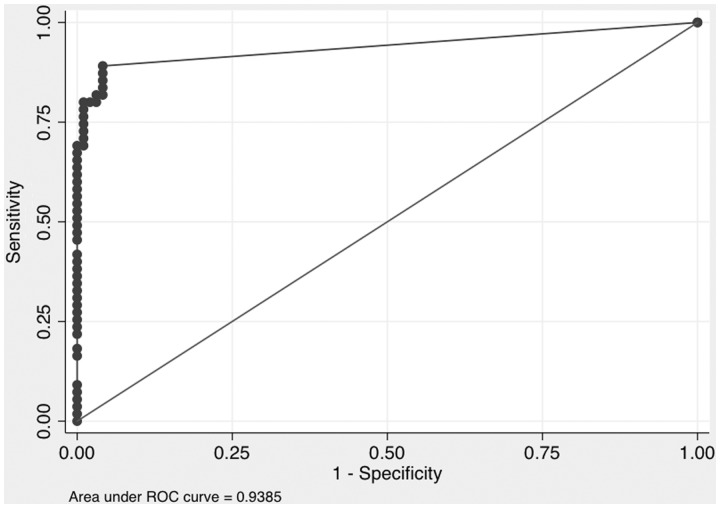
The anti-SRP ELISA was positive in 88.4% (23/26) of anti-SRP antibody immunoprecipitation-positive subjects as compared with 0% (0/78) of anti-SRP antibody negative controls (P < 0.001) (Table 1 and Fig. 1). Median (IQR) anti-SRP antibody levels by ELISA in subjects with positive and negative anti-SRP antibody immunoprecipitation results were 113.3 (15.6–128) and 2 (2–2) U/ml, respectively (P < 0.001) (Fig. 1). The immunoprecipitation and ELISA results concurred in 97.1% (101/104) of subjects, yielding a strong agreement between the two anti-SRP detection methods (κ = 0.94). The overall accuracy of the ELISA was 97.0%. A ROC curve identified a cut-off of 4 U/ml for ELISA positivity (Fig. 2), with an AUC of 0.94. Test–retest reliability was strong, with a Pearson correlation of 0.91 (P < 0.001). The anti-SRP antibody ELISA (using a cut-off of 4 U/ml) had 88% sensitivity, 100% specificity, 100% PPV, 96% NPV and 97% overall accuracy (Table 2).
Table 1.
Frequency of anti-signal recognition particle antibody-positive subjects by ELISA using a cut-off of 4 U/ml
| Myositis SRP + by immunoprecipitation | Control groups immunoprecipitationnegative for anti-SRP autoantibody |
|||||
|---|---|---|---|---|---|---|
| SSc (n = 7) | SLE (n = 4) | IMNM (n = 38) | Myositis Jo-1 (n = 14) | Myositis others (n = 15) | ||
| ELISA positive | 23 | 0 | 0 | 0 | 0 | 0 |
| ELISA negative | 3 | 7 | 4 | 38 | 14 | 15 |
| Total | 26 | 7 | 4 | 38 | 14 | 15 |
Other: 9 anti-TIF-1γ, 4 anti-Mi-2, 1 anti-MJ, 1 PL-7 autoantibodies; SRP: signal recognition particle; IMNM: immune-mediated necrotizing myopathy.
Fig. 1.
Anti-SRP antibody serum levels by ELISA in myositis and control subjects
IP: Protein immunoprecipitation; Ab: Autoantibody; IMNM: immune mediated necrotizing myopathy; Anti-SRP: anti-signal recognition particle.
Fig. 2.
Receiver operating characteristic curve for anti-signal recognition particle antibodies detected by ELISA vs protein immunoprecipitation
ROC: receiver operating characteristic.
Table 2.
Performance characteristics of anti-signal recognition particle antibody ELISA using a cut-off of 4 U/ml
| Sensitivity, % | Specificity, % | Positive predictive value, % | Negative predictive value, % | Overall accuracy, % |
|---|---|---|---|---|
| 88 | 100 | 100 | 96 | 97 |
Association of anti-SRP and anti-HMGCR autoantibodies in myositis subjects
None (0/24) of the anti-SRP antibody IMNM subjects were positive for anti-HMGCR antibodies. However, 60% (22/37) of non-SRP antibody IMNM controls were positive for anti-HMGCR antibodies. Therefore, anti-SRP and anti-HMGCR were mutually exclusive in subjects with IMNM and there were no false-positive anti-SRP antibodies detected by ELISA in subjects with HMGCR antibody-associated IMNM. The other 15 IMNM patients had no recognizable autoantibody detected.
Longitudinal changes in anti-SRP ELISA levels and relationship to myositis disease activity measures
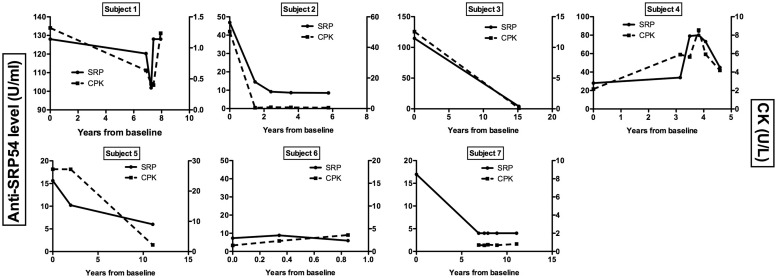
Serum samples from seven subjects with anti-SRP antibodies were collected at multiple time points (range: 3–6) and measured by ELISA with CK and MMT obtained at each time point. Patients had variable times of follow-up (median of 8 years). Overall, longitudinal changes in anti-SRP levels were associated with longitudinal changes in CK (Fig. 3; P < 0.01), and similar trends were seen with changes in MMT. There was significant variability in the anti-SRP levels among these seven subjects, although in most subjects anti-SRP levels decreased to very low (or undetectable) levels following prolonged treatment, with anti-SRP levels that paralleled decreases in CK and increases in MMT.
Fig. 3.
Longitudinal changes in anti-signal recognition particle 54-kDa subunit antibody levels and serum creatine kinase levels over time in seven subjects with positive anti-SRP by protein and RNA immunoprecipitation
Anti-SRP54: anti-signal recognition particle 54-kDa subunit antibody; CPK: creatine phosphokinase (also creatine kinase); SRP: signal recognition particle.
Discussion
This is the first report describing development and validation of an ELISA for the semi-quantitative measurement of serum anti-SRP autoantibodies. The ELISA is simple to perform and has high sensitivity (88%) and specificity (100%) and low false-positive and negative rates. We found excellent agreement in detecting anti-SRP autoantibodies by ELISA compared with immunoprecipitation methods. The SRP ELISA can facilitate assessment of the IMNM subset of myositis by differentiating between anti-SRP(+) vs anti-HMGCR-associated NM. This is clinically relevant, since distinguishing these myopathies often poses a significant challenge to physicians. One of the strengths of the reported ELISA is its 100% specificity. None of the 67 non-SRP(+) myositis patients (including 38 patients with muscle histopathology similar to anti-SRP patients (i.e. NM) were positive using this ELISA. All anti-SRP antibody (+) patients (including the three false negative by anti-SRP ELISA) were indeed negative for anti-HMGCR antibodies. Among the three false-negative (immunoprecipitation+/ELISA−) subjects, one subject, a 33-year-old African-American female, had classic NM on muscle biopsy with severe muscle weakness, very high muscle enzyme levels and refractory disease consistent with classic anti-SRP NM. One subject, a 30-year-old Caucasian female, had severe refractory muscle weakness with muscle biopsy consistent with DM (without the skin rash of DM) that was not consistent with the typical phenotype seen in anti-SRP-positive patients. The third subject, a 32-year-old African-American female, had PM with severe refractory disease that was not entirely consistent with the typical phenotype seen with anti-SRP positivity.
Our results are similar to a recent study using addressable laser bead immunoassay for semi-quantitative anti-SRP antibody detection, in which 31 anti-SRP subjects were studied [13]. Eight subjects followed longitudinally showed an association of anti-SRP levels with CK. In addition to demonstrating an association of ELISA-based anti-SRP levels with CK, we also demonstrated that anti-SRP levels over time paralleled changes in MMT, both being important measures of disease activity in NM patients.
Anti-SRP-positive NM patients generally have severe, treatment-resistant myositis with a NM and a high serum CK. Although little is known regarding the pathogenesis of this NM, an in vitro study suggests that anti-SRP autoantibodies purified from anti-SRP-positive myositis subjects have a pronounced and specific inhibitory effect on the translocation of secretory proteins from endoplasmic reticulum [12]. Given that the serum levels of muscle enzymes, especially CK, reflect muscle necrosis and disease activity in anti-SRP-positive patients, the significant association between anti-SRP antibody levels and muscle enzymes in our study supports the role of anti-SRP antibodies as a surrogate marker for disease activity and as a mediator of disease pathogenesis in this NM subset of IIM patients. B-cell-depleting therapies like rituximab have shown excellent efficacy in patients possessing anti-SRP antibodies, again suggesting a possible role of anti-SRP in the pathogenesis of myositis [7, 18]. The clinical improvement of such patients may be due to decreased levels of anti-SRP autoantibodies following rituximab. Our study suggests that anti-SRP levels may serve as a biomarker of disease activity, and future clinical trials should quantify anti-SRP autoantibody levels in myositis.
While the development, validation and longitudinal correlation of our ELISA results with disease activity were major strengths of our study, there are several potential limitations. Although one might argue that stored serum samples may have degraded over time, we have noted that autoantibodies are stable for many years in appropriately stored serum samples. In addition, the immunoprecipitation on all of the older samples was performed close to the time that the patients presented to our centre, and the ELISA that was done on the appropriately stored samples yielded the same results as those noted by immunoprecipitation. There was no association between the age of the serum sample and the quantitative ELISA result for that sample. Another potential limitation of our study is the small sample size of the longitudinal data, which limits the conclusions that can be drawn from this analysis. Nevertheless, our longitudinal data provide preliminary data for additional larger longitudinal studies. A third potential limitation of our study is related to our development of an ELISA that only detects the SRP54 subunit of the SRP antigen complex, which may limit detection of anti-SRP autoantibodies that bind other parts of the SRP complex. This may explain the finding of three false-negative subjects among the anti-SRP subjects detected by immunoprecipitation. However, even if true, this is a minor limitation, since the false-negative rate was only 3.7% with a highly specific assay that provides semi-quantitative results that can be used as a biomarker for disease activity in some patients. Another potential limitation of our study is that we only analysed a small number of non-myositis control subjects and no healthy control subjects. However, we did this to focus our analysis on a control population that only included subjects with clinical phenotypes for which this autoantibody is likely to be tested.
In summary, we report the development, validation and reliability of an ELISA-based semi-quantitative assay for measurement of serum anti-SRP autoantibodies in a US cohort. The ELISA had excellent sensitivity and specificity, and there was substantial agreement of results between the ELISA and non-quantitative immunoprecipitation techniques. We propose that this semi-quantitative ELISA measurement would provide a simple yet reliable method for anti-SRP detection in clinical rheumatology practice since ELISA represents a significant advancement over the non-quantitative, cumbersome and costly immunoprecipitation techniques that are currently available. We believe that anti-SRP autoantibodies are valuable clinical biomarkers with the potential to provide pathogenic insight into the link between these autoantibodies and NM. From a clinical perspective, we would recommend ELISA testing for anti-SRP and anti-HMGCR autoantibodies in patients with a biopsy demonstrating NM. If ELISA testing is negative it should be followed by more comprehensive testing by immunoprecipitation for myositis autoantibodies. Moreover, longitudinal measurements of anti-SRP autoantibody levels may provide valuable clues regarding the prognosis of IMNM and treatment responsiveness associated with a decline or increase in serum levels of anti-SRP antibody. We anticipate the commercial development and widespread use of an anti-SRP ELISA to measure this antibody in patients with suspected IMNM. However, additional studies are needed to establish a link between longitudinal changes in serum levels of anti-SRP autoantibodies and myositis disease activity.
Funding: This project was supported by NIAMS grant number 5R01AR061298.
Disclosure statement: During the conduct of this research, M.C.L. was an employee of the University of Pittsburgh. He received grant support from Genentech, was a consultant for Genentech, UCB, Crescendo and Baxter, and an expert witness for Abbott. During preparation of this manuscript, M.C.L. was an employee of Abbvie. C.V.O. has received research support for a clinical trial with Genentech. R.A. has received research grants from Questcor and Pfizer, and has served as a consultant to Questcor and aTyr Pharma Inc. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Bernstein RM, Morgan SH, Chapman J, et al. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br Med J (Clin Res Ed) 1984;289:151–2. doi: 10.1136/bmj.289.6438.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis. 2001;60:116–23. doi: 10.1136/ard.60.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hengstman GJ, ter Laak HJ, Vree Egberts WT, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006;65:1635–8. doi: 10.1136/ard.2006.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990;33:1361–70. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- 7.Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res. 2010;62:1328–34. doi: 10.1002/acr.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Rosell M, Moore S, Pattanaik D, et al. Signal recognition antibody-positive myopathy and response to intravenous immunoglobulin G (IVIG) J Clin Rheumatol. 2013;19:214–7. doi: 10.1097/RHU.0b013e31828e6442. [DOI] [PubMed] [Google Scholar]
- 9.Reeves WH, Nigam SK, Blobel G. Human autoantibodies reactive with the signal-recognition particle. Proc Natl Acad Sci U S A. 1986;83:9507–11. doi: 10.1073/pnas.83.24.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada N, Mimori T, Mukai R, Kashiwagi H, Hardin JA. Characterization of human autoantibodies that selectively precipitate the 7SL RNA component of the signal recognition particle. J Immunol. 1987;138:3219–23. [PubMed] [Google Scholar]
- 11.Janda CY, Li J, Oubridge C, et al. Recognition of a signal peptide by the signal recognition particle. Nature. 2010;465:507–10. doi: 10.1038/nature08870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romisch K, Miller FW, Dobberstein B, High S. Human autoantibodies against the 54 kDa protein of the signal recognition particle block function at multiple stages. Arthritis Res Ther. 2006;8:R39. doi: 10.1186/ar1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benveniste O, Drouot L, Jouen F, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2011;63:1961–71. doi: 10.1002/art.30344. [DOI] [PubMed] [Google Scholar]
- 14.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:337–45. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal R, Lucas M, Fertig N, Oddis CV, Medsger TA., Jr Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009;60:1112–8. doi: 10.1002/art.24409. [DOI] [PubMed] [Google Scholar]
- 16.Okano Y, Medsger TA., Jr Novel human autoantibodies reactive with 5′-terminal trimethylguanosine cap structures of U small nuclear RNA. J Immunol. 1992;149:1093–8. [PubMed] [Google Scholar]
- 17.Mitri GM, Lucas M, Fertig N, Steen VD, Medsger TA., Jr A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum. 2003;48:203–9. doi: 10.1002/art.10760. [DOI] [PubMed] [Google Scholar]
- 18.Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–24. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.