Abstract
Objective. The aim of this study was to assess the prognostic value of systolic pulmonary artery pressure (sPAP) estimated by echocardiography in the multinational European League Against Rheumatism Scleroderma Trial and Research (EUSTAR) cohort.
Methods. Data for patients with echocardiography documented between 1 January 2005 and 31 December 2011 were extracted from the EUSTAR database. Stepwise forward multivariable statistical Cox pulmonary hypertension analysis was used to examine the independent effect on survival of selected variables.
Results. Based on our selection criteria, 1476 patients were included in the analysis; 87% of patients were female, with a mean age of 56.3 years (s.d. 13.5) and 31% had diffuse SSc. The mean duration of follow-up was 2.0 years (s.d. 1.2, median 1.9). Taking index sPAP of <30 mmHg as reference, the hazard ratio (HR) for death was 1.67 (95% CI 0.92, 2.96) if the index sPAP was between 30 and 36 mmHg, 2.37 (95% CI 1.14, 4.93) for sPAP between 36 and 40 mmHg, 3.72 (95% CI 1.61, 8.60) for sPAP between 40 and 50 mmHg and 9.75 (95% CI 4.98, 19.09) if sPAP was >50 mmHg. In a multivariable Cox model, sPAP and the diffusing capacity for carbon monoxide (DLCO) were independently associated with the risk of death [HR 1.833 (95% CI 1.035, 3.247) and HR 0.973 (95% CI 0.955, 0.991), respectively]. sPAP was an independent risk factor for death with a HR of 3.02 (95% CI 1.91, 4.78) for sPAP ≥36 mmHg.
Conclusion. An estimated sPAP >36 mmHg at baseline echocardiography was significantly and independently associated with reduced survival, regardless of the presence of pulmonary hypertension based on right heart catheterization.
Keywords: systemic sclerosis, tricuspid regurgitant jet velocity, systolic pulmonary arterial pressure, pulmonary hypertension, survival
Rheumatology key messages.
The hazard ratio for death was 3.02 (95% CI 1.91, 4.78) for systolic pulmonary artery pressure ≥ 36 mmHg in SSc patients.
The hazard ratio for death was 4.94 (95% CI 2.66, 9.17) for DLCO <64% of predicted in SSc patients.
Right heart catheterization should be considered each time pulmonary hypertension is suspected in SSc patients.
Introduction
Pulmonary arterial hypertension (PAH) is a frequent complication of SSc with a prevalence ∼10% [1, 2]. Right heart catheterization (RHC) is mandatory for the diagnosis of PAH. Pulmonary hypertension (PH) is defined on RHC by a mean pulmonary artery pressure (mPAP) of ≥25 mmHg at rest with a mean pulmonary arterial wedge pressure (mPAWP) of <15 mmHg [3]. Within the last two decades, interstitial lung disease (ILD) and PAH have become the leading causes of death in SSc patients [4, 5]. The estimated 3 year survival among patients with PAH associated with SSc without therapy is ∼50–60% [6–9] and is worse than the prognosis observed in idiopathic PAH. Many factors can explain this dismal prognosis. Among them, older age, cardiomyopathy and the high prevalence of pulmonary veno-occlusive disease have been proposed. Moreover, PAH is often diagnosed in an advanced stage in SSc, with up to 75% of patients being in New York Heart Association functional class III or IV. To improve this prognosis, and taking the high prevalence of PAH into account, yearly screening for PAH in SSc patients is suggested by various guidelines [10]. Thus far, echocardiography is the most used screening tool for suspect PH in SSc. Based on the tricuspid regurgitant jet (TRJ) velocity, estimated systolic pulmonary artery pressure (sPAP) can be calculated using the Bernoulli equation and adding estimated right atrial pressure (RAP). The European Society of Cardiology/European Respiratory Society (ESC/ERS) diagnostic algorithm in SSc patients with suspected PH is based on the maximal TRJ [3]: a patient with a TRJ of ≤2.8 m/s (estimated sPAP ≤36 mmHg, assuming mean RAP (mRAP) ≤5 mmHg) is unlikely to have PH. Based on this algorithm, RHC should be considered in SSc patients with suspected PH if the TRJ is between 2.9 and 3.4 m/s (corresponding to an estimate sPAP of between 37 and 50 mmHg, assuming RAP is 5 mmHg) in symptomatic patients and if the TRJ is >3.4 m/s (corresponding to an estimate sPAP >50 mmHg, assuming RAP is 5 mmHg) in non-symptomatic patients. We have previously shown in a large nationwide prospective study that if RHC is performed in the case of a TRJ >2.8 m/s, it is possible to identify at an early stage SSc patients suffering from PAH and survival can be improved with early therapeutic intervention [10, 11]. In the trans-sectional study performed initially, we used a TRJ cut-off of 2.5 m/s, which seemed too low to identify SSc patients at risk of PH and produced a relatively high percentage of false-positive results [12]. Unfortunately, in real practice, RHC is not systematically performed in all SSc patients in whom PH is suspected. Therefore, while the prognosis of RHC-proven PAH is known, there are no data on the prognostic value of estimate sPAP, regardless of the presence of RCH-proven PH, whatever the mechanism of PH. Thus the aim of the present study was to assess the prognostic value of sPAP estimated from the TRJ measured by echocardiography to predict 5 year survival in patients with SSc using the multinational European League Against Rheumatism Scleroderma Trial and Research (EUSTAR) cohort.
Methods
Data collection
Population
From June 2004 onwards, EUSTAR centres were requested to enter patient data into a web database, provided that the patients had given their informed written consent. Data were to be collected at each visit and at least once a year. Since the aim of our study was to evaluate the prognostic value of estimated sPAP in the modern PAH therapeutic era, data collected from 2005 to 31 December 2011 were extracted from the EUSTAR database. The database started in 2004, but information about sPAP was requested from 2007 with the development of the online database. It was also possible from 2007 to enter patients with a record of previous echocardiographies performed before 2007. Patients were selected if they had a first echocardiography after 1 January 2005 with at least one follow-up visit between the 1 January 2005 and the 31 December 2011. The first echocardiography performed after 1 January 2005 with an evaluation of sPAP was termed the index echocardiography. Data of all subsequent visits were used provided that at least one echocardiography and/or RHC was performed. Patients with PH or PAH documented by a RHC before 1 January 2005 were also excluded from the study. The EUSTAR scientific committee approved the evaluation of the prognostic value of sPAP in patients suffering from SSc included in the EUSTAR database. Ethics committee approval for the study was obtained from each participating EUSTAR centre (based on Ethics Committee Approval, Basel, Switzerland, no. 323/09).
Collected data
Collected data included demographics, history of RP, history of the first non-RP symptom of SSc, subtype of SSc (diffuse, limited or other), modified Rodnan skin score (mRSS), history of renal crisis, immunological status, dyspnoea level based on the NYHA classification, history of digital ulcers, systemic treatments and co-morbidity with systemic hypertension. Available data regarding echocardiography [TRJ, sPAP, left ventricular ejection fraction (LVEF), diastolic dysfunction, pericardial effusion], RHC (right ventricular systolic pressure, mPAP, pulmonary resistance, PAWP, cardiac index), 6 min walking test (distance walked, oxygen saturation at rest and during exercise), pulmonary function tests, diffusion capacity for carbon monoxide (DLCO), forced vital capacity (FVC), total lung capacity (TLC) and forced expiratory volume in 1 s were collected at the date of the index echocardiography. The different sPAP categories were based on the 2009 ESC/ERS recommendations [3]: if sPAP is <36 mmHg, PH is unlikely; if sPAP is >36 mmHg but <50 mmHg, PH is possible and if sPAP is >50 mmHg, PH is likely. A restrictive lung syndrome was defined as a FVC or TLC <70% of predicted. If available, data regarding lung fibrosis from a high-resolution CT scan were entered in the database.
Survival status of the patient was evaluated at the date of the last available information. If the patient died during the follow-up period, the date of death was provided by the centre. We also asked all centres with at least one patient meeting inclusion criteria whether RHC was performed during follow-up and to provide the results, in case the information was missing in the EUSTAR database. A data review was conducted prior to analysis in order to determine whether the patient could be included in the analysis.
Data analysis
Results are expressed as mean (s.d.) for continuous variables and as n (%) for categorical variables. All-cause mortality was used for analyses. The date of index echocardiography was considered the baseline from which survival was measured. Individual analyses based on the Cox PH model were used to examine relationships between survival and selected variables measured at index echocardiography. Events were right-censored at 5 years of follow-up. Stepwise forward multivariable Cox PH analysis was used to examine the independent effect on survival of selected variables (those with a P-value ≤ 0.20 from individual analysis with sufficient documentation), controlling for possible confounders. Assumptions on comparable characteristics of censored and uncensored patients and were tested. The constancy of the HR over time was evaluated. The DLCO threshold was derived from logistic regression models and receiver operating characteristic (ROC) curves. In addition, we performed regression analysis using sPAP at the index echocardiography as a continuous variable and we then conducted multivariate analysis limited to sPAP and one of the other potentially explanatory variables. We examined the regression coefficient for each millimetre of mercury of sPAP and considered that the other variable added information if it led to a change in the sPAP regression coefficient of at least 10%. Statistical analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Population characteristics
A total of 24 405 visits involving 9616 patients were extracted from the EUSTAR database. Among these patients, 420 had only one documented visit before 1 January 2005, 4789 had no documented sPAP at any visit, 13 were <18 years old and 12 died at the first visit. In addition, 404 did not meet the ACR criteria, 1965 had their first visit before 1 January 2005 and 67 had a diagnosis of PAH before the first visit recorded in the database. For the remaining 1946 patients, all centres were contacted to obtain the survival status and to determine if RHC was performed at any time during follow-up; we received a response from the centres for 1476 patients. Therefore 1476 patients from 54 centres were included in the analysis.
The characteristics of the analysed patients at baseline are summarized in Table 1. Mean duration of follow-up was 2.0 years (s.d. 1.2, median 1.9). During this period of follow-up, of the 273 patients who had an index sPAP ≥36 mmHg, 44 (16.1%) underwent RHC: 24 were found to have PAH, 6 to have post-capillary PH and 4 to have PH related to ILD. In total, 42 (2.8%) patients were diagnosed with PAH during this period, whereas post-capillary PH was found in 14 (0.9%) patients and PH related to ILD was found in 6 (0.4%) patients.
Table 1.
Characteristics of the 1476 patients analysed
| Total number documented | Data | |
|---|---|---|
| Female | 1476 | 1280 (86.7) |
| BMI, kg/m2 | 742 | 24.4 (4.4) |
| Age, years | 1476 | 56.3 (13.5) |
| Time since RP, years | 1457 | 13.9 (11.1) |
| Time since non-RP first symptom, years | 1350 | 9.5 (7.6) |
| dcSSc | 1476 | 462 (31.3) |
| lcSSc | 1476 | 984 (66.7) |
| Limited SSc | 1476 | 30 (2.0) |
| Modified Rodnan skin score | 1298 | 8.9 (7.3) |
| Hypertension | 1454 | 309 (21.3) |
| History of renal crisis | 1384 | 20 (1.5) |
| Active digital ulcers | 1457 | 520 (35.7) |
| Lung fibrosis (on HRCT) | 862 | 439 (50.9) |
| Restrictive syndrome (FVC and/or TLC <70%) | 1402 | 332 (23.7) |
| ANAs | 1392 | 1345 (96.6) |
| ACAs | 1342 | 499 (37.2) |
| Anti-topoisomerase antibodies | 1343 | 469 (34.9) |
| NYHA functional class | 1379 | |
| I | 771 (55.9) | |
| II | 474 (34.4) | |
| III | 116 (8.4) | |
| IV | 18 (1.3) | |
| sPAP, mmHg | 1476 | 29.8 (9.5) |
| DLCO, % | 1340 | 68.7 (19.5) |
| FVC, % | 1343 | 96.0 (21.7) |
| TLC, % | 692 | 92.6 (19.4) |
| LVEF, % | 1339 | 62.0 (5.9) |
| Diastolic dysfunction | 1374 | 294 (21.4) |
| Pericardial effusion | 1252 | 91 (7.3) |
Results are the number of documented patients expressed as the mean (s.d.) for continuous variables or as n (%) for categorical variables. Percentages are calculated on the number of documented variables. DLCO: diffusing capacity for carbon monoxide; FVC: forced vital capacity; HRCT: high-resolution computed tomography; LVEF: , left ventricular ejection fraction; NYHA: New York Heart Association; sPAP: systolic pulmonary artery pressure; TLC: total lung capacity.
Prognostic value of estimated sPAP at baseline and other risk factors of death
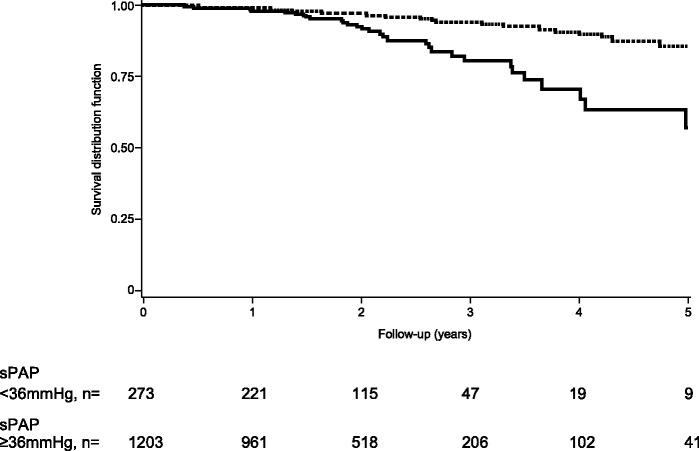
During the follow-up period, 76 patients died (only 15 deaths were recorded to be related to SSc, but data were missing). Whether death was related to PH was not provided due to the structure of the database. The overall 5 year survival rate was 79.7% (95% CI 72.6%-, 85.1%). Kaplan–Meier estimates of the survival distribution were significantly different across index sPAP values, as shown in Fig. 1 (log-rank test P < 0.0001). The estimated 5 year survival rate decreased from 88.2% (95% CI 78.9%-, 93.5%) in patients with an index sPAP <30 mmHg to 28.4% in patients with an index sPAP ≥50 mmHg. Assuming a sPAP threshold of 36 mmHg, the estimated 5 year survival rate was 85.7% (95% CI 78.5, 90.6) in patients with an sPAP <36 mmHg and 57.3% (95% CI 38.4%-, 72.3%) in patients with an sPAP ≥36 mmHg. The 1, 2 and 3 year survival rates are provided in Table 2.
Fig. 1.
Five-year survival distribution function according to sPAP at index echocardiographyDashed line: sPAP <36 mmHg; solid line: sPAP ≥36 mmHg (values at index echocardiography). sPAP: systolic pulmonary arterial pressure.
Table 2.
Risk of death associated with sPAP at index echocardiography
| sPAP <36 mmHg (n = 1203) | 36 ≤ sPAP ≤ 50 mmHg (n = 213) | sPAP >50 mmHg (n = 48) | |
|---|---|---|---|
| 1 year survival estimate, % (95% CI) | 99.4 (98.7, 99.7) | 98.9 (95.8, 99.7) | 93.4 (81.0, 97.8) |
| 2 year survival estimate, % (95% CI) | 97.0 (95.6, 98.0) | 93.5 (87.8, 96.6) | 86.5 (69.7, 92.0) |
| 3 year survival estimate, % (95% CI) | 93.8 (91.1, 95.7) | 84.5 (73.7, 91.2) | 66.5 (42.8, 82.1) |
| Hazard ratio (95% CI) | 1.44 (1.06, 1.96) | 1.83 (1.43, 2.33) |
Data are right-censored at 60 months. hazard ratios are derived from a Cox model. The 1, 2 and 3 year survival rates were estimated with a Kaplan–Meier model. sPAP: systolic pulmonary arterial pressure.
Taking the index estimated sPAP <30 mmHg as reference, a Cox model showed that HRs for death were 1.67 (95% CI 0.94, 3.06) if the index sPAP was ≥30 and <36 mmHg, 2.31 (95% CI 1.04, 5.12) for an sPAP ≥36 and <40 mmHg, 2.79 (95% CI 1.30, 5.97) for an sPAP ≥40 and <50 mmHg and 8.39 (95% CI 4.40, 16.17) if the sPAP was ≥50. Taking the index estimated sPAP <36 mmHg as a reference, SSc patients with an sPAP ≥36 mmHg had a poorer prognosis. Survival rates were 99.4% (95% CI 98.7%, 99.7%), 97.0% (95% CI 95.6%, 98.0%) and 93.8% (95% CI 91.1%, 95.7%) at the 1, 2 and 3 year follow-ups in patients with an index sPAP <36 mmHg, compared with 98.0% (95% CI 95.3%, 99.2%), 92.5% (95% CI 87.5%, 95.5%) and 80.6% (95% CI 71.3%, 87.2%) for patients with an index sPAP ≥36 mmHg. HR for death was 3.02 (95% CI 1.91, 4.78) for PAPs ≥36 mmHg.
Finally, DLCO <60% was associated with a HR of 8.30 (95% CI 2.96, 23.25) for death vs DLCO ≥80%. Using logistic regression modelling, the vital status of the patient at the end of follow-up and plotting a ROC curve, we found 64% of predicted DLCO to be the best cut-off value. This threshold was associated with a HR of 4.94 (95% CI 2.66, 9.17) for death. The 3 year survival rate was 77.5% (95% CI 63.6%-, 86.6%) if the DLCO was <64% vs 88.4% (95% CI 62.9%-, 96.8%) if the DLCO was ≥64%.
Besides the index sPAP and DLCO, numerous characteristics of patients at the index visit were associated with survival (Table 3). In a multivariate Cox model with stepwise selection, DLCO, age, TLC and index sPAP were independently associated with the risk of death: for each point lost in the index DLCO (expressed as a percentage of the theoretical value), the 5 year risk of death increased by 2.7% (95% CI 0.9%-, 4.5%); for each year of age, the risk increased by 6.5% (95% CI 3.9%-, 9.3%); for a loss of 1% of predicted TLC, the risk increased by 2.6% (95% CI 1.1%, 4.0%) and the risk was 1.83-fold greater (95% CI 1.035, 3.25) if the index sPAP was ≥36 mmHg (Table 4). Other variables (i.e. gender, diffuse SSc, mRSS, LVEF, diastolic dysfunction and pericardial effusion) were not retained in the multivariate model. In addition, we performed regression analysis using sPAP at the index echocardiography as a continuous variable and then conducted multivariate analysis limited to sPAP and one of the other potentially explanatory variables. We examined the regression coefficient for each millimetre of mercury of sPAP and considered that the other variable added information if it led to a change in the sPAP regression coefficient of at least 10%. Only patient age and DLCO at the index visit met this criterion.
Table 3.
Patient characteristics at the index visit associated with risk of death (univariate analysis)
| Parameter | s.e. | HR (95% CI) | P-value | |
|---|---|---|---|---|
| Age, years | 0.05 | 0.01 | 1.05 (1.03, 1.08) | <0.0001 |
| Male | 0.75 | 0.26 | 2.13 (1.28, 3.55) | 0.004 |
| Diffuse SSc | 0.63 | 0.23 | 1.87 (1.19, 2.95) | 0.007 |
| sPAP, mmHg | 0.05 | 0.007 | 1.06 (1.04, 1.07) | <0.0001 |
| DLCO, % | −0.05 | 0.008 | 0.95 (0.94, 0.97) | <0.0001 |
| FVC, % | −0.04 | 0.006 | 0.97 (0.95, 0.98) | <0.0001 |
| TLC, % | −0.04 | 0.009 | 0.96 (0.95, 0.98) | <0.0001 |
| mRSS, per unit | 0.04 | 0.01 | 1.04 (1.01, 1.07) | 0.005 |
| LVEF, % | −0.05 | 0.02 | 0.95 (0.92, 0.98) | 0.003 |
| Diastolic dysfunction | 0.40 | 0.26 | 1.50 (0.89, 2.51) | 0.13 |
| Pericardial effusion | 0.83 | 0.36 | 2.29 (1.13, 4.63) | 0.02 |
| Lung fibrosis | 0.47 | 0.28 | 1.60 (0.92, 2.77) | 0.10 |
| PAH or PH | 1.49 | 0.27 | 4.43 (2.63, 7.48) | <0.0001 |
DLCO, FVC and TLC are expressed as a percentage of the theoretical value. DLCO: diffusion capacity for carbon monoxide; FVC: forced vital capacity; HR: hazard ratio; LVEF: left ventricular ejection fraction; mRSS: modified Rodnan skin score; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; sPAP: systolic pulmonary arterial pressure; TLC: total lung capacity.
Table 4.
Patient characteristics at the index visit associated with risk of death (multivariate analysis)
| Parameter | s.e. | HR (95% CI) | P-value | |
|---|---|---|---|---|
| DLCO, % predicted | −0.02765 | 0.00939 | 0.973 (0.955, 0.991) | 0.003 |
| Age, year | 0.06330 | 0.01296 | 1.065 (1.039, 1.093) | <0.0001 |
| TLC, % predicted | −0.02638 | 0.00763 | 0.974 (0.960, 0.989) | 0.0005 |
| sPAP ≥36 mmHg | 0.60604 | 0.29161 | 1.833 (1.035, 3.247) | 0.04 |
DLCO and TLC are expressed as a percentage of the theoretical value. DLCO: diffusion capacity for carbon monoxide; HR: hazard ratio; sPAP: systolic pulmonary arterial pressure; TLC: total lung capacity.
Discussion
The international guidelines recommend echocardiographic screening in SSc for the detection of PH in symptomatic patients [World Health Organization (WHO) class I, level B] [13] and may be considered in asymptomatic patients (WHO class IIb, level C). Moreover, experts recommend that systematic screening of PH should be done at least yearly in all SSc patients because it is the only way to detect early PAH [11, 14], allowing early management with better survival [15].
In the present study we have demonstrated that the best cut-off for sPAP in terms of survival prognostic factor is 36 mmHg (corresponding to a TRJ of 2.8 m/s, assuming mRAP is 5 mmHg). sPAP was an independent risk factor for death in patients with SSc who were not known to be suffering from PH or PAH at the time of the index echocardiography, with a HR of 3.02 (95% CI 1.91, 4.78) for sPAP ≥36 mmHg. In a smaller, single-centre study (n = 115), Kiatchoosakun et al. [16] showed that sPAP was independently associated with decreased survival, with a 3 year survival rate of 67%, which is very close to the present analysis.
While it was interesting to demonstrate that sPAP is an independent risk factor for death, irrespective of the presence of PH, and irrespective of the patient’s status and the final diagnosis, the fact that 16.1% of patients with sPAP >36 mmHg (corresponding to a TRJ >2.8 m/s, assuming the mRAP is 5 mmHg) at the index echocardiography never underwent RHC over the follow-up period is surprising (each centre was individually contacted to determine whether, from the date of the index echocardiography to 31 December 2011, patients underwent RHC and, if yes, what was the result). Indeed, as a patient with a TRJ >3.4 m/s (or sPAP >50 mmHg) is likely to have PH, RHC is indicated to confirm the diagnosis according to ESC/ERS recommendations [3]. It seems likely that sometimes poor access to RHC and the fear of asking for RHC may contribute to this low number of patients with a clear indication for RHC but with no RHC performed. As a limitation, we know that echocardiography has some weakness in identifying SSc patients at risk of PH. Notably, TRJ of measurable quality was ∼81–86% when the Doppler evaluation was performed by a highly experienced echocardiographer [12, 17]. We previously found that in SSc patients with TRJ >2.5 m/s, 55% had PAH on RHC, another 10% had post-capillary PH and 18% had borderline mPAP (between 21 and 24 mmHg) [12]. Moreover, Coghlan et al. [18] recently showed that 20% of SSc patients with PAH had TRJ ≤2.5 m/s and 36% had TRJ ≤2.8 m/s.
Based on the EUSTAR database, Tyndall et al. [5] found that suspected PH based on echocardiography was an independent risk factor of death. In the study performed by Fransen et al. [19], which was also based on the EUSTAR database, age, ESR and DLCO <70% of predicted were independent factors of death, with an OR of 1.94 for DLCO <70%. Our results suggest that while these three disease factors may predict 5 year survival at SSc diagnosis, sPAP (and TRJ) should now also be considered as another independent survival prognostic factor.
As reported by Steen et al. [20], DLCO may be significantly decreased (i.e. <60% of normal) for many years prior to the occurrence of PAH. In the DETECT study [18], among SSc patients with >3 years of evolution and a DLCO <60% of predicted without severe ILD or severe known left heart disease, it was shown that of 466 patients who underwent RHC, 31% (n = 144) had PH, 19% (n = 87) had PAH, 6% (n = 30) had post-capillary PH and 6% (n = 27) had PH associated with ILD. In the present study we have demonstrated that a DLCO <64%, independent of pulmonary or cardiac status, is an independent risk factor for death in SSc. An index DLCO of <64% was also associated with a 4.87-fold risk of death (95% CI 1.3, 17.6). From the DETECT study, we know that 31% of SSc patients with a DLCO <60% of predicted have PH. Consequently we should consider that all SSc patients with a sPAP >36 mmHg (corresponding to a TRJ >2.8 m/s, assuming mRAP is 5 mmHg) and/or a DLCO <64% are at a high risk of PH and subsequently of death. These patients should be monitored carefully and the question of performing RHC should be discussed on a regular basis. As ∼50% of the patients in the EUSTAR database had no documented sPAP, we recommend that regular echocardiography screening should be performed. Moreover, since 83.6% of the patients who had sPAP >36 mmHg (corresponding to TRJ >2.8 m/s, assuming mRAP is 5 mmHg) never underwent RHC during the follow-up period, this should be considered a red flag pointing to a life-threatening situation that needs to be investigated. In such cases, RHC is mandatory to confirm or exclude PH and to clearly categorize the mechanism of PH.
There are some strengths and limitations of our study. The strengths are the large number of patients analysed, the real-life conditions and the confirmation of data regarding survival status by each centre for all analysed patients. In contrast, the key limitations of our (real-life) study are that patients were selected on available sPAP. This may have induced a selection bias towards patients exhibiting symptoms, thus impairing inference to the whole population of SSc patients. Selection bias is more likely at earlier times, where regular screening assessment was not the rule, but is less likely after the recommendations regarding regular echocardiographic screening for PAH were published. There are some missing data (e.g. the 6 min walking test was documented in only 268 patients and could not be used for the multivariate analysis) and individual practices in each centre varied regarding the decision to perform RHC in patients with sPAP ≥36 mmHg, which certainly impacted the delay of PH diagnosis. In addition, because the centre did not answer our query, we were able to analyse only 1476 patients of 1946 meeting the inclusion criteria.
In conclusion, our data support the idea that in routine clinical management of SSc patients, sPAP ≥36 mmHg should be seen as a red flag indicating a high risk of death, resulting in immediate RHC to identify or exclude PH.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contribution of Nicholas Barton, MD, for his advice in editing the manuscript. All authors contributed to the concept, writing and revision of the manuscript.
Funding: This study was supported by an unrestricted research grant from GlaxoSmithKline and by a research grant from EULAR to EUSTAR. The sponsors had no role in the design of the study, the collection and analysis of data or in the preparation of the manuscript.
Disclosure statement: Y.A. has provided consultancy services and/or received funding from Bayer Pharma, Actelion, Pfizer, Sanofi-Aventis, CSL Behring and Roche in the area of potential treatments for SSc. O.D. had consultancy relationships with and/or has received research funding from Actelion, Pfizer, Ergonex, BMS, Sanofi-Aventis, United BioSource, Roche/Genentech, Medac, Biovitrium, Boehringer Ingelheim Pharma, Novartis, 4D Science, Active Biotec, Sinoxa, Serodapharm, Bayer, GSK and EpiPharm in the area of potential treatments for scleroderma and its complications. In addition, O.D. has a patent mir-29 licensed for the treatment of SSc. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol. 2010;37:2540–7. doi: 10.3899/jrheum.100245. [DOI] [PubMed] [Google Scholar]
- 2.Meier F, Frommer KW, Dinser R, et al. Update on the profile of the EUSTAR cohort: an analysis of the EUSTAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2012;71:1355–60. doi: 10.1136/annrheumdis-2011-200742. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Bogaard HJ, Condiffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl 25):D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972−2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 6.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–50. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 7.Hachulla E, Launay D, Mouthon L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009;136:1211–9. doi: 10.1378/chest.08-3042. [DOI] [PubMed] [Google Scholar]
- 8.Launay D, Sitbon O, Hachulla E, et al. Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann Rheum Dis. 2013;72:1940–6. doi: 10.1136/annrheumdis-2012-202489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefevre G, Dauchet L, Hachulla E, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 2013;65:2412–23. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Hachulla E, de Groote P, Gressin V, et al. The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multi-center nationwide longitudinal study in France. Arthritis Rheum. 2009;60:1831–9. doi: 10.1002/art.24525. [DOI] [PubMed] [Google Scholar]
- 12.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multi-center study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension’. The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 14.Vachiéry JL, Coghan G. Screening for pulmonary arterial hypertension in systemic sclerosis. Eur Respir Rev. 2009;18:162–9. doi: 10.1183/09059180.00003209. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011;63:3522–30. doi: 10.1002/art.30541. [DOI] [PubMed] [Google Scholar]
- 16.Kiatchoosakun S, Wongvipaporn C, Nanagara R, et al. Right ventricular systolic pressure assessed by echocardiography: a predictive factor of mortality in patients with scleroderma. Clin Cardiol. 2011;34:488–93. doi: 10.1002/clc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgeson D, Seward J, Miller FJ, et al. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–7. doi: 10.1016/s0894-7317(96)90475-7. [DOI] [PubMed] [Google Scholar]
- 18.Coghlan JG, Denton C, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73:1340–9. doi: 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fransen J, Popa-Diaconu D, Hesselstrand R, et al. Clinical prediction of 5-year survival in systemic sclerosis: validation of a simple prognostic model in EUSTAR centres. Ann Rheum Dis. 2011;70:1788–92. doi: 10.1136/ard.2010.144360. [DOI] [PubMed] [Google Scholar]
- 20.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516–22. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.