In 2006, CDC issued recommendations that routine opt-out HIV screening be performed in all health care settings in the United States for all individuals aged 13 to 64 years (except in communities in which the prevalence of undiagnosed HIV is less than 0.1%).1 Shortly afterward, the American College of Emergency Physicians issued a policy statement, with conditional support for HIV screening in EDs.2 Although some literature suggests a trend toward increased rates of ED engagement,3 a very recent article found that overall rates of HIV testing in EDs across the United States remain very low (0.2% of all visits).4 Numerous reasons have been conjectured, but pragmatic challenges associated with integrating testing into ED practice have been cited as most prominent.
The accompanying CDC article5 highlights findings from one ED that implemented a new HIV diagnostic testing algorithm,6 advanced by experts at CDC and now awaiting official federal approval.7 The algorithm is already being used in a handful of EDs across the United States. Although the driving force for the CDC’s creation of the new algorithm was to address technical shortfalls intrinsic to the traditional testing algorithm,8,9 use of this new algorithm affords opportunities for more easily integrating HIV testing into ED practice, in addition to improving early recognition of HIV. Understanding the new algorithm and the associated advanced diagnostic assays used helps put this article into perspective for EDs grappling with whether and how HIV screening should be adopted locally.
The traditional algorithm for HIV testing (used in clinical settings and most EDs today) includes a 2-step process with initial screening by either a conventional IA (ie, enzyme-linked immunosorbent assay)10 or one of many FDA rapid point-of-care HIV IAs,11 followed by confirmatory testing, typically WB. WB does not yield a positive result until approximately 5 to 6 weeks postinfection, whereas enzyme-linked immunosorbent assay or point-of-care tests will yield positive results from about 1 day to several weeks earlier than WB (depending on the particular assay used). In practice, use of this traditional algorithm for patients with acute HIV infection can yield discordant results (between the screening IA and WB), leading to some patients being told that they are HIV negative because of technical limitations of the old algorithm.12 The new algorithm, designed to address these technical limitations, uses a fourth-generation Ag/Ab (Combo) test, with supplemental RNA testing as needed, for resolution of discordant cases. This shortens the window period and permits diagnosis of very early (acute) HIV infections (Figure).
Figure.
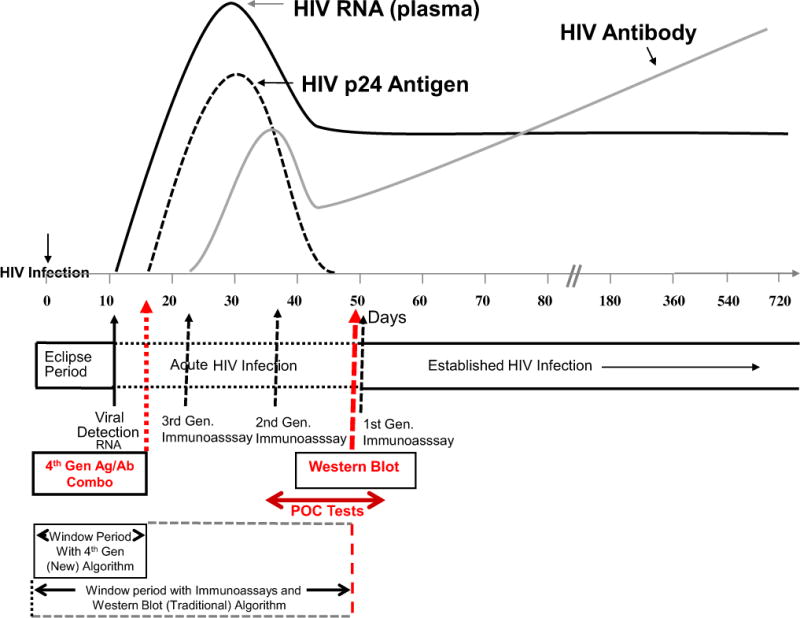
Schematic showing narrowing of the window for diagnosis of HIV infection, using the new diagnostic algorithm that incorporates fourth-generation antigen/antibody testing. Arrows represent approximate days relative to HIV infection when various test results turn positive, recognizing that each assay has a range of days in which the test results are positive. POC, Point-of-care tests. Figure adapted in colloabration with B. Branson, with permission from Branson BM. The future of HIV testing. J AIDS. 2010;55:S102–S105.
Although the tools and algorithms described in this article have been available for several years and tested retrospectively, findings from the Phoenix ED provides the first direct evidence of effect when they are used in clinical settings. The key findings from the ED site include not only that nearly one third of the newly identified infections were acute (ie, in the seroconversion window) and would have been missed had the traditional testing algorithm been used but also that use of the new algorithm results in clinically relevant interventions (eg, a pregnant mother immediately linked to care and beginning to receive therapy, avoiding HIV transmission to the unborn child) (K. Geren, MD, personal communication, October 2013, Maricopa Integrated Health Systems, Phoenix, Az).
What is the overall perspective of this new algorithm for HIV testing in EDs? Several features of the algorithm and processes associated with testing implementation with a fourth-generation assay deserve attention. First, rates of identification of acute HIV were unexpectedly high; possible explanations include the fact that viral-like syndromes are a frequent reason for ED visits or that ED patients with viral-like syndromes may have been preferentially tested. Regardless of why, these findings highlight the utility of EDs adopting the new algorithm because previous studies show that patients with acute HIV infections have markedly higher rates of transmission (up to 20-fold) and are responsible for disproportionate rates of HIV transmissions (estimated at 15% to 50%).13 Furthermore, early detection of HIV increases overall rates of awareness of HIV serostatus (known to substantially reduce risky behavior and new transmissions14) and provides opportunities for rapid linkage to care and initiation of antiretroviral treatment, which translates into decreased likelihood of future HIV-related complications. Practically speaking, early experiences with fourth-generation rapid blood-based testing have proven conducive to streamlining screening with ED flow and easy scale up, permitting testing as part of routine care. Specifically, opt-out consent and test orders can be placed at triage, venipuncture for HIV can be integrated with other blood drawing, and results can be entered into the electronic medical record, permitting easy access and delivery of results by the clinical team. Direct evidence of effect comes from this Phoenix ED, where well over 90% of the eligible population there who do not opt out receives testing (K. Geren, MD, personal communication, October 2013, Maricopa Integrated Health Systems, Phoenix, Az). This represents a significantly higher proportion of the ED population being tested than most other ED-based HIV screening models.15
Challenges do exist, however. First, not all EDs have access to the fourth-generation testing methods.
Second, the initial labor and time associated with program start-up are not insignificant and require close coordination and cooperation between the ED, the laboratory, and the institutional information technologist.
Third, although test turnaround time is relatively rapid, processing delays can occur, meaning test results may not always be ready before the patient is discharged, which represent a challenge for patients with positive results.
Fourth, although use of the blood-based fourth-generation testing algorithm streamlines and affords opportunities to scale up testing, patients who are otherwise not receiving a venipuncture will not be tested, meaning that one third to one half of the ED population would not receive testing unless blood were drawn separately exclusively for an HIV test. Individual EDs will need to establish their own approach to this challenge.
Two essential take-home messages exist for us in the ED from this CDC article. First, emergency physicians should be aware of the window associated with the traditional HIV testing algorithm and the utility of the new HIV testing algorithm for narrowing the period and detecting acute infections, which affects both the individual patient and the public health.
Second, these new algorithms open a window for EDs seeking to build integrated streamlined models for HIV screening and offer potential for long-term sustainability. Longitudinal experiences with these new algorithms from EDs across the United States will be informative going forward.
References
- 1.Branson B, Handsfield H, Lampe M, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 2.American College of Emergency Physicians. HIV testing and screening in the emergency department. Ann Emerg Med. 2007;50:209. doi: 10.1016/j.annemergmed.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Rothman R, Hsieh Y-H, Harvey L, et al. 2009 US emergency department HIV testing practices. Ann Emerg Med. 2011;58:S3–S9.e4. doi: 10.1016/j.annemergmed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Hoover J, Tao G, Heffelfinger J. Monitoring HIV testing at visits to emergency departments in the United States: very-low rate of HIV testing. J Acquir Immune Defic Syndr. 2013;62:90–94. doi: 10.1097/QAI.0b013e3182742933. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2013;62:489–494. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. DRAFT Recommendations: Diagnostic Laboratory Testing for HIV Infection in the United States. Centers for Disease Control & Prevention; 2012. Available at: http://www.cdc.gov/hiv/pdf/policies_Draft_HIV_Testing_Alg_Rec_508.2.pdf. Accessed September 17, 2013. [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Criteria for Laboratory Testing and Diagnosis of Human Immunodeficiency Virus Infection; Approved Guideline. Wayne, PA: Clinical & Laboratory Standards Institute; 2011. [Google Scholar]
- 8.Branson B. The future of HIV testing. J Acquir Immune Defic Syndr. 2010;55:S102–S105. doi: 10.1097/QAI.0b013e3181fbca44. [DOI] [PubMed] [Google Scholar]
- 9.Branson B, Mermin J. Establishing the diagnosis of HIV infection: new tests and a new algorithm for the United States. J Clin Virol. 2011;52:S3–S4. doi: 10.1016/j.jcv.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Hoxhaj S, Davila J, Modi P, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58:S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Haukoos J, Hopkins E, Hull A, et al. HIV testing in emergency departments in the United States: a national survey. Ann Emerg Med. 2011;58:S10–S6.e8. doi: 10.1016/j.annemergmed.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Wesolowski L, Delaney K, Hart C, et al. Performance of an alternative laboratory-based algorithm for diagnosis of HIV infection utilizing a third generation immunoassay, a rapid HIV-1/HIV-2 differentiation test and a DNA or RNA-based nucleic acid amplification test in persons with established HIV-1 infection and blood donors. J Clin Virol. 2011;52:S45–S49. doi: 10.1016/j.jcv.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Prabhu V, Hutchinson A, Farnham P, et al. Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: an update. AIDS. 2009;23:1792–1794. doi: 10.1097/QAD.0b013e32832e7d04. [DOI] [PubMed] [Google Scholar]
- 14.Marks G, Crepaz N, Janssen R. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 15.Kelen G, Rothman R. Emergency department–based HIV testing: too little, but not too late. Ann Emerg Med. 2009;54:65–71. doi: 10.1016/j.annemergmed.2009.03.027. [DOI] [PubMed] [Google Scholar]


