Abstract
While fear and anxiety can grow over time in anxiety disorders, most efforts to model this phenomenon with fear conditioning in rodents causes fear that remains stable or decreases across weeks or months. Here, we describe several methods to induce conditioned fear that grows over the course of 1 month and is sustained for at least 2 months using an extended fear conditioning approach. These methods include a very reliable standard method that causes multiple fear measures to increase over months, as well as alternative methods.
Keywords: fear incubation, fear conditioning, delayed-onset fear, PTSD, anxiety
INTRODUCTION
An unfortunate aspect of post-traumatic stress disorder (PTSD) is that, under certain conditions, PTSD-related fear and anxiety can grow over extended periods following the cessation of traumatic events, now termed delayed-onset PTSD (Andrews et al., 2007). This prompted the search for novel animal models to probe the neurobiological basis of delayed-onset PTSD and to facilitate the search for potential therapeutic strategies and treatments. One promising avenue for studying anxiety disorders in laboratory animals is to use Pavlovian fear conditioning, which has long been hypothesized to be related to anxiety disorders (Eysenck, 1979; Mowrer, 1939; Watson and Rayner, 1920). In fear conditioning, an initially neutral cue (e.g. a tone or an environment) is paired with an aversive stimulus (e.g. a footshock). However, under most conditions, conditioned fear in rodents remains stable or decreases slightly in the weeks and months following fear conditioning (Gale et al., 2004; Gleitman and Holmes, 1967; Hendersen, 1978; Quirk, 2002). In addition, several previous demonstrations of incubation of contextual fear have reported inconsistent results across rodent strains, age or experiment procedures (Balogh and Wehner, 2003; Frankland et al., 2004; Houston et al., 1999), or cause fear that increases up to 14 days and then fades (Balogh et al., 2002). Other fear conditioning procedures involve conditioned contextual fear training and cause increased fear generalization to other contextual or discrete auditory cues, but do not increase fear to the trained context (‘generalization’ rather than ‘incubation’ of fear to the conditioned cue) (Siegmund and Wotjak, 2007; Wiltgen and Silva, 2007). However, we recently found that an extended fear conditioning procedure, in which rats receive 100 shocks paired with tones over 10 days, causes reliable fear incubation that emerges 1 month after fear conditioning and is sustained for at least 2 months after the end of fear training (Pickens et al., 2009a; Pickens et al., 2009b; Pickens et al., 2010).
The protocols within this unit describe procedures to produce fear incubation. Basic Protocol 1 very reliably produces fear incubation, with multiple demonstrations of this phenomenon in several papers (Pickens et al., 2009a; Pickens et al., 2009b; Pickens et al., 2010). Alternate Protocol 1 gives details of a procedure to produce fear incubation with longer cues that may more closely model the human clinical problem of maladaptive anxiety, rather than a fear response which may be an adaptive response to an imminent threat (Davis et al., 2009; Waddell et al., 2006; Walker and Davis, 2008). Alternate Protocol 2 gives details of a procedure to produce fear incubation in rats that are not performing a concurrent lever-press task to earn food, which has greater similarity to the fear conditioning procedure commonly used in other laboratories (Fanselow and Gale, 2003; LeDoux, 2000; Maren, 2001).The most crucial factor appears to be the extended fear training; it seems likely that other experimental parameters can be adjusted according to the needs of a particular lab (as described below in the Critical Parameters and Troubleshooting section).
BASIC PROTOCOL 1: CONCURRENT FREEZING/CONDITIONED SUPPRESSION INCUBATION WITH SHORT CUES
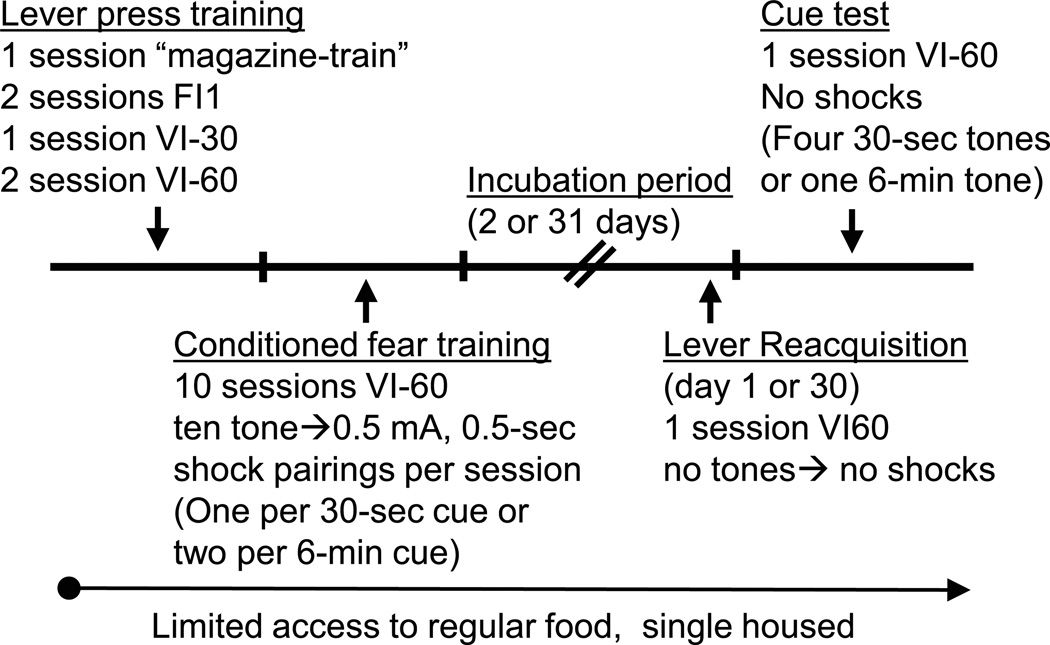
This is the standard protocol that has been used repeatedly for pharmacological testing and investigations into the time-course and mechanisms underlying fear incubation (Pickens et al., 2009a; Pickens et al., 2009b; Pickens et al., 2010). It has reliably produced fear incubation under a wide variety of feeding conditions and after other manipulations were given (e.g.: i.p., s.c. or i.c.v. injections) and allows for two measures of fear conditioning: conditioned suppression of lever-pressing for food (Estes and Skinner, 1941) and conditioned freezing. A schematic of the experimental timeline for Basic Protocol 1 is depicted in Figure 1.
Figure 1.
Time-line for Basic Protocol 1 and Alternate Protocol 1.
Materials
Rats (ex: Long-Evans rats from Charles River Laboratory weighing 250–500 g single housed for the duration of the experiment). All experiments must be approved by the appropriate institutional and or national review boards.
Food chow
Operant chambers (ex: previous experiments have used Med Associates chambers with a retractable “active lever”, pellet dispenser to deliver 45-mg pellets, Sonalert tone generator, red houselight and shock generators programmed to deliver scrambled shocks)
A video camera for closed captioned viewing/recording of behavior (ex: a system from Coulbourn Instruments mounted to the top of each chamber, extending 2 cm into the chamber with software to record behavior, has been used)
Software to control operant chambers installed on a desktop computer (ex: Med Associates software- Med-PC-IV and Trans-IV. Sample Trans-IV programs for all experimental phases for all 3 protocols are available by emailing the authors or at www.pickenslab.net).
A computer with software to view videos of rat behavior
A metronome (mechanical or a metronome program)
45 mg food pellets (ex: # F00021, 5.5% fat, 60% carbohydrate, 4.5% fiber; Bioserv)
A scale with gram sensitivity to weigh rats and food chow
Phase I- Food restriction
-
1
Let rats acclimate to the colony room for at least one week and ensure that they weigh 300 g or more before food restriction begins.
-
2
Weigh rats to determine their initial weight, and multiply this weight by 0.85 to determine their target weight.
-
3
Food restrict rats to 85% of their free-feeding weight by giving rats 4 g of food chow per day until their weight reaches their target weight.
-
4Begin maintenance feeding to keep the rats at their 85% of their free feeding weight.Notes: The definition of “85% of free feeding weight” can have multiple interpretations-either 85% of initial weight at the start of food restriction (without subsequent growth) or 85% of initial weight with subsequent growth to track the normal increase in weight that occurs as the rats age. If the goal is to maintain the rats at 85% of their initial weight, give the rats 14 g of food chow for maintenance feeding if they are at their appropriate weight. For every gram overweight, give 1 g less with a minimum of 4 g per day. For every gram underweight, give rats 0.5 g more with a maximum of 21 g. If the goal is to allow the rats to slowly grow from their 85% weight, give rats 18–20 g of feed per day for maintenance feeding. On days where rats earn food in the operant chambers, subtract the weight of the food earned from the daily food ration. Both methods of maintenance feeding maintain lever-pressing at a constant level across a 1-month incubation interval. These feeding conditions were determined in male Long-Evans rats, and some adjustments may need to be made for other rat strains.
-
5
Keep rats on maintenance feeding for at least 4 days before beginning conditioning sessions and continue maintenance feeding for the duration of the experiment.
Phase II- Magazine training and lever press training
-
6
Give rats a session of magazine training in order to learn the location of food delivery and any clicking noises or vibrations that accompany the delivery of food (ex: a sample program that delivers a single food pellet every 125 seconds during a 60-min session with the active lever retracted).
-
7On the following day, train rats to lever-press for food pellets under a schedule where all or most lever-presses lead to food pellet delivery.Notes: A fixed-interval-1 (FI-1) schedule, in which lever-presses can earn a pellet each sec, is effective in teaching rats to lever-press with steady reinforcement while minimizing the occurrence of multiple food pellet deliveries within seconds caused by rats “playing with” the lever. Two sessions in a single day (morning and afternoon) with a maximum duration of 60 min or 50 food pellets work well. Any rat that does not reach 50 reinforcements with the afternoon session should be given an additional 3-h session with a water bottle present in the chamber immediately after the afternoon session.
-
8Train the rats to lever-press on a variable interval (VI) schedule, in which there is reinforcement for the first lever-press after an unpredictable interval from the time of the last pellet delivery.Notes: VI schedules maintain a steady level of responding and movement while preventing the rats from earning a large number of pellets that would cause satiation and lower levels of responding at the end of the session (~120–150 food pellets within a 90-min session). Rats can be given a single session on a VI-30 schedule (pellet availability for lever presses ranging from 1 to 59 s), followed by two once-daily sessions on a VI-60 schedule (pellet availability for lever-presses ranging from 1 to 119 s).
Phase III- Fear conditioning
-
9Give rats 10 days of fear conditioning with a discrete cue that predicts a mild footshock, with the lever available and lever-pressing reinforced on a VI schedule.Notes: This extended “overtraining” is necessary to produce a low level of fear that grows over time, since a single day of fear conditioning tends to cause fear that is high initially and does not increase over time. To achieve this, daily 90-min sessions, each involving 10 × 30-sec tones that co-terminate with a mild footshock (0.5-sec, 0.5-mA), are recommended.
-
10Measure conditioned fear during fear conditioning for each session using the measures of conditioned suppression of lever-pressing or conditioned freezing.Notes: Conditioned suppression can be measured with a formula (Armony et al., 1997) to adjust lever pressing during the 30-sec tone with baseline lever-pressing in the 30-sec period immediately before tone delivery:The maximum value on this scale is 1.00 and the minimum value is −1.00. A positive suppression ratio indicates a decrease in lever-pressing during the tone and a negative suppression ratio indicates an increase in lever-pressing during the tone. A suppression ratio of 1 indicates complete suppression of lever-pressing during the tone (high fear), a suppression ratio of 0 indicates that there is no change in the lever-pressing during the tone (no fear). Analyzing conditioned suppression using this measure gives identical statistical results as analyzing conditioned suppression using the Annau-Kamin ratio (Annau and Kamin, 1961), in which lower scores indicate higher fear. To minimize variability due to trial-by-trial differences in pre-cue lever-pressing or trials in which rats may not make any lever-presses (and would require dividing by 0), responses during the pre-cue and tone periods can be summed to calculate a single suppression ratio value for each conditioning session.If a video camera is present in the chamber, it is also possible to measure conditioned freezing across the conditioning days. In this case, videos should be scored for freezing (immobility except for movement related to breathing (Fanselow, 1980), at regular timed intervals (ex: every 2 sec as indicated by a metronome or stopwatch).Different feeding conditions lead to different fear acquisition curves. Keeping the rats at 85% of their initial weight tends to cause conditioned suppression to peak around sessions 2–4 and decrease as further sessions are conducted. Feeding rats so that they grow over time from their 85% weights tends to cause an asymptotic level of conditioned suppression by day 2–3 that does not decrease. However, both procedures cause fear to incubate during testing.
Phase IV- Fear incubation testing
-
11Weigh and feed rats daily during the incubation interval according to the maintenance feeding procedure until the test days.Note: For maximum fear incubation, rats should be tested for conditioned fear on post-conditioning days 1–2 and after one month (ex: day 30 and 31). Fear incubation does not appear within 15 days after conditioning, and fear appears to have reached its maximum after 1 month and does not further increase at 2 months (see Figure 2). However, fear is similar between 1 and 2 months and a 2-month test could be used if experimental timing or intervening manipulations make it necessary. In addition, fear incubation is reliably seen in both between-subjects (ex: one group tested for the first time on days 1–2 and another group tested for the first time on day 30–31) and within-subjects (e.g. one group tested on day 1–2 and then retested on day 30–31) comparisons.
-
12On the last day of the incubation interval (e.g. day 1 or day 30), give rats a lever-press reacquisition session, in which they can lever-press for food pellets without any fear cues or shocks in the context where cued fear testing will occur.Note: For a maximal fear incubation effect, all testing should take place in the chamber where fear conditioning occurred. In previous studies (cites?), this session has been equivalent to the VI-60 lever-press training session that occurs before fear conditioning (rats lever-press on a VI-60 schedule for 90 min, with no tones or shocks present). This acts to stabilize the lever-pressing to a steady level so that suppression of lever-pressing can be measured against a steady baseline. This training also functions to extinguish any contextual fear conditioning so that fear to the cue can be measured with minimal contributions from contextual fear. While this is technically also a contextual fear test, no significant differences in lever-press rates have been seen either in the first 5-min block or across the entire 90-min session. No systematic measurement of conditioned freezing has been done in the lever-press reacquisition session.
-
13On the day after lever reacquisition session, give rats a session where the fear cue is repeatedly presented without shock. Measure conditioned freezing and/or conditioned suppression and compare the behavior at the different time points to determine the fear incubation effect.Note: In past experiments (cites?), the rats were presented with 4 30-sec tones during a 35-min test, but no shocks were presented (extinction testing). The measures of fear are the suppression ratio and conditioned freezing during the tone. Depending on the particular procedure and fear measure used, differences in fear responding may be maximal on the first trial and grow smaller or may be hidden by ceiling effects and may emerge across the course of the extinction test. However, differences in fear responses between day 2 and day 31 tend to disappear after ~3–4 trials.
Figure 2.
Time-course of fear incubation using the procedure described in Basic Protocol 1. The measure of fear is conditioned suppression of lever-pressing (mean+SEM). * significantly different from day 2 (p < 0.05). Adapted, with permission, from (Pickens et al., 2009b).
ALTERNATE PROTOCOL 1: FEAR/ANXIETY INCUBATION WITH LONG CUES
The majority of fear incubation studies have used relatively short cues (30-sec long) with highly predictable shocks to induce relatively strong emotional responses (proposed to model “fear”). However, it is also possible to cause incubation of a more diffuse emotional state by pairing longer cues (minutes long) with unpredictable shocks (proposed to model “conditioned anxiety”) (Pickens et al., 2010). The procedure is very similar to those used in the basic protocol (see Figure 1), with differences in the length of the tone cue and the timing of the shocks.
Materials
The materials are the same as in Basic Protocol 1.
Phase I and Phase II
-
1
Conduct Phase I (food restriction) and Phase II (magazine training and lever-press training) as in Basic Protocol 1.
Phase III- Fear conditioning
-
2Give rats 10 days of fear conditioning in which shocks occur at unpredictable times (specify parameters of unpredictability, e.g., ITI range) during extended (minutes long) cues.Note: In a previous experiment (cite?), each session contained 5 6-min long tones with 2 × 0.5-sec, 0.5-mA footshocks occurring at unpredictable times during each tone presentation (10 shocks total per session).
-
3Measure conditioned fear during fear conditioning for each conditioning session using the measures of conditioned suppression of lever-pressing and/or conditioned freezing.Note: For this manipulation, suppression ratios for conditioned suppression can be calculated based on the rate of responding (responses/min) during a 2-min pre-cue period and compared to the rate of responding during the tone itself.
Phase IV- Fear incubation testing
-
4
Conduct the incubation interval feeding and lever-press reacquisition session as in Basic Protocol 1.
-
5On the day after lever reacquisition session, give rats a session where the fear cue is repeatedly presented without shock. Measure conditioned freezing and/or conditioned suppression and compare the behavior at the different time points to determine the fear incubation effect.Note: In a previous experiment (cite?), a 35-min test with 2 × 6-min cues but no footshocks was given. An increase in fear was seen in both between-subjects (e.g.: one group tested for the first time on days 1–2 and another group tested for the first time on day 30–31) and within-subjects (e.g. one group tested on day 1–2 and then retested on day 30–31) comparisons. This study demonstrated that fear incubation occurs primarily during the first cue presentation, so a single cue test is a reasonable option. The progression of the fear response during the fear extinction test can be determined on a minute-by-minute basis by calculating a suppression ratio based on the rate of responding in the pre-cue period and the rate of responding during each minute of the cue. Conditioned freezing can also be measured for an additional measure of conditioned fear.
ALTERNATE PROTOCOL 2: FEAR INCUBATION MEASURED WITHOUT A CONCURRENT OPERANT TASK
While the majority of fear incubation studies using the extended fear conditioning method have involved rats lever-pressing for food pellets to allow for multiple fear measures and to increase baseline activity, it is possible to observe fear incubation using the more common approach in which conditioned freezing is measured without a concurrent lever-press task (Pickens et al., 2010). This procedure also involves 10 fear conditioning sessions. However, the fear incubation effect is smaller than that seen with a concurrent lever-press task and it may require a greater N to observe a significant incubation effect in certain cases.
Materials
The materials for this protocol are the same as those in Basic Protocol 1, with 2 exceptions. First, this procedure does not require the experimental chamber to contain an active lever. If an active lever is present, it should be retracted during all training sessions. Second, 45-mg food pellets are not required for this protocol.
Phase I- Food restriction
-
1Food restrict the rats as described in Basic Protocol I, with 18–20 g/ day for maintenance feeding.Note: Even without a concurrent lever-press task, fear incubation appears to be more robust under conditions of mild food restriction than when rats are free-fed. A direct comparison of rats restricted to 85% of their free feeding weights then given 18–20 g per day against free-fed rats revealed no significant interaction between feeding conditions and the incubation effect (unpublished data?). However, the incubation effect was observed in post-hoc tests both within-subjects and between-subjects in the food-restricted rats, and was not observed in either comparison in the free-fed rats. Therefore, it is preferable to food restrict the rats.
Phase II- Experimental chamber habituation
-
2Place the rats into the experimental chamber without any tones, shocks, pellets or active lever for a single chamber habituation session.Note: Previous experiments have used 90-min conditioning sessions, so this habituation session was also 90-min long. This session acts to minimize any non-associative anxiety or novelty effects that the experimental chamber might cause.
Phase III- Fear conditioning
-
3Give rats 10 days of fear conditioning in sessions without an active lever or food pellets available.Notes: In a previous experiment (cite?), the rats were presented with 10 × 30-s long shocks which each co-terminated with a 0.5-sec, 0.5-mA footshock.
-
4Measure conditioned fear during fear conditioning for each conditioning session using conditioned freezing.Note: A previous experiment (cite?) found that conditioned freezing is stable across conditioning days with this protocol, with no increases or decreases.
Phase IV- Fear incubation testing
-
5
Conduct the incubation interval feeding as in Basic Protocol 1.
-
6On the last day of the incubation interval, give the rats a 90-min session with no discrete fear cues or shocks in the context where the rats will be tested for their cued fear.Notes: In a previous experiment, this session was 90-min long and occurred on day 1 or day 30. This session serves to extinguish fear to the context so that incubation of fear to the discrete cues can be tested with minimal contribution from contextual fear. It can also serve as a contextual fear test. For this purpose, the rats’ freezing behavior in the first 5 min of the session can be used as a measure of fear before extinction occurs.
-
7On the day after the context exposure, test the rats for fear responses to the fear cue.Notes: In a previous experiment, the rats were presented with 4 × 30-s tones during a 35-min test, but no shocks were presented (extinction testing). Higher fear was seen in testing after a month compared with 2 days after fear conditioning.
COMMENTARY
Background Information
The extended fear conditioning method of obtaining incubation was largely based on a report of a single rat that exhibited fear that decreased across 60 sessions of cue-shock pairing and reappeared after a 30 day shock-free period (Millenson and Dent, 1971) (also see Rosas and Alonso (1997) for a related finding).
There are other procedures used to obtain fear that grows over weeks. For example, there are several reports of contextual fear growing from low levels seen 1–3 days after conditioning to day 15 (Balogh et al., 2002; Balogh and Wehner, 2003). There is also a related finding in which fear to a context previously paired with shock does not incubate or increase, but this fear generalizes to other contextual or auditory cues that were never paired with shock (Siegmund and Wotjak, 2007; Wiltgen and Silva, 2007). However, to the best of our knowledge, the extended fear conditioning method is the only method known to cause conditioned fear to a conditioned cue that incubates over an extended period and is maintained for more than a month.
Critical Parameters and Troubleshooting
Extended training and incubation interval
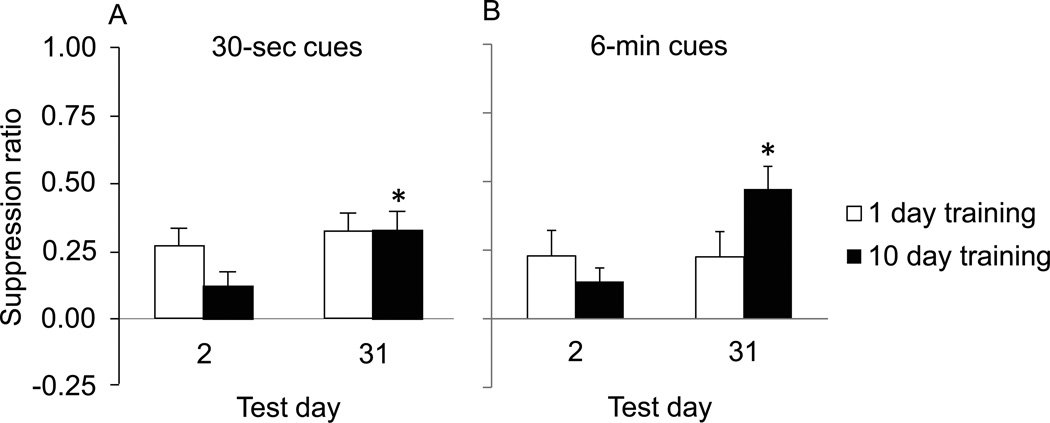
The two most critical parameters are the length of fear training and the incubation interval. Conditioned fear rarely incubates over weeks or months in other laboratory set-ups (Gale et al., 2004; Gleitman and Holmes, 1967; Hendersen, 1978; Quirk, 2002), likely due to the fact that most fear conditioning procedures involve a single day of fear conditioning. This is corroborated by experiments run in parallel with the fear incubation procedure, in which fear conditioning parameters identical to those used for 10 day training result in the acquisition of stable fear behavior that does not grow over time if used for only one day of fear conditioning (Figure 3) (Pickens et al., 2009b). In addition, significant fear incubation does not appear within 14–15 days, but appears within 30 days after extended fear conditioning. However, if extended fear conditioning is given and a long incubation interval is used, other parameters may be less critical. For example, one group observed fear incubation 33 vs. 3 days after extended fear conditioning in a different rat strain (Sprague-Dawley rather than Long-Evans), with a longer shock (1 s rather than 0.5 sec), and with a shorter inter-trial interval (average 2 min rather than average 8.5 min) than the ones used in previous fear incubation experiments (Morrow et al., 2012).
Figure 3.
Comparison of fear expression after limited (1 day) or extended (10 day) training. A: Fear expression after training with 30-sec cues (as in Basic Protocol 1). B: Fear expression after training with 6-min cues (as in Alternate Protocol 1). The measure of fear is conditioned suppression of lever-pressing (mean+SEM). * significantly different from day 2 (p < 0.05). Adapted, with permission, from (Pickens et al., 2009b) and (Pickens et al., 2010).
Feeding conditions
While fear incubation occurs under a wide variety of feeding conditions, the magnitude, persistence, and particular pattern of the test data depends upon the feeding conditions used, particularly in the conditioned suppression measure (Pickens et al., 2009b; Pickens et al., 2010). When looking at conditioned suppression in the Basic Protocol (30-sec cue in rats lever-pressing for food), smaller incubation effects that are largely driven by differential responding on a single trial have been seen in rats restricted to 17 g/day or 20 g/day without initial restriction to 85% of free-feeding weight (unpublished data and (Pickens et al., 2010)). Conversely, rats restricted to 85% of initial free-feeding weight and then maintained at that weight have consistently demonstrated fear incubation differences in conditioned suppression that last for 2–3 test trials with no difference left on the 4th trial (Pickens et al., 2009a; Pickens et al., 2009b). Notably, restriction to 85% of initial weight followed by a daily food ration of 20 g per day causes the most durable incubation effect, in which conditioned suppression is different between day 2 and day 31 for all 4 test trials.
The conditioned freezing measure appears to be less sensitive to the food restriction conditions (Pickens et al., 2010), as rats fed 20 g either with or without the initial restriction to 85% of free-feeding weight exhibited differences in conditioned freezing between day 2 and day 31 that lasted for 3 test trials. Similarly, in an experiment run using Alternative Protocol 2, although food restricted rats (85% then 18–20 g) trended towards greater fear incubation than free-fed rats that were not lever-pressing for food, there was no significant effect of the food restriction conditions on the incubation effect (no interaction of feeding conditions with test day).
Test context
We recommend that the fear test be done in the same context as the fear training, although no specific parametric study has been done on the interaction of the test context with fear incubation using the protocols described here. A study in which 150-sec tones were paired with a shock for 16 fear conditioning sessions demonstrated that fear responses increased from 3 to 20 days after the end of conditioning, regardless of whether testing occurred in the fear conditioning context or an alternative context (Rosas and Alonso, 1997). However, fear was higher on day 3 in the group tested in a different context, which suggests that testing in an alternative context could lead to smaller incubation effects. This is in accord with a study by Bouton (Bouton et al., 2008) in which cues that were extensively paired with shock caused low fear when tested one day later in the context where the cue had been presented, and high fear when tested 1 day later in an alternative context where the cue was not previously presented. This suggests that fear could be higher 2 days after extended fear conditioning if the testing occurs in an alternative context, and that this high fear on day 2 might limit the magnitude of the incubation effect. Thus, although it is possible to test fear incubation in an alternative context (Rosas and Alonso, 1997), it is recommended that fear incubation be tested in the context used for fear conditioning.
Comparisons with immediate-onset fear
It is possible to directly compare delayed-onset fear, which is initially low and increases (incubates) over weeks or month, with fear that is high soon after the end of fear training. A single fear conditioning session causes fear that does not incubate. A 2 × 2 design, in which rats are trained with 1 or 10 fear conditioning sessions and then tested after 2 days or 1 month, has been tested twice (Figure 3). In rats trained with 30-sec cues and a concurrent lever-press task (as in Basic Protocol 1), conditioned suppression was equally high 2 days and 1 month after a single day of fear conditioning. Conversely, conditioned suppression in rats given 10 sessions of fear conditioning was low on day 2 and increased after 1 month to be equal to the high level seen in the rats given a single day of fear conditioning (Pickens et al., 2009b). The equivalent level of fear in the different groups makes it possible to compare the neurobiological basis of incubated fear and fear that is high soon after conditioning and does not need to incubate.
The pattern is slightly different after training with long (6-min) cues paired with shocks at unpredictable times. With this training regimen, 10 days of training causes low conditioned suppression 2 days later that increases after 1 month, and 1 day of fear conditioning causes fear that does not incubate. However, the conditioned response after 1 day of fear conditioning is lower than that seen 1 month after extended training (10 conditioning sessions) (Pickens et al., 2010). The different levels of fear/anxiety in the different conditions could complicate efforts to directly compare the neurobiological basis of the conditioned response. Development of a procedure that causes equivalent conditioned responses in the two conditions would be very helpful for future comparisons.
Shock intensity
The shock level used for these protocols was based on previous experiments in the literature using 0.5-sec, 0.5-mA shock, but not shocks stronger than 0.5-mA, to cause a non-monotonic fear acquisition curve. Under these conditions, extended training causes less fear expression than limited training. (Annau and Kamin, 1961; Millenson and Hendry, 1967). This non-monotonic acquisition curve was originally hypothesized to be necessary to produce relatively low fear expression 2 days after the end of training, so that fear would be able to increase from this initially low level. However, fear incubation can be observed in training conditions where this non-monotonic fear acquisition curve is not seen (Pickens et al., 2010). Fear incubation has also been demonstrated after training with a longer shock (1-sec, 0.5-mA) (Morrow et al., 2012), but a parametric study of the effects of shock intensity on fear incubation is yet to be performed. If stronger shocks are used for training and conditioned fear is very high on test day 2, a lower shock intensity is suggested.
Anticipated Results
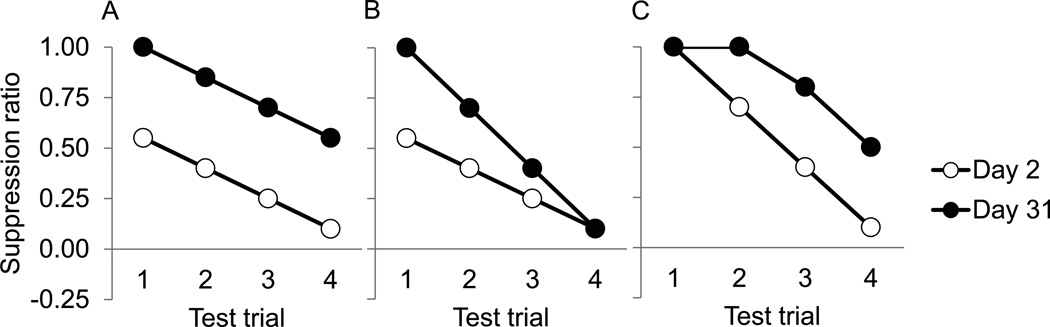
Fear incubation after extended fear conditioning is a very robust phenomenon which has been demonstrated under a wide variety of feeding conditions, cue lengths and in several fear measures in both between-subjects and within-subjects comparisons. The trial-by-trial pattern of the difference in fear expression between low pre-incubated fear and high pre-incubated fear in the extinction test, however, may differ. Patterns of test data have included those with incubated fear higher than pre-incubated fear on every test trial (Figure 4A), those with higher incubated fear on the initial trials with the difference disappearing on later trials (Figure 4B), and those in which there is no difference on the first trial (likely due to ceiling effects) but differences appear on later trials (Figure 4C). Regardless of the particular pattern, fear across a 3- or 4-trial test is reliably higher 1 month after extended training than 2 days later.
Figure 4.
Different trial-by-trial patterns of fear incubation (idealized data). A: Higher fear expression on day 31 on every trial during the fear test. B: Higher fear expression on day 31 early in the test with the differences disappearing on later trials. C: Similar early in the test due to ceiling effects, with fear expression on day 31 higher on later trials.
Time Considerations
If run every day, the fear incubation procedure takes ~50–55 days to complete. This includes 10 days for food restriction and allowing the rats to acclimate to maintenance food restriction conditions, 5 days for magazine and lever-press training, 10 days for fear conditioning and ~30 days for the incubation period and test. The fear conditioning procedure without lever-press training (Alternate Protocol 2) requires 4 fewer days, since no lever-press training is required.
The incubation interval lasts about 4 weeks, in which the rats are not exposed to the experimental chambers and require little more than daily handling and feeding. This time period allows the experimenter to give an additional group of rats lever-press training, fear conditioning, and day 1–2 testing in the experimental chambers, and have this group in their incubation period (and the operant chambers free) in time for the original group to be tested for fear responding on day 30–31.
ACKNOWLEDGEMENT
This work was supported by the Intramural Programs of the National Institute on Drug Abuse. These sponsors had no influence on the protocol design and in the collection, analysis and interpretation of data.
Footnotes
INTERNET RESOURCES
Website for the Pickens lab (Kansas State University) starting in August 2013. This site contains sample programs for all 3 protocols.
LITERATURE CITED
- Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164:1319–1326. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci. 2002;116:947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Frohardt RJ, Sunsay C, Waddell J, Morris RW. Contextual control of inhibition with reinforcement: Adaptation and timing mechanisms. J Exp Psychol Anim Behav Process. 2008;34:223–236. doi: 10.1037/0097-7403.34.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390–400. [Google Scholar]
- Eysenck HJ. The conditioning model of neurosis. Behav Brain Sci. 1979;2:155–199. [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitman H, Holmes PA. Retention of incompletely learned CER in rats. Psychon Sci. 1967;7:19–20. [Google Scholar]
- Hendersen RW. Forgetting and conditioned fear inhibition. Learn Motiv. 1978;9:16–30. [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Millenson JR, Dent JG. Habituation of conditioned suppression. Q J Exp Psychol. 1971;23:126–134. doi: 10.1080/00335557143000130. [DOI] [PubMed] [Google Scholar]
- Millenson JR, Hendry DP. Quantification of response suppression in conditioned anxiety training. Can J Psychol. 1967;21:242–252. doi: 10.1037/h0082981. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Pavlovian conditioned approach to a reward cue predicts fear incubation. Society for Neuroscience conference; New Orleans, LA. 2012. [Google Scholar]
- Mowrer OH. A stimulus-response analysis of anxiety and its role as a reinforcing agent. Psychol Rev. 1939;46:553–565. [Google Scholar]
- Pickens CL, Adams-Deutsch T, Nair SG, Navarre BM, Heilig M, Shaham Y. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009a;164:1398–1406. doi: 10.1016/j.neuroscience.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009b;65:881–886. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Navarre BM, Nair SG. Incubation of conditioned fear in the conditioned suppression model in rats: role of food-restriction conditions, length of conditioned cue, and generality to conditioned freezing. Neuroscience. 2010;169:1501–1510. doi: 10.1016/j.neuroscience.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas JM, Alonso G. The effect of context change upon long-term memory of CS duration. Behav Processes. 1997;39:69–76. doi: 10.1016/s0376-6357(96)00045-9. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Watson JB, Rayner R. Conditioned emotional responses. J Exp Psychol. 1920;3:1–14. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]