Abstract
Objectives
Reflecting on the self and on others activates specific brain areas and contributes to metacognition and social cognition. The aim of the current study is to investigate brain activation during self- and other-reflection in patients with bipolar disorder (BD). In addition, we examined whether potential abnormal brain activation in BD patients could distinguish BD from patients with schizophrenia (SZ).
Methods
During functional magnetic resonance imaging (fMRI), 17 BD patients, 17 SZ patients and 21 healthy controls (HCs) performed a self-reflection task. The task consisted of sentences divided into three conditions: self-reflection, other-reflection and semantic control.
Results
BD patients showed less activation in the posterior cingulate cortex (PCC) extending to the precuneus during other-reflection compared to HCs (p = 0.028 FWE corrected on cluster-level within the regions of interest). In SZ patients, the level of activation in this area was in between BD patients and HCs, with no significant differences between patients with SZ and BD. There were no group differences in brain activation during self-reflection. Moreover, there was a positive correlation between the PCC/precuneus activation during other-reflection and cognitive insight in SZ patients, but not in BD patients.
Conclusions
BD patients showed less activation in the PCC/precuneus during other-reflection. This may support an account of impaired integration of emotion and memory (evaluation of past and current other-related information) in BD patients. Correlation differences of the PCC/precuneus activation with the cognitive insight in patients with BD and SZ might reflect an important difference between these disorders, which may help to further explore potentially distinguishing markers.
Keywords: Self-reflection, Other-reflection, Bipolar disorder, Schizophrenia, Posterior cingulate cortex, Precuneus
Highlights
-
•
We investigated self-reflection and other-reflection in bipolar disorder.
-
•
Bipolar had less PCC/precuneus activation during other-reflection than controls.
-
•
PCC/precuneus activation was unrelated to cognitive insight in bipolar patients.
1. Introduction
Several studies have demonstrated disturbed metacognitive processing in patients with bipolar disorder (BD) (Batmaz et al., 2014; Samamé, 2013; Thaler et al., 2013). Self- and other-reflection can be considered metacognitive processes and refer to the evaluation process by which people determine to what extent certain cues (e.g. traits and attitudes) apply to themselves or others respectively (Van der Meer et al., 2010). Patients with BD may show comparable impairments in several metacognitive domains as patients with schizophrenia (SZ) (Brüne, 2005; Rabin et al., 2014; Thaler et al., 2013) in whom self- and other-reflection have been investigated more abundantly (Bedford et al., 2012; Holt et al., 2011; Murphy et al., 2010; Pauly et al., 2013; Van der Meer et al., 2013). However, little is known about self- and other-reflection and the underlying neural correlates in BD patients.
Self-reflection and other-reflection are related to the function of cortical midline structures (CMS). The CMS consist of the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), dorsal medial prefrontal cortex (DMPFC) and ventral medial prefrontal cortex (VMPFC) (Northoff & Bermpohl, 2004). The cognitive neuropsychiatric self-reflection/self-appraisal model (Van der Meer et al., 2010) suggests that these different structures each have a specific contribution to reflective processing. According to this model, directing attention is associated with activation of the ACC. The PCC is involved in consultation of autobiographical memory to facilitate decisions whether the confronted stimuli apply to self or others. Ultimately, the final decision is proposed to be related to activation of the DMPFC. In addition, the insula seems to be involved in reflective processing and is associated with evaluation of internal self-state and somatic feedback (Craig, 2009). These processes are similar in both self- and other-reflection (Van der Meer et al., 2010). Moreover, a specific role in tagging self-relevant information has been hypothesized for the VMPFC. Murray et al. (2012) have reported evidence that the level of activation in the VMPFC is mediated by the degree of self-relatedness. They demonstrated that the VMPFC is activated by stimuli related to self and, to a lesser extent, a close other, but not by stimuli referring to a public other.
There are currently, to our knowledge, no studies investigating the neural correlates of self- and other-reflection in BD patients. However, studies conducted in SZ patients have revealed abnormal brain activations during self- and other-reflection that occur during psychotic periods and seem to persist during stable periods after psychotic symptoms disappear. Compared to healthy individuals, SZ patients have shown less activation during self-reflection in the PCC (Van der Meer et al., 2013), inferior temporal gyrus extending to the middle temporal gyrus (Pauly et al., 2013) and precuneus (Murphy et al., 2010), but also hyper-activation in the PCC has been reported (Holt et al., 2011). During other-reflection, less activation in the VMPFC, ACC, insula and cuneus (Pauly et al., 2013) and PCC/precuneus (Van der Meer et al., 2013) has been reported. In addition, comparing self to other has shown less activation in the DMPFC (Bedford et al., 2012), and higher activation in the PCC/precuneus (Shad et al., 2012). These studies suggest that the self- and other-reflective network is abnormally activated in SZ patients during reflective processing, even in the absence of current symptoms (Holt et al., 2011).
Lichtenstein et al. (2009) have reported that the same genes could lead to vulnerability for both of these disorders and some authors even suggest that BD and SZ are an expression of different levels of severity on the same disease continuum rather than two completely different diseases (Craddock & Owen, 2005). It has indeed been shown that patients with BD and SZ share several clinical features (e.g. anhedonia, psychotic symptoms; American Psychiatric Association, 2013; Kempf et al., 2005). Moreover, patients with both BD and SZ have shown disturbed metacognitive processing in social and emotional domains such as theory of mind (e.g. Brüne, 2005; Rabin et al., 2014; Thaler et al., 2013) which is closely associated with self- and other-reflection (Happé, 2003; Mitchell et al., 2005; Saxe et al., 2006). In addition, while healthy individuals tend to attribute more positive than negative events to themselves during self-reflection (self-serving bias; Mezulis et al., 2004), this tendency is disturbed in both SZ patients (Mehl et al., 2014; Mizrahi et al., 2008) and BD patients (Lyon et al., 1999). However, BD and SZ have been classified as two different disorders for a long time. SZ patients have shown worse cognitive functioning than BD patients (Krabbendam et al., 2005), which also implies differences between BD and SZ. Therefore, it would be interesting to explore whether potential abnormal activations during self- and other-reflection in BD patients are comparable to those in SZ patients or whether these represent unique neurobiological features distinguishing BD patients from SZ patients.
In order to maximize comparability between BD and SZ groups, and also potentially get more pronounced differences compared to healthy individuals, we recruited a relatively homogeneous group of BD patients who had a history of psychosis, but were not currently experiencing a psychosis. Notably, it has been found that activation during reflective processing is associated with level of illness insight (Van der Meer et al., 2013). BD patients have generally a good level of illness insight when they are not in a manic episode (Cassidy, 2010), while in SZ patients illness insight could be low during all phases of illness (Arango & Amador, 2011; Yen et al., 2002). Therefore, we aimed to investigate differences between BD and SZ in brain activation during self- and other-reflection in patients with good insight only.
In the present study, we aimed to investigate the neural correlates during a self-reflection task in BD patients with a history of psychotic symptoms. Based on the importance of the self-reflective network during both self- and other-reflective processing and their established deficiencies in relation with psychotic symptomatology, we hypothesized that BD patients would show abnormal activation within the self-reflection network compared to healthy individuals. Additionally, differences between patients with BD and SZ in brain activation during reflective processes, as well as correlations with insight, were explored in order to examine whether the potential abnormalities in BD were specific for BD.
2. Methods
2.1. Participants
This study included BD patients, SZ patients and healthy controls (HCs). The following inclusion criteria were applied to both BD and SZ patients: (1) Being stable on current medication and no medication change in the week before scanning; (2) no electroconvulsive therapy in the year prior to the scan; (3) having no psychiatric disorders other than BD or SZ (e.g. substance use disorder); and (4) for BD patients, having a history of an episode with psychotic symptoms, lifetime. A specific inclusion criterion for HCs was no current or past psychiatric disorders. Additional inclusion criteria for all participants were: (1) No somatic or neurological disorders that may have impacted the central nervous system; and (2) no MRI-incompatibilities (e.g. metal implants, claustrophobia or pregnancy).
Following these criteria, 21 BD patients (8 males, 13 females) were recruited from several mental health care institutions in the North of the Netherlands. Diagnosis of BD was confirmed with the Mini International Neuropsychiatric Interview-Plus 5.0.0 (MINI-Plus; Sheehan et al., 1998).
Based on the final BD patient sample (see Section 3.1 Patient disposition), 17 SZ patients (11 males, 6 females) were selected from a sample of a previous study (Van der Meer et al., 2013). These participants were not significantly different from the BD sample on intelligence (measured with the Dutch reading test for adults (NLV); Schmand et al., 1991), age, gender and level of education. Diagnosis of SZ was confirmed with the MINI-Plus. Because self-reflection is also associated with insight into illness (both clinical insight and cognitive insight) (Van der Meer et al., 2013), it is important to match patients with BD and SZ on insight to exclude alternative explanations for any potential group differences. We matched SZ patients with BD patients on illness insight, based on the Schedule of Assessment of Insight-Expanded version (SAI-E, clinical insight; Kemp & David, 1997) and the Beck Cognitive Insight Scale (BCIS, cognitive insight; Beck et al., 2004). The BCIS is composed of a self-reflectiveness subscale and a self-certainty subscale. Cognitive insight is measured by a composite score of these two sub-scales (i.e. score on the self-reflectiveness minus the self-certainty score). BD and SZ patients were matched on the BCIS subscales and the BCIS composite score. Higher scores on the SAI-E and BCIS indicate better insight.
In addition, we included a group of 21 HCs (12 males, 9 females) matched on intelligence, gender and level of education with both patient groups. The HC group was the same as in a previous study (Van der Meer et al., 2013).
The present study was performed in accordance with the Helsinki Declaration of 1975 and was approved by the Medical Ethics Committee of the University Medical Center Groningen (UMCG). All participants gave written informed consent and received monetary compensation (€45) for participation.
2.2. Clinical assessment and measures
Current severity of depression and mania was measured by the Quick Inventory of Depressive Symptomatology (QIDS-SR; Rush et al., 2003) and Young Mania Rating Scale (YMRS; Young et al., 1978), respectively. A depressive state was defined as a score of >10 on the QIDS-SR (Rush et al., 2006) and a mania state was defined as a score of >8 on the YMRS (Mercer & Becerra, 2013). Current psychotic symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). The PANSS is composed of three dimensions: positive, negative and general psychopathology. Lower scores on QIDS, YMRS and PANSS indicate lower symptom severity.
2.3. Self-reflection task
The self-reflection task consisted of three conditions: (1) self-reflection (self): sentences referring to “I/me” which were balanced for positive/negative valence and mental/physical quality (e.g. “It's always fun with me”, “I am a liar”, “I look youthful”, “I am fat”); (2) other-reflection (other): sentences referring to a relative or close friend who remained the same throughout the whole experiment, also balanced for positive/negative valence and mental/physical quality (e.g. “(other) has respect for others”, “(other) sometimes says stupid things”, “(other) is healthy”, “(other) has wrinkles”). Prior to scanning, the name of a close other was provided to match the familiarity and difficulty of accessing stored knowledge compared to the self and would replace the “other” in the given examples; (3) semantic control (semantic): sentences containing equal number of false and true general knowledge statements (e.g. “snow is black”, “dogs run faster than snails”). Before scanning, participants received instructions and explanations about the task. Participants were instructed to define on a four-point continuum how much they agreed with the presented sentence (1-fully disagree; 4-fully agree). Each trial consisted of a sentence presented for 4000 ms followed by a 500 ms fixation cross.
For each condition, sixty sentences were presented. Sentences were presented in a block design, with each block consisting of five trials. The three conditions were counterbalanced in a semi-random order to avoid consecutive presentation of the same condition. Reaction times (RT) were recorded. The procedure lasted about 15 min.
2.4. Image acquisition
fMRI data were collected using a 3.0 Tesla whole body scanner (Philips Intera, Best, NL). Stimuli were rear-projected on a screen, which was visible via a mirror attached to the sense-8 head coil. The head was kept in position by an elastic band and foam cushions on each side of the head. A T1-weighted 3D fast field echo anatomical image was acquired parallel to the bicommissural plane, covering the whole brain (170 slices; TR = 9.00 ms; TE = 3.50 ms; FOV = 232.00 × 170.00 × 256.00 mm; voxel size: 1 × 1 × 1 mm). The functional images were acquired using T2*-weighted echo planar imaging sequences. Each functional image consisted of 37 interleaved axial slices of 3.5 mm thickness (slice gap = 0 mm; TR = 2.00 s; TE = 30 ms; FOV = 224.00 × 129.50 × 224.00 mm; 64 × 64 matrix of 3.50 × 3.50 × 3.50 voxels). To prevent artefacts due to nasal cavities, images were tilted approximately 10° to the AC−PC transverse plane.
2.5. Data analysis
2.5.1. Behavioural analysis
In order to check the self-serving bias in BD patients, we recoded the 4-point scale to agreed and disagreed items. Items scoring 1 or 2 on the four-point continuum were considered as items disagreed, while those scoring 3 or 4 were considered as items agreed. We calculated a value corresponding to the self-serving bias by subtracting the number of negative sentences participants attributed to themselves from the number of positive sentences, yielding one score per participant. In addition, we calculated this value for the other-condition (other-serving bias). Behavioural group differences were assessed with a one-way analysis of variance (ANOVA) for self-serving bias and other-serving bias separately.
In addition, differences in RT were tested using a repeated measures ANOVA, with condition (self and other) and valence (positive and negative) as within-subjects factors, and group (BD, SZ and HC) as between-subjects factor. Significance level was set at p < .05 (two-sided) for behavioural analyses.
2.5.2. fMRI analysis
fMRI data were pre-processed and analysed using statistical parametric mapping (SPM 8) (http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 7.13 (The Math Works Inc., Natick, MA). Before pre-processing, Philips PAR-files were converted to analyse data by MRIcro. Functional images were slice-time corrected, realigned and co-registered to the anatomical image. Following this, the fMRI images were normalized into standard Montreal Neurological Institute (MNI) space and smoothed with a 3D 10 mm full-width/half-maximum Gaussian Kernel.
In the first-level analysis, separate regressors were defined for self, other and semantic sentences. In order to avoid systematic low-frequency noise, a high-pass filter was defined based on the following formula: 1.1 times the longest period between two subsequent trials of the same condition. For each participant, two contrasts were defined: self > semantic and other > semantic.
For second-level analyses, contrast images were entered in a full factorial model, with condition (self > semantic and other > semantic) as within-subjects variable and group (BD, SZ and HC) as between-subjects variable. Age was added as covariate of no interest. Because the behavioural results showed that only a few sentences were regarded as negative, we did not investigate differences related to valence on a neural level.
The main task effects were investigated in the three groups separately for the contrasts self > semantic, other > semantic, [(self > semantic) > (other > semantic)] and [(other > semantic) > (self > semantic)]. The threshold was set at p < .05 family wise error (FWE) corrected for multiple comparisons on voxel-level and an extended threshold of k ≥ 10 voxels.
In order to assess reflective processing in BD patients, t-tests were conducted between BD patients and HCs for each contrast. Following this, to investigate whether patients with BD and SZ would show different or comparable activations, t-tests were conducted comparing BD with SZ patients. Furthermore, in order to check whether we could get comparable results as observed in previous studies of reflective processing in SZ patients (Bedford et al., 2012; Holt et al., 2011; Murphy et al., 2010; Pauly et al., 2013; Shad et al., 2012; Van der Meer et al., 2013), we also performed t-tests between SZ patients and HCs. For all t-tests, significance level was set at p < .05 FWE corrected on cluster-level for the spatial extent of our regions of interest (ROIs).
Limiting the regions of interest to the self- and other-reflection network, four ROI-masks were created by drawing a sphere of 20 mm (radius) around the cluster centre coordinates reported in quantitative meta-analyses of self- and other-reflection tasks by Murray et al. (2012), in accordance with Van der Meer et al. (2013). Regarding the masks for the contrasts other > semantic and [(self > semantic) > (other > semantic)], we used the coordinates for the close-other reported by Murray et al. (2012). For the mask for the contrast [(other > semantic) > (self > semantic)], we took the coordinates of close-other and public-other combined reported by Murray et al. (2012), because no significant clusters were reported for close-other only.
For the purpose of visualizing the trend of activation level in the three groups, mean activation values of the area(s) resulting from the t-tests were extracted for each participant using the MarsBaR toolbox (http://marsbar.sourceforge.net) and were plotted for each group.
We repeated these analyses with the self-serving bias and other-serving bias added as covariates of no interest during testing of self- and other-reflection respectively, to test whether the findings would be related to behavioural performance. Moreover, we performed correlation analyses between the mean activation values of the areas showing a difference (extracted with the MarsBaR toolbox) and the scores on SAI-E, BCIS (the two subscales and the composite score), as well as scores on PANSS, YMRS and QIDS to assess whether the potential different activations were related to insight and symptom severity. Correlation analyses were first performed in all groups together, and then in the subgroups separately in order to explore whether there were group differences in correlations. This exploratory analysis might shed some light on our understanding of the neural mechanisms underlying the same symptoms in affective psychotic disorder (BD) and non-affective psychotic disorder (SZ). The Benjamini–Hochberg procedure was applied to correct for multiple tests in order to control for the false discovery rate. In case there were group differences in correlation, Fisher's r to z transformation (Kenny, 1987) was applied to compare whether the correlations in each group differed significantly from each other.
3. Results
3.1. Patient disposition
Demographic and clinical data of participants are shown in Table 1. Three BD patients were excluded because of excessive head movements (more than 3 mm and/or 3° in any direction) and one BD patient was excluded because of missing behavioural data. A total of 17 BD patients, 17 SZ patients and 21 HCs were included in the analyses.
Table 1.
Demographic and clinical characteristics for BD patients, SZ patients and HCs.
| Variables | BDa | SZb | HCc | p |
|---|---|---|---|---|
| Age, M (SD) (years) | 41.29 (11.82) | 35.53 (9.74) | 29.95 (10.99) | .009** |
| Male/female (N) | 7/10 | 11/6 | 12/9 | .37 |
| Education leveld | 6.00 (0.87) | 5.65 (0.86) | 5.76 (0.83) | .47 |
| Intelligence (NLV) (score) | ||||
| Total correct | 42.88 (3.72) | 38.50 (6.84) | 41.15 (6.80) | .14 |
| Number of manic episodes | 7.93 (11.06) | |||
| Number of depressive episodes | 5.57 (4.42) | |||
| QIDS | 6.35 (6.17) | 7.94 (3.88) | 2.00 (1.18) | .002** |
| YMRS | 1.31 (1.49) | 1.71 (1.86) | .52 | |
| PANSS | ||||
| Total | 40.81 (6.27) | 52.59 (13.52) | .003** | |
| Positive | 9.50 (2.63) | 12.71 (4.63) | .021* | |
| Negative | 9.56 (2.94) | 13.47 (4.81) | .009** | |
| General psychopathology | 21.75 (3.57) | 26.41 (6.96) | .023* | |
| Insight | ||||
| SAI-E | 22.43 (2.03) | 21.64 (1.93) | .26 | |
| BCIS | ||||
| Composite score | 7.75 (5.43) | 7.29 (4.27) | .79 | |
| Self-reflectiveness | 15.13 (4.44) | 16.41 (5.29) | .46 | |
| Self-certainty | 7.38 (2.58) | 9.12 (4.41) | .18 | |
| Psychopharmacological drug (N) | ||||
| Haloperidol | 1 | |||
| Pimozide | 1 | |||
| Aripiprazole | 9 | |||
| Clozapine | 2 | |||
| Olanzapine | 2 | 5 | ||
| Quetiapine | 6 | 4 | ||
| Risperidone | 1 | |||
| Clomipramine | 1 | |||
| Lithium | 7 | |||
| Sertraline | 1 | |||
| Trazodone | 1 | |||
| Valproic acid | 3 | |||
| Bupropion | 1 | |||
| Oxazepam | 1 | |||
| None | 1 | 1 |
Abbreviations: NLV = Dutch Reading Test for Adults; QIDS = Quick Inventory of Depressive Symptoms-Self Report; YMRS = Young Mania Rating Scale; PANSS = Positive and Negative Syndrome Scale; SAI-E = Schedule of Assessment of Insight-Expanded version; BCIS = Beck Cognitive Insight Scale.
p < .05.
p < .01.
One BD patient withdrew from the study before the MRI scan, therefore we do not have clinical interview data for this patient. The number of depressive/manic episodes was unavailable for another two BD patients.
Three SZ patients were recruited from another study in which these measures were not included.
Six HCs were recruited from another study and one HC withdrew from the study before the MRI scan, therefore they do not have clinical interview data. Another HC did not have data for NLV, because he was familiar with this test.
Education level was based on Verhage (1964).
Of the 17 included BD patients, 13 were in a euthymic state and 4 were in a depressed state. Moreover, 14 out of the 17 BD patients had a BD type I diagnosis and 2 had a BD type II diagnosis. For one of the 17 BD patients, we did not have information on the type of BD, because he did not participate in the clinical interviews. His treating psychiatrist confirmed the diagnosis BD of this patient, so we included this patient in the final analysis.
There was a group difference on age (F(2, 52) = 5.106, p = .009). Post-hoc t-tests showed that HCs were younger than BD patients (p = .002, mean difference in age = −11.34, 95% CI of the mean difference in age: −18.47 ~ −4.21). There was no significant difference in age between BD and SZ patients (p = .129) or between HCs and SZ patients (p = .123).
Regarding the clinical measures, both patients with BD and SZ had higher QIDS-scores than HC, with no significant differences between them. Patients with BD and SZ also did not differ on YMRS-scores. In addition, SZ patients had higher PANSS-scores compared to BD patients (positive symptom score, negative symptom score and general psychopathology score).
3.2. Behavioural results
For the self-serving bias (Table 2), a main effect of group was present (F(2, 52) = 10.021, p < .001): compared to HCs, both BD patients (p < .001, mean difference in self-serving bias = −9.43, 95% CI: −14.47~−4.38) and SZ patients (p < .001, mean difference in self-serving bias = −9.72, 95% CI: −14.77~−4.67) showed a reduced self-serving bias, indicating that patients with BD and SZ attributed more negative and less positive events to themselves. Patients with BD and SZ did not differ in self-serving bias (p = .91). Regarding the other-serving bias, no significant differences were seen (p = .22). RTs did not significantly differ among groups (p = .12).
Table 2.
Behavioural results of bias value and reaction times for BD patients, SZ patients and HCs.
| BD | SZ | HC | |
|---|---|---|---|
| Items agreed M (SD) | |||
| Self_pos | 18.53 (6.55) | 16.41 (6.95) | 24.43 (3.65) |
| Self_neg | 7.76 (4.44) | 5.94 (3.51) | 4.24 (2.86) |
| Self-serving bias | 10.76 (9.77) | 10.47 (7.87) | 20.19 (5.34) |
| Other_pos | 21.76 (4.85) | 19.41 (6.10) | 23.57 (3.91) |
| Other_neg | 4.24 (3.42) | 4.59 (3.14) | 5.00 (2.76) |
| Other-serving bias | 17.53 (6.36) | 14.82 (7.57) | 18.57 (5.92) |
| Semantic | 32.88 (3.85) | 32.29 (3.67) | 34.48 (1.33) |
| Reaction times in seconds M (SD) | |||
| Self_pos | 2.20 (0.35) | 2.26 (0.34) | 2.06 (0.32) |
| Self_neg | 2.18 (0.29) | 2.26 (0.30) | 2.03 (0.29) |
| Other_pos | 2.25 (0.37) | 2.31 (0.35) | 2.06 (0.39) |
| Other_neg | 2.12 (0.29) | 2.28 (0.39) | 2.07 (0.40) |
| Semantic | 2.28 (0.31) | 2.41 (0.23) | 2.08 (0.38) |
Abbreviations: pos = positive; neg = negative.
3.3. Neuroimaging results
3.3.1. Main effects of task
Visual inspection showed similar patterns of activation in the three groups (see Fig. 1). In HCs, the contrast self > semantic revealed activations in the VMPFC, DMPFC, ACC, PCC, precuneus, angular gyrus, supramarginal gyrus (SMG), inferior parietal lobule (IPL), cerebellum, superior parietal lobule (SPL) and postcentral gyrus. A similar pattern of activation as for self > semantic was seen for other > semantic. The contrast [(other > semantic) > (self > semantic)] revealed activation differences in the PCC, precuneus, and the cuneus, while the contrast [(self > semantic) > (other > semantic)] did not show any effect. Fig. 1 and Table S1 (see Supplementary material) summarize these task effects.
Fig. 1.
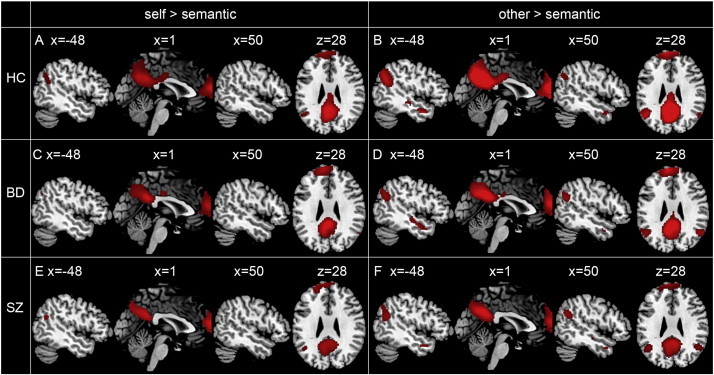
Main task effects of brain activation during self > semantic in HCs (A), BD patients (C) and SZ patients (E); during other > semantic in HCs (B), BD patients (D) and SZ patients (F).
3.3.2. Group comparisons
When comparing BD patients to HCs, BD patients showed less activation in the PCC/precuneus during other > semantic (Fig. 2; peak MNI-coordinates: x = 2, y = −60, z = 22, t = 3.77, k = 128, p = .028 FWE corrected on cluster-level). There were no significant differences in other areas. In addition, no significant group differences were observed for the contrasts self > semantic, [(self > semantic) > (other > semantic)] and [(other > semantic) > (self > semantic)].
Fig. 2.
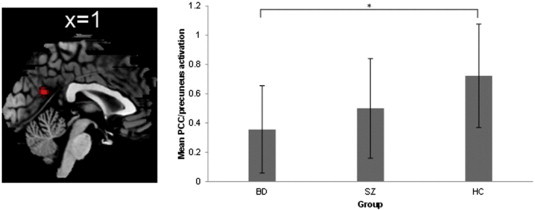
Group comparison between HCs and BD patients for contrast other > semantic. BD patients show reduced activation in the PCC/precuneus compared to HCs. On the right, bar graph shows mean PCC/precuneus activation. Error bar = 1 S.D. *p < .05.
Additionally, even though SZ patients displayed an intermediate level of activation in the PCC/precuneus between BD and HC during other-reflection (see Fig. 2), the difference between BD patients and SZ patients was not significant (p = .20). Nor did we detect significant differences between patients with BD and SZ on the other three contrasts. In addition, no significant differences were seen between SZ patients and HCs.
3.3.3. Additional neuroimaging analyses
The hypo-activation in the PCC/precuneus during other-reflection in BD patients remained significant after adding other-serving bias as covariate of no interest (peak MNI-coordinates: x = 2, y = −60, z = 22, t = 3.79, k = 124, and p = .03 FWE corrected on cluster-level). The lack of group difference during self-reflection was not changed by adding self-serving bias as covariate of no interest. Furthermore, no significant correlations were seen between the degree of activation in the PCC/precuneus cluster and scores on the SAI-E, BCIS, PANSS, YMRS, or QIDS (ps > .05). However, at a more liberal threshold (i.e. without multiple comparison correction), we found a positive correlation between the PCC/precuneus activation and the BCIS composite score (cognitive insight) in SZ patients (r = .508, p = .038), but not in BD patients (r = −.250, p = .35) (see Fig. 3). This apparent interaction was significant (Z = 2.124, p = .034).
Fig. 3.
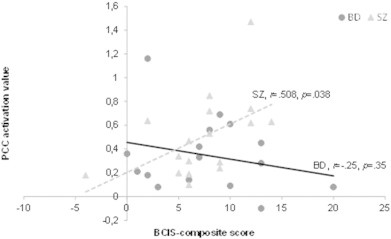
Correlation between the cognitive insight (BCIS composite score) and PCC/precuneus activation during other-reflection in patients with BD and SZ. A positive correlation is seen in SZ patients, but not in BD patients.
4. Discussion
In the present study, our main aim was to investigate brain activation during self- and other-reflection in patients with bipolar disorder (BD) with a history of psychotic symptoms compared to healthy controls (HCs). In addition, we compared BD patients to patients with schizophrenia (SZ) to investigate whether observed abnormal activations were unique features of BD. Most importantly, BD patients showed less activation in the posterior cingulate cortex (PCC) extending to the precuneus during other-reflection compared to HCs. No significant differences were observed between BD and SZ patients, with SZ patients showing a level of activation in between BD patients and HCs.
4.1. Comparison between BD patients and HCs during other-reflection
During other-reflection, hypo-activation in the PCC/precuneus was observed in BD patients compared to HCs, which was not induced by differences in activation in response to the semantic control condition (see Supplement material) or by behavioural performance during other-reflection. Moreover, since most participants were stable during scanning, the observed hypo-activation is likely irrespective of current residual psychotic, depressive and manic symptoms. Altogether, this suggests that the hypo-activation in the PCC/precuneus was due to differences of other-reflection per se rather than any other confounding factor.
Both the PCC and precuneus have been shown to be important areas underlying autobiographical memory (Fink et al., 1996; Maddock et al., 2001; Maguire & Mummery, 1999). It has been suggested that autobiographical memory may play a critical role in self-reflective processing (Dimaggio et al., 2009), and also in other-reflective processing (Van der Meer et al., 2010). Indeed, according to the self-reflection/self-appraisal model (Van der Meer et al., 2010), the role of the PCC and precuneus during self- and other-reflection is associated with autobiographical memory processing. For the current results, it could be suggested that less activation in the PCC/precuneus reflects reduced integration between past and current information, and more specifically regarding to information about other people. In line with this suggestion, previous literature has shown that autobiographical memory is indeed impaired in BD patients, e.g. they provide less details during autobiographical memory recall than healthy individuals (Scott et al., 2000; Shimizu et al., 2009).
In addition, the PCC is an important structure for emotional processing. For example, Maddock et al. (2003) have shown that healthy individuals have more activation in the PCC in response to both positive and negative words compared to neutral words. Interestingly, BD patients have shown a lack of PCC/precuneus activation during emotionally modulated cognitive processing (i.e. emotional Stroop task) (Malhi et al., 2005). In addition, it has been found that BD patients show reduced PCC activation during emotion regulation compared to HCs (Townsend et al., 2013). Combined with the role of the PCC/precuneus in autobiographical memory, we propose that the PCC/precuneus may be an interaction unit of processing emotional information and autobiographical memory. A recent study has indeed confirmed that the PCC and precuneus are activated during emotional autobiographical memory processing (Bado et al., 2014). Therefore, it might be suggested that the hypo-activation in the PCC/precuneus during other-reflection in BD patients implies disturbed integration of emotional information and autobiographical memory related to a close other. This might result in inappropriate or inaccurate knowledge about the close other.
This disturbed processing of close-other information might be related to disturbed social cognition (e.g. theory of mind) in BD patients, and hence disturbed social functioning. BD patients indeed have shown impairments in social cognition (Donohoe et al., 2012; Elgie & Morselli, 2007; Samamé et al., 2012). Interestingly, the PCC and precuneus have been shown to be involved during social cognition, for instance during theory of mind (Abu-Akel & Shamay-Tsoory, 2011; Carrington & Bailey, 2009; Spreng et al., 2009), and processing of social-self (e.g. judging self with social-value traits, such as “am I intelligent”) (Sugiura, 2013). We therefore postulate that less activation in the PCC/precuneus during other-reflection in BD patients might be related to the commonly observed impairments in social cognition.
4.2. Comparison between BD patients and HCs during self-reflection
Concerning self-reflection, our behavioural results showed that BD patients attributed less positive sentences and more negative sentences to themselves compared to HCs (smaller self-serving bias). Nonetheless, we did not find any significant differences in neural activation during self-reflection. Several potential explanations could be suggested. One possible explanation is that the circuitry for self-reflection is not impaired in BD. Instead, the reduced self-serving bias in BD patients might be related to depressive realism, which can be the results of proper insight regarding their situation, such as less social support and stigma (for a review, see Hawke et al., 2013). Another possible explanation could be that neural abnormalities are valence-specific or opposed between different valences. Due to a limited number of negative sentences attributed to the self or other, we are unable to make any statements on the valence effects related to the neuroimaging findings. More studies are necessary to clarify these.
4.3. Comparison of SZ patients with BD patients and HCs
In order to identify whether abnormal activation of the PCC/precuneus during other-reflection was specific for BD patients, we compared BD patients to SZ patients. The intermediate level of activation in the PCC/precuneus in SZ patients reported in the present study may imply that activation during other-reflection in SZ patients tends to be less abnormal than in BD patients. This is remarkable, because SZ patients in general perform worse than BD patients on tasks measuring cognitive performance (e.g. verbal memory, verbal fluency and verbal working memory; Krabbendam et al., 2005). Moreover, we also compared SZ patients with HCs in order to replicate previous findings, and no differences were seen. In previous studies to SZ, disturbances in brain activation during self- and other-reflection have been seen in different areas, including the medial prefrontal cortex (dorsal and ventral), ACC, precuneus, cuneus, PCC, angular gyrus, lingual gyrus, temporal gyrus (superior and inferior/middle) and insula (Bedford et al., 2012; Holt et al., 2011; Murphy et al., 2010; Pauly et al., 2013; Shad et al., 2012; Van der Meer et al., 2013). The reported abnormalities also differ on hypo- or hyper-activation. Thus, there are quite some inconsistent findings among previous studies. We think a selection bias could explain these results. We selected SZ patients matched on illness insight to the BD patients. BD patients with a history of psychotic symptoms were selected to represent the more severe BD patients. These BD patients were in general scanned during a euthymic period and had good insight. As such, the SZ patients also had good insight and might therefore represent a less severe group of SZ patients, with relatively preserved ability of reflective processing. This could lead to not finding differences between SZ and both BD and HC. This is underscored by the finding of a positive correlation between cognitive insight and PCC/precuneus activation in SZ patients, which indicates that a lower level of activation in the PCC/precuneus was related to worse insight in SZ. Some other factors such as sample size, control condition and illness state may also have contributed to this inconsistency with previous studies.
4.4. Correlations with insight
Notably, we found a positive relationship between PCC/precuneus activation and cognitive insight in SZ patients, but not in BD patients. This differential pattern is remarkable, as patients with BD and SZ did not differ on cognitive insight at a group level. Caution is needed in interpreting this correlation, however, as it did not survive the correction for multiple comparisons. Nonetheless, it may serve as a heuristic for future research. Cognitive insight is defined as the ability to reflect on abnormal experiences and to correct incorrect interpretations (Beck et al., 2004). The observed correlation might imply that better cognitive insight in SZ is associated with more retrieval of autobiographical memory during reflection on a close other. More information from autobiographical memory could be helpful for differentiating a close other to a larger extent from the self, from which SZ patients may benefit to re-evaluate and correct sensations coming from psychotic symptoms (e.g. hallucination). Because patients included in this study generally had a good level of insight, differences are not likely driven by considerable levels of cognitive impairments. The SZ patients had more severe psychotic symptoms than BD patients though. Better insight may be more important for normalizing metacognitive brain activation in SZ patients, but not in BD patients. Taken together, this finding of a difference in correlation with insight is interesting in terms of that the neural correlates underlying metacognition could differentiate between BD and SZ patients, which might help pave the way to further explore potentially distinguishing markers for BD and SZ.
4.5. Limitations
There were some limitations of the current study. Firstly, most patients in our study were taking medication, which may have confounded our results. Psychotropic medication has been shown to have an ameliorative effect on functional abnormalities in BD patients (Hafeman et al., 2012; Phillips et al., 2008). Because most of the BD patients in our study were stable during scanning, existing differences between BD and HC groups might have been obscured by the psychotropic medication. Secondly, our sample size was modest. However, it has been shown that a sample size of 16 has a kappa index around .62 (Thirion et al., 2007), which indicates substantial power for reproducibility (kappa index = .61–.80) (Landis & Koch, 1977). In addition, our sample size was comparable to most of previous self- and other-reflection studies in SZ patients (with samples between 11 and 19 patients in five out of six studies, and one with a group of 47 SZ patients) (Bedford et al., 2012; Holt et al., 2011; Murphy et al., 2010; Pauly et al., 2013; Shad et al., 2012; Van der Meer et al., 2013). Thirdly, the age difference between groups could have influenced our results, but this was controlled for by adding age as covariate in the second level analyses. Although extremely difficult to perform, future research with larger, age matched samples, and medication naïve patients is needed to confirm and extend our results. Fourthly, the results of correlation with insight were observed without multiple comparisons correction. Fifthly, BD is characterized by emotional problems; therefore, it would be interesting to investigate the valence-specific difference in self- and other-reflection. However, the number of negative attributions was quite small in the present study. Therefore, we were not able to investigate the effect of valence on a neural level. Finally, BD patients in our study were mostly euthymic. Although one previous study with bipolar manic and depressive patients, including those with psychosis, has shown no, even reverse self-serving bias (Lyon et al., 1999), it would be interesting to explore the behavioural changes in self- and other-reflection in BD among Euthymia, past psychosis and current psychosis in one study. Future studies are needed to shed light on this.
5. Conclusions
In conclusion, we observed less activation in the PCC extending to the precuneus during other-reflection in BD patients compared to HCs. BD patients did not differ in brain activation from SZ patients, and SZ patients showed a level of activation in the PCC/precuneus in between BD patients and HCs. This hypo-activation of the PCC/precuneus could contribute to a disturbance in coupling information from autobiographical memory and current other-related stimuli. This may be of relevance for the impairments in social cognition observed in patients with BD and SZ. Future studies may include an additional BD group without a history of psychosis to investigate whether the observed disturbance in our BD sample is related to psychosis.
Conflicts of interest
None of the authors have commercial or other relationships that might pose a conflict of interest in connection with the present study.
Acknowledgements
We thank Dr. H.G. Knegtering, Dr. R. Bruggeman, L. Bais, Drs. B. Haarman and prof. dr. W.A. Nolen for their help with patient inclusion. We thank Dr. R. Renken for his help with the study design. In addition, we thank A. Sibeijn-Kuiper and J. Streurman for scanning the participants, and thank Dr. J-B. Marsman for his help with the analyses. This study was supported by a grant from the European Science Foundation EURYI (NWO grant number 044.035.001) awarded to A. Aleman. H.G. Ruhé was supported by a VENI grant (NWO/ZonMW grant number 016.126.059).
Footnotes
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nicl.2015.04.010.
Appendix A. Supplementary data
Supplementary material.
References
- Abu-Akel A., Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49(11):2971–2984. doi: 10.1016/j.neuropsychologia.2011.07.012. 21803062 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. fifth edition edition. American Psychiatric Publishing, Inc.; Washington, DC: 2013. [Google Scholar]
- Arango C., Amador X. Lessons learned about poor insight. Schizophr. Bull. 2011;37(1):27–28. doi: 10.1093/schbul/sbq143. 21163898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bado P., Engel A., de Oliveira-Souza R., Bramati I.E., Paiva F.F., Basilio R., Sato J.R., Tovar-Moll F., Moll J. Functional dissociation of ventral frontal and dorsomedial default mode network components during resting state and emotional autobiographical recall. Hum. Brain Mapp. 2014;35(7):3302–3313. doi: 10.1002/hbm.22403. 25050426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batmaz S., Ulusoy Kaymak S.U., Kocbiyik S., Turkcapar M.H. Metacognitions and emotional schemas: a new cognitive perspective for the distinction between unipolar and bipolar depression. Compr. Psychiatry. 2014;55(7):1546–1555. doi: 10.1016/j.comppsych.2014.05.016. 24974282 [DOI] [PubMed] [Google Scholar]
- Beck A.T., Baruch E., Balter J.M., Steer R.A., Warman D.M. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr. Res. 2004;68(2–3):319–329. doi: 10.1016/S0920-9964(03)00189-0. 15099613 [DOI] [PubMed] [Google Scholar]
- Bedford N.J., Surguladze S., Giampietro V., Brammer M.J., David A.S. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. B.M.C. Psychiatry. 2012;12(106):106. doi: 10.1186/1471-244X-12-106. 22876974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr. Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. 15888423 [DOI] [PubMed] [Google Scholar]
- Carrington S.J., Bailey A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. 19034900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy F. Insight in bipolar disorder: relationship to episode subtypes and symptom dimensions. Neuropsychiatr. Dis. Treat. 2010;6:627–631. doi: 10.2147/NDT.S12663. 20957122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N., Owen M.J. The beginning of the end for the Kraepelinian dichotomy. Br. J. Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. 15863738 [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel — now? the anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. 19096369 [DOI] [PubMed] [Google Scholar]
- Dimaggio G., Vanheule S., Lysaker P.H., Carcione A., Nicolò G. Impaired self-reflection in psychiatric disorders among adults: a proposal for the existence of a network of semi independent functions. Conscious. Cogn. 2009;18(3):653–664. doi: 10.1016/j.concog.2009.06.003. 19615919 [DOI] [PubMed] [Google Scholar]
- Donohoe G., Duignan A., Hargreaves A., Morris D.W., Rose E., Robertson D., Cummings E., Moore S., Gill M., Corvin A. Social cognition in bipolar disorder versus schizophrenia: comparability in mental state decoding deficits. Bipolar Disord. 2012;14(7):743–748. doi: 10.1111/bdi.12011. 23020773 [DOI] [PubMed] [Google Scholar]
- Elgie R., Morselli P.L. Social functioning in bipolar patients: the perception and perspective of patients, relatives and advocacy organizations - a review. Bipolar Disord. 2007;9(1–2):144–157. doi: 10.1111/j.1399-5618.2007.00339.x. 17391357 [DOI] [PubMed] [Google Scholar]
- Fink G.R., Markowitsch H.J., Reinkemeier M., Bruckbauer T., Kessler J., Heiss W.D. Cerebral representation of one's own past: neural networks involved in autobiographical memory. J. Neurosci. 1996;16(13):4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. 8753888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D.M., Chang K.D., Garrett A.S., Sanders E.M., Phillips M.L. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. 22631621 [DOI] [PubMed] [Google Scholar]
- Happé F. Theory of mind and the self. Ann. N. Y. Acad. Sci. 2003;1001:134–144. doi: 10.1196/annals.1279.008. 14625359 [DOI] [PubMed] [Google Scholar]
- Hawke L.D., Parikh S.V., Michalak E.E. Stigma and bipolar disorder: a review of the literature. J. Affect. Disord. 2013;150(2):181–191. doi: 10.1016/j.jad.2013.05.030. 23759420 [DOI] [PubMed] [Google Scholar]
- Holt D.J., Cassidy B.S., Andrews-Hanna J.R., Lee S.M., Coombs G., Goff D.C., Gabrieli J.D., Moran J.M. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol. Psychiatry. 2011;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. 21144498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis A.H., Abramson L.Y., Hyde J.S., Hankin B.L. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychol. Bull. 2004;130(5):711–747. doi: 10.1037/0033-2909.130.5.711. 15367078 [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Kemp R., David A.S. Insight and compliance. Treatment compliance and the therapeutic alliance. Harwood; Amsterdam: 1997. pp. 61–84. [Google Scholar]
- Kempf L., Hussain N., Potash J.B. Mood disorder with psychotic features, schizoaffective disorder, and schizophrenia with mood features: trouble at the borders. Int Rev Psychiatry. 2005;17(1):9–19. doi: 10.1080/09540260500064959. 16194767 [DOI] [PubMed] [Google Scholar]
- Kenny D.A. Statistics for the Social and Behavioral Sciences. Little, Brown; Boston: 1987. [Google Scholar]
- Krabbendam L., Arts B., Van Os J., Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr. Res. 2005;80(2–3):137–149. doi: 10.1016/j.schres.2005.08.004. 16183257 [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. 843571 [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., Yip B.H., Björk C., Pawitan Y., Cannon T.D., Sullivan P.F., Hultman C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. 19150704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon H.M., Startup M., Bentall R.P. Social cognition and the manic defense: attributions, selective attention, and self-schema in bipolar affective disorder. J. Abnorm. Psychol. 1999;108(2):273–282. doi: 10.1037/0021-843X.108.2.273. 10369037 [DOI] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/S0306-4522(01)00108-7. 11440800 [DOI] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. 12454910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Mummery C.J. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. 10088900 [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Lagopoulos J., Sachdev P.S., Ivanovski B., Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7(Suppl. 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. 16225562 [DOI] [PubMed] [Google Scholar]
- Mehl S., Landsberg M.W., Schmidt A.C., Cabanis M., Bechdolf A., Herrlich J., Loos-Jankowiak S., Kircher T., Kiszkenow S., Klingberg S., Kommescher M., Moritz S., Müller B.W., Sartory G., Wiedemann G., Wittorf A., Wölwer W., Wagner M. Why do bad things happen to me? Attributional style, depressed mood, and persecutory delusions in patients with schizophrenia. Schizophr. Bull. 2014;40(6):1338–1346. doi: 10.1093/schbul/sbu040. 24743864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer L., Becerra R. A unique emotional processing profile of euthymic bipolar disorder? A critical review. J. Affect. Disord. 2013;146(3):295–309. doi: 10.1016/j.jad.2012.10.030. 23218848 [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. The link between social cognition and self-referential thought in the medial prefrontal cortex. J. Cogn. Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. 16197685 [DOI] [PubMed] [Google Scholar]
- Mizrahi R., Addington J., Remington G., Kapur S. Attribution style as a factor in psychosis and symptom resolution. Schizophr. Res. 2008;104(1–3):220–227. doi: 10.1016/j.schres.2008.05.003. 18632256 [DOI] [PubMed] [Google Scholar]
- Murphy E.R., Brent B.K., Benton M., Pruitt P., Diwadkar V., Rajarethinam R.P., Keshavan M.S. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr. Res. 2010;116(2–3):252–258. doi: 10.1016/j.schres.2009.11.009. 20051318 [DOI] [PubMed] [Google Scholar]
- Murray R.J., Schaer M., Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci. Biobehav. Rev. 2012;36(3):1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. 22230705 [DOI] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. 15301749 [DOI] [PubMed] [Google Scholar]
- Pauly K.D., Kircher T.T., Schneider F., Habel U. Me, myself and I: temporal dysfunctions during self-evaluation in patients with schizophrenia. Soc. Cogn. Affect. Neurosci. 2013;9(11):1779–1788. doi: 10.1093/scan/nst174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Travis M.J., Fagiolini A., Kupfer D.J. Medication effects in neuroimaging studies of bipolar disorder. Am. J. Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. 18245175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin S.J., Hasson-Ohayon I., Avidan M., Rozencwaig S., Shalev H., Kravetz S. Metacognition in schizophrenia and schizotypy: relation to symptoms of schizophrenia, traits of schizotypy and social quality of life. Isr. J. Psychiatry Relat. Sci. 2014;51(1):44–53. 24858634 [PubMed] [Google Scholar]
- Rush A.J., Bernstein I.H., Trivedi M.H., Carmody T.J., Wisniewski S., Mundt J.C., Shores-Wilson K., Biggs M.M., Woo A., Nierenberg A.A., Fava M. An evaluation of the quick inventory of depressive symptomatology and the Hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol. Psychiatry. 2006;59(6):493–501. doi: 10.1016/j.biopsych.2005.08.022. 16199008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N., Markowitz J.C., Ninan P.T., Kornstein S., Manber R., Thase M.E., Kocsis J.H., Keller M.B. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54(5):573–583. doi: 10.1016/S0006-3223(02)01866-8. 12946886 [DOI] [PubMed] [Google Scholar]
- Samamé C. Social cognition throughout the three phases of bipolar disorder: a state-of-the-art overview. Psychiatry Res. 2013;210(3):1275–1286. doi: 10.1016/j.psychres.2013.08.012. 24075306 [DOI] [PubMed] [Google Scholar]
- Samamé C., Martino D.J., Strejilevich S.A. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatr. Scand. 2012;125(4):266–280. doi: 10.1111/j.1600-0447.2011.01808.x. 22211280 [DOI] [PubMed] [Google Scholar]
- Saxe R., Moran J.M., Scholz J., Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc. Cogn. Affect. Neurosci. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. 18985110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B., Bakker D., Saan R., Louman J. [The Dutch Reading Test for Adults: a measure of premorbid intelligence level] Tijdschr Gerontol Geriatr. 1991;22(1):15–19. 1877068 [PubMed] [Google Scholar]
- Scott J., Stanton B., Garland A., Ferrier I.N. Cognitive vulnerability in patients with bipolar disorder. Psychol. Med. 2000;30(2):467–472. doi: 10.1017/S0033291799008879. 10824667 [DOI] [PubMed] [Google Scholar]
- Shad M.U., Keshavan M.S., Steinberg J.L., Mihalakos P., Thomas B.P., Motes M.A., Soares J.C., Tamminga C.A. Neurobiology of self-awareness in schizophrenia: an fMRI study. Schizophr. Res. 2012;138(2–3):113–119. doi: 10.1016/j.schres.2012.03.016. 22480958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20)):22–33. quiz 34Pubmed: 9881538] [PubMed] [Google Scholar]
- Shimizu M., Kubota Y., Mason R., Baba H., Calabrese J.R., Toichi M. Selective deficit of autobiographical incident memory in subjects with bipolar disorder. Psychopathology. 2009;42(5):318–324. doi: 10.1159/000232974. 19672134 [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. 18510452 [DOI] [PubMed] [Google Scholar]
- Sugiura M. Associative account of self-cognition: extended forward model and multi-layer structure. Front. Hum. Neurosci. 2013;7:535. doi: 10.3389/fnhum.2013.00535. 24009578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler N.S., Allen D.N., Sutton G.P., Vertinski M., Ringdahl E.N. Differential impairment of social cognition factors in bipolar disorder with and without psychotic features and schizophrenia. J. Psychiatr. Res. 2013;47(12):2004–2010. doi: 10.1016/j.jpsychires.2013.09.010. 24112946 [DOI] [PubMed] [Google Scholar]
- Thirion B., Pinel P., Mériaux S., Roche A., Dehaene S., Poline J.B. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. NeuroImage. 2007;35(1):105–120. doi: 10.1016/j.neuroimage.2006.11.054. 17239619 [DOI] [PubMed] [Google Scholar]
- Townsend J.D., Torrisi S.J., Lieberman M.D., Sugar C.A., Bookheimer S.Y., Altshuler L.L. Frontal–amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol. Psychiatry. 2013;73(2):127–135. doi: 10.1016/j.biopsych.2012.06.030. 22858151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. 20015455 [DOI] [PubMed] [Google Scholar]
- Van der Meer L., De Vos A.E., Stiekema A.P.M., Pijnenborg G.H.M., Van Tol M.J., Nolen W.A., David A.S., Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 2013;39(6):1288–1295. doi: 10.1093/schbul/sbs122. 23104865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.F., Chen C.S., Yeh M.L., Yen J.Y., Ker J.H., Yang S.J. Comparison of insight in patients with schizophrenia and bipolar disorder in remission. J. Nerv. Ment. Dis. 2002;190(12):847–849. doi: 10.1097/01.NMD.0000034519.08746.91. 12486373 [DOI] [PubMed] [Google Scholar]
- Verhage F. Van Gorcum; Assen: 1964. Intelligentie en leeftijd bij volwassenen en bejaarden. [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. 728692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


