Abstract
Background
The International Classification of Headache Disorders provides criteria for the diagnosis and subclassification of migraine. Since there is no objective gold standard by which to test these diagnostic criteria, the criteria are based on the consensus opinion of content experts. Accurate migraine classifiers consisting of brain structural measures could serve as an objective gold standard by which to test and revise diagnostic criteria. The objectives of this study were to utilize magnetic resonance imaging measures of brain structure for constructing classifiers: 1) that accurately identify individuals as having chronic vs. episodic migraine vs. being a healthy control; and 2) that test the currently used threshold of 15 headache days/month for differentiating chronic migraine from episodic migraine.
Methods
Study participants underwent magnetic resonance imaging for determination of regional cortical thickness, cortical surface area, and volume. Principal components analysis combined structural measurements into principal components accounting for 85% of variability in brain structure. Models consisting of these principal components were developed to achieve the classification objectives. Ten-fold cross validation assessed classification accuracy within each of the ten runs, with data from 90% of participants randomly selected for classifier development and data from the remaining 10% of participants used to test classification performance. Headache frequency thresholds ranging from 5–15 headache days/month were evaluated to determine the threshold allowing for the most accurate subclassification of individuals into lower and higher frequency subgroups.
Results
Participants were 66 migraineurs and 54 healthy controls, 75.8% female, with an average age of 36 +/− 11 years. Average classifier accuracies were: a) 68% for migraine (episodic + chronic) vs. healthy controls; b) 67.2% for episodic migraine vs. healthy controls; c) 86.3% for chronic migraine vs. healthy controls; and d) 84.2% for chronic migraine vs. episodic migraine. The classifiers contained principal components consisting of several structural measures, commonly including the temporal pole, anterior cingulate cortex, superior temporal lobe, entorhinal cortex, medial orbital frontal gyrus, and pars triangularis. A threshold of 15 headache days/month allowed for the most accurate subclassification of migraineurs into lower frequency and higher frequency subgroups.
Conclusions
Classifiers consisting of cortical surface area, cortical thickness, and regional volumes were highly accurate for determining if individuals have chronic migraine. Furthermore, results provide objective support for the current use of 15 headache days/month as a threshold for dividing migraineurs into lower frequency (i.e. episodic migraine) and higher frequency (i.e. chronic migraine) subgroups.
Keywords: Migraine, Cortical Thickness, Cortical Surface Area, Diagnostic Classifier, Magnetic Resonance Imaging
Introduction
The diagnosis and sub-classification of migraine are based upon a patient’s report of symptoms and exclusion of secondary headache disorders. Formal diagnostic criteria for migraine are available in the International Classification of Headache Disorders (ICHD), the latest version being the ICHD 3 beta.1 These diagnostic criteria were devised according to the consensus opinion of a group of headache experts who are members of the International Headache Society Classification Committee. Publication of criteria for diagnosing migraine and other headache disorders was a substantial advance in the field, providing standardization of migraine diagnoses when performing research and guiding clinicians when evaluating patients. However, a major limitation during the development of formal diagnostic criteria for migraine is that there is not an objective “gold-standard” for making a migraine diagnosis that can be used to test the value of individual components of the criteria. Thus, some aspects of the diagnostic criteria are mostly arbitrary, such as the division of chronic migraine (CM) from episodic migraine (EM) based upon a headache frequency of 15 headache days per month. Objective biomarkers for diagnosing and subclassifying migraine would allow for optimization of migraine diagnostic criteria.
The goal of this study was to utilize brain magnetic resonance imaging (MRI) structural data to develop classifiers that can differentiate the brain structure of an individual patient with migraine from that of a healthy control subject and that differentiate the brain structure of an individual CM patient from that of a patient with EM. This study also investigated the headache frequency threshold that allowed for a classifier to most accurately assign individual migraine patients to a lower frequency migraine subgroup or a higher frequency migraine subgroup based upon brain structure. In doing so, this study investigated whether the threshold of 15 headache days per month that is currently used to differentiate CM from EM is supported by brain structural differences between these two headache frequency subgroups.
Methods
Approvals
Approvals were obtained from the Institutional Review Boards of the Mayo Clinic and Washington University in St. Louis. Each subject underwent an informed consent process and provided written informed consent prior to participation.
Subject Inclusion and Exclusion Criteria
Healthy controls without migraine, people with EM, and people with CM were enrolled as participants. Headache diagnoses were made according to ICHD 2 diagnostic criteria. Potential participants were excluded if they had acute or chronic pain conditions other than migraine, if they had contraindications to MRI, if they had neurologic disorders other than migraine, if they used daily medications that could be considered migraine prophylactic medications (e.g. anti-seizure medications, anti-depressants, blood pressure medications), if they used opioids, if they met criteria for medication overuse, and if they had abnormal brain MRI scans according to usual clinical interpretation.
Collection and Analyses of Participant Characteristics
Participants were studied when they were in their usual state of health. Data collected from all participants included age, sex, medication use, medical history, Beck Depression Inventory-II (BDI-II) score, and State-Trait Anxiety Inventory (STAI) scores.2–4 Additional data collected from migraine participants included headache frequency, number of years with migraine, and Migraine Disability Assessment (MIDAS) score.5 Data were compared amongst subject groups using two-tailed t-tests or Fisher’s exact test, as appropriate.
Imaging Parameters
Participants were imaged on one of two Siemens (Erlangen, Germany) MRI machines, each at a different institution: 1) MAGNETOM Trio 3T scanner using a 12-channel head matrix coil; or 2) MAGNETOM Skyra 3T scanner using a 20-channel head matrix coil. Structural scans included a high-resolution 3D T1-weighted sagittal magnetization prepared rapid gradient echo (MP-RAGE) series (Trio parameters: TE=3.16 ms, TR=2.4 s, 1x1x1 mm voxels, 256x256 mm field of view (FOV), acquisition matrix 256 x 256; Skyra parameters: TE=3.03 ms; TR=2.4 s; 1x1x1.3 mm voxels; 256x256 mm FOV, acquisition matrix 256 x 256) and T2-weighted images in axial plane (Trio parameters: TE=88 ms, TR=6280 ms, 1x1x4 mm voxels, 256x256 mm FOV, acquisition matrix 256 x 256; Skyra parameters: TE=84 ms; TR=6800 ms; 1x1x4 mm voxels; 256x256 mm FOV, acquisition matrix 256 x 256). Nearly equal proportions of migraine and healthy control participants were imaged on each of the two MRI scanners: 32 of 54 (59%) healthy control participants were imaged on scanner one and 38 of 66 (58%) migraine participants were imaged on scanner one, including 28 of 51 (55%) EM participants and 10 of 15 (66%) CM participants.
Cortical Reconstruction and Segmentation
T1 MP-RAGE sequence image processing was performed using the automated FreeSurfer image analysis suite (version 5.3, http://surfer.nmr.mgh.harvard.edu/). All image post-processing was conducted using a single Mac workstation running OS X Lion 10.7.5 software, so as to prevent post-processing irregularities derived from using multiple workstations.6 FreeSurfer methodology is well described in prior papers.7 Briefly, processing includes skull stripping, automated Talairach transformation, segmentation of subcortical gray and white matter, intensity normalization, and gray-white mater boundary tessellation and surface deformation. 7–10 This automatic segmentation and parcellation process provides information used to calculate regional volumes, cortical surface areas, and cortical thicknesses over the left and right hemispheres.
In order to validate the accuracy of the brain reconstruction process and to avoid inclusion of erroneous datasets into the final analysis, the automated segmentations and parcellations of each individual participant were manually inspected for errors before including the data for statistical analysis.
Mean thickness, surface area, and volume estimates were then extracted from FreeSurfer and exported to MATLAB (2007a, MathWorks) for further analyses. Overall, there were 204 structural measures including 68 measures of cortical thickness, 68 measures of cortical surface area, and 68 measures of regional volume.
Statistical analysis
Statistical analyses aimed to accomplish the following tasks: (1) classify migraine patients (CM and EM together, EM alone, and CM alone) vs. healthy controls; (2) classify CM vs. EM using the ICHD criteria of 15 headache days per month for assigning participants to CM or EM groups and determine the actual headache frequency threshold that allows for the most accurate classification of individuals to a higher frequency vs. a lower frequency migraine subgroup. The same analysis pipeline was used for each of the classification tasks. The pipeline consisted of four major steps, as follows:
Balancing class sample sizes: Some of the classification problems in the two tasks had imbalanced class sample sizes. For example, using 15 headache days per month as the headache frequency threshold, the dataset consisted of 51 EM patients but only 15 CM patients. A well-known oversampling approach called Synthetic Minority Oversampling Technique (SMOTE) was used to handle class imbalance and match the minority and majority class sample sizes.11
Dimension reduction: Since a total of 204 features were used in the classification, the dimensionality of the features exceeded the sample size, creating difficulty in classification. Thus, principal components analysis (PCA) was used to achieve dimension reduction. PCA works by finding linear combinations of features, called principal components (PCs). Usually, a few PCs sufficiently account for the majority of the variability in the original feature space, leading to dimension reduction. In this study PCs for the area, thickness, and volume features were derived separately (i.e., three sets of PCs). The PCs that accounted for 85% of the variability in the area, thickness, and volume were kept for further analyses.
Classification: The PCs produced from (ii) were used to build classification models. Four different classification algorithms, including diagonal linear discriminate analysis (DLDA), diagonal quadratic discriminate analysis (DQDA), support vector machine (SVM), and decision tree (DT), were used so that results could be cross-referenced. To avoid over-fitting, 10-fold cross validation was used to assess classification accuracy. Specifically, in each of the 10 cross validation runs, 10% of the subjects (randomly) were put aside to test the classification performance, and the remaining 90% of the subjects were used to develop the classifier. The average performance of the 10 runs and the best performance amongst the 10 runs were collected and reported.
Interpretation: To facilitate interpretation of the classification results, for each classification algorithm, a step-wise search was performed to identify the best subset of PCs. The search started with finding the PC that achieved the highest cross validation accuracy (e.g. PC1). Next, the remaining PCs were searched for the one PC that when used in conjunction with PC1, improved the cross validation accuracy the most (e.g. PC2). The search continued until adding more PCs did not improve the cross validation accuracy by 1% or more.
Since each PC is a linear combination of the original features, the combination coefficients of the original features were assessed for contributions to the PC and further to the classification. Specifically, for each original feature, the mean and standard deviation of its coefficient were calculated. According to the three-sigma rule, nearly 95% of the coefficients lie within two standard deviations of the mean. As a result, the original features whose coefficient exceeded two standard deviations were considered to be significant, contributing to the PCs and the classification.
Results
Subject Characteristics
Data from 120 subjects were available for this study, including 54 healthy controls, 51 EM patients, and 15 CM patients. (Table 1) Mean age of the entire cohort was 36.3 +/− 11.1 years. Ninety-one participants were female and 29 were male. There were not differences in age or sex distribution between the subject cohorts. There were not differences in BDI-II scores, state anxiety scores, and trait anxiety scores between subject groups with the exception of a slightly higher BDI-II score in the migraine group compared to healthy participants. However, the mean BDI-II scores were within normal ranges (not indicative of depression) in both groups. As expected, MIDAS scores and headache frequency were higher in the CM group compared to the EM group.
Table 1.
Subject Characteristics.
| Healthy Control (n=54) | Migraine (n=66) | Control vs. Migraine p-value | EM (n=51) | CM (n=15) | EM vs. CM p-value | |
|---|---|---|---|---|---|---|
| Age in years | 37 (11) | 36 (11) | .82 | 37 (12) | 35 (6) | .41 |
| Female | 39 (72.2%) | 52 (78.8%) | .52 | 39 (76.5%) | 13 (86.7%) | .49 |
| BDI-II score | 2.2 (4) | 4.1 (4.3) | .01 | 3.8 (4.1) | 5.3 (4.9) | .31 |
| State Anxiety | 24.8 (5.3) | 26.8 (7.1) | .08 | 26.4 (6.8) | 28 (8.3) | .51 |
| Trait Anxiety | 28.8 (7.9) | 31.6 (8.9) | .08 | 31.4 (9.5) | 32.1 (6.9) | .77 |
| Headache Frequency (days/month) | N/A | 9 (6) | N/A | 6 (3) | 19 (5) | <.001 |
| Years with Migraine | N/A | 16 (10) | N/A | 17 (11) | 14 (9) | .22 |
| MIDAS | N/A | 20.5 (19) | N/A | 14.7 (10.2) | 40.2 (27.5) | .003 |
The “migraine” cohort includes EM and CM participants. Values are means followed by standard deviation in parentheses, except for “Female” that is reported as an absolute number followed by the percentage of the cohort that is female. Subject cohorts were similar for age, sex, and anxiety scores. Depression scores were slightly higher in the migraine group, but average scores for the migraine group and control group were within normal/non-depressed ranges.
Experiment I: Classify Migraine Patients vs. Healthy Controls
There were 66 migraine patients (EM + CM) and 54 healthy controls. This imbalance in number of people in each cohort was considered to be unsubstantial, and thereby step (i), balancing class sample sizes, was skipped in the analysis pipeline. The remaining steps in the analysis pipeline were applied. The classification accuracy is summarized in Table 2. Note that among the four classification algorithms in (iii), DQDA and DT produced the best results, yielding average overall classification accuracies of 68% and 64.7% respectively. Since these accuracies were less than optimal, the second part of step (iv), i.e., examining the contributions of original features to the classification, was not performed. We suspected that the unsatisfactory classification accuracy might be due to heterogeneity within the migraine cohort, such as would be seen if there were subgroups within the migraine cohort.
Table 2.
Migraine vs. Healthy Control and Episodic Migraine vs. Healthy Control classification accuracies.
| DQDA | DT | |
|---|---|---|
| Migraine (Episodic Migraine + Chronic Migraine) vs. Healthy Control | ||
| Average accuracy ± SD over 10 runs | ||
| Overall accuracy | 68.0%±2.3% | 64.7%±2.4% |
| Migraine accuracy | 77.9%±4.2% | 69.6%±4.1% |
| Healthy Control accuracy | 55.9%±9.7% | 58.7%±6.6% |
| vBest accuracy among 10 runs | ||
| Overall accuracy | 72.5% | 70% |
| Migraine accuracy | 74.2% | 71.2% |
| Healthy Control accuracy | 70.4% | 68.5% |
| Episodic Migraine vs. Healthy Control | ||
| Average accuracy ± SD over 10 runs | ||
| Overall accuracy | 67.2%±2.4% | 66.5%±6.0% |
| EM accuracy | 57.5%±5.5% | 65.7%±5.4% |
| Healthy Control accuracy | 76.5%±2.6% | 67.2%±8.3% |
| Best accuracy among 10 runs | ||
| Overall accuracy | 73.3% | 77.1% |
| EM accuracy | 70.6% | 72.5% |
| Healthy Control accuracy | 75.9% | 81.5% |
The average and best accuracies for classifying migraine (EM + CM) vs. healthy controls and EM vs. healthy controls are listed when using DQDA and DT. For example, when using DQDA the average overall accuracy for classifying migraine vs. healthy control was 68% while the best accuracy achieved was 72.5%.
To test the possibility of there being headache frequency subgroups within the entire migraine cohort, we went on to classify CM vs. healthy controls and EM vs. healthy controls. The average overall accuracy for classifying CM vs. healthy controls was 86.3% in the DQDA model and 74.6% in the DT model. (Table 3) Although DQDA produced significantly better classification results, we chose to still present the results by DT for consistency with our other experiments. Table 4 and Figure 1 show the features that contribute to the classification of CM vs. healthy controls. Although structural measures of several regions contribute to the classification, structure of the anterior cingulate cortex, entorhinal cortex, temporal pole, and transverse temporal gyrus were frequently represented. The average overall accuracy for classifying EM vs. healthy controls was 67.2% in the DQDA model and 66.5% in the DT model. (Table 2) Since these accuracies were less than optimal, we chose not to proceed with the second part of step (iv), i.e., examining the contributions of original features to the classification.
Table 3.
Chronic Migraine vs. Healthy Control and Chronic Migraine vs. Episodic Migraine classification accuracies.
| DQDA | DT | |
|---|---|---|
| Chronic Migraine vs. Healthy Control | ||
| Average accuracy ± SD over 10 runs | ||
| Overall accuracy | 86.3%±1.9% | 74.6%±3.5% |
| CM accuracy | 90.6%±2.9% | 74.7%±5.9% |
| Healthy Control accuracy | 82.2%±2.6% | 74.4%±4.4% |
| Best accuracy among 10 runs | ||
| Overall accuracy | 88.6% | 80.0% |
| CM accuracy | 94.1% | 78.4% |
| Healthy Control accuracy | 83.3% | 81.5% |
| Chronic Migraine vs. Episodic Migraine | ||
| Average accuracy ± SD over 10 runs | ||
| Overall accuracy | 84.2%±4.2% | 83.0%±5.2% |
| EM accuracy | 81.8%±4.9% | 84.3%±8.3% |
| CM accuracy | 86.7%±4.2% | 81.8%±4.7% |
| Best accuracy among 10 runs | ||
| Overall accuracy | 91.2% | 90.2% |
| EM accuracy | 88.2% | 96.1% |
| CM accuracy | 94.1% | 84.3% |
The average and best accuracies for classifying CM vs. healthy controls and CM vs. EM are listed when using DQDA and DT. For example, when using DQDA the average overall accuracy for classifying CM vs. EM was 84.2% while the best accuracy achieved was 91.2%.
Table 4.
Principal components of the Chronic Migraine vs. Healthy Control classification model.
| DQDA | DT | Brain Hemisphere | MRI Features in PCs | |
|---|---|---|---|---|
| Cortical Surface Area PCs | ||||
| 7 | X | R | Caudal Anterior Cingulate | |
| R | Entorhinal | |||
| R | Transverse Temporal | |||
| L | Transverse Temporal | |||
| 8 | X | R | Entorhinal | |
| R | Rostral Anterior Cingulate | |||
| 9 | X | R | Transverse Temporal | |
| L | Temporal Pole | |||
| 10 | X | R | Caudal Anterior Cingulate | |
| R | Rostral Anterior Cingulate | |||
| L | Entorhinal | |||
| L | Transverse Temporal | |||
| 11 | X | X | R | Transverse Temporal |
| R | Insula | |||
| L | Parahippocampal | |||
| L | Insula | |||
| 15 | X | X | R | Superior Temporal |
| R | Temporal Pole | |||
| L | Pars Triangularis | |||
| L | Temporal Pole | |||
| 17 | X | R | Frontal Pole | |
| L | Superior Temporal | |||
| 18 | X | L | Entorhinal | |
| L | Supramarginal | |||
| Cortical Thickness PCs | ||||
| 21 | X | X | R | Caudal Anterior Cingulate |
| R | Isthmus Cingulate | |||
| R | Posterior Cingulate | |||
| 23 | X | R | Isthmus Cingulate | |
| R | Parahippocampal | |||
| R | Pars Orbitalis | |||
| R | Rostral Anterior Cingulate | |||
| R | Temporal Pole | |||
| L | Parahippocampal | |||
| L | Rostral Anterior Cingulate | |||
| 27 | X | R | Medial Orbital Frontal | |
| L | Entorhinal | |||
| L | Isthmus Cingulate | |||
| 28 | X | R | Entorhinal | |
| R | Insula | |||
| L | Superior Temporal | |||
| 29 | X | R | Pars Opercularis | |
| R | Pars Triangularis | |||
| L | Caudal Middle Frontal | |||
| 30 | X | R | Superior Temporal | |
| R | Frontal Pole | |||
| L | Entorhinal | |||
| L | Parahippocampal | |||
| 32 | X | X | R | Pericalcarine |
| L | Pars Triangularis | |||
| L | Temporal Pole | |||
| Volume PCs | ||||
| 37 | X | L | Caudal Middle Frontal | |
| L | Cuneus | |||
| L | Lingual | |||
| L | Paracentral | |||
| L | Pericalcarine | |||
| 38 | X | R | Entorhinal | |
| R | Pericalcarine | |||
| L | Cuneus | |||
| L | Pericalcarine | |||
| L | Temporal Pole | |||
| 40 | X | R | Entorhinal | |
| R | Parahippocampal | |||
| R | Rostral Anterior Cingulate | |||
| R | Transverse Temporal | |||
The PCs that comprise the CM vs. Healthy Control classifiers derived via DQDA and DT are presented. The classifiers contained PCs consisting of cortical surface area, cortical thickness, and regional volume measurements. The left-most column contains the name of the PC. An “X” under “DQDA” or “DT” indicates that the PC was part of the classifier derived using DQDA or DT analyses in at least one of the ten iterations. The right-most column lists the brain regions for which structural measures comprise that PC, named according to FreeSurfer terminology. For example, PC8 is comprised of cortical surface area measurements of the entorhinal cortex and rostral anterior cingulate. R = right; L = left.
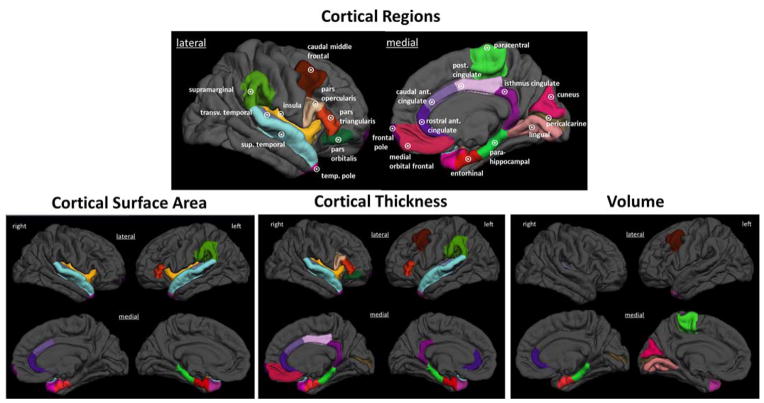
Figure 1. Regions that comprise the chronic migraine vs. healthy control classifiers.
Brain regions for which surface area, thickness or volume measures contributed to a classifier differentiating patients with CM from healthy controls are demonstrated on a 3-D rendering of the brain. The principal components to which these regions contribute are listed in Table 4. transv = transverse; sup = superior; temp = temporal; ant = anterior; post = posterior.
Experiment II: Classify CM vs. EM and Determine the Headache Frequency Threshold that Allows for the Most Accurate Classification of Higher Frequency vs. Lower Frequency Migraine
Different headache frequency (headache days/month) thresholds were investigated to find the number of headache days that most accurately divided the migraine participants into lower frequency and higher frequency subpopulations based upon measures of brain structure. Headache thresholds of 5, 6, 7, 8, 9, 10, 11, 12, and 15 days per month were explored. Thresholds of 13 and 14 days resulted in the same partition of patients as 15 days and thus were not separately analyzed. Table 5 summarizes the class sample sizes corresponding to each threshold. It can be seen that class imbalance existed for most of the thresholds. Therefore, we applied SMOTE, i.e., step (i) in the analysis pipeline, to the analysis of those thresholds. Then, we applied steps (ii) and (iii). Figure 2 shows the 10-fold cross validation classification accuracies with respect to the different thresholds produced by four classification algorithms. As shown in Figure 2, using a headache frequency of 15 headache days per month to divide the migraine patients into two headache frequency subgroups allowed for the most accurate classification. This observation was consistently obtained from all four different classification algorithms. Nine days with headache per month was the second most optimal headache frequency threshold for differentiating migraine subpopulations. It is our intention to explore the sensitivity of this threshold (9 days) in future studies.
Table 5.
Class sample sizes for different headache frequency thresholds.
| Thresholds (headache days/month) | Number of participants in low frequency class (headache frequency<threshold) | Number of participants in high frequency class (headache frequency>=threshold) |
|---|---|---|
| 5 | 18 | 48 |
| 6 | 27 | 39 |
| 7 | 33 | 33 |
| 8 | 34 | 32 |
| 9 | 42 | 24 |
| 10 | 43 | 23 |
| 11 | 46 | 20 |
| 12 | 47 | 19 |
| 15 | 51 | 15 |
The number of study participants in “lower frequency” and “higher frequency” migraine subgroups depended upon the chosen headache frequency threshold for dividing the migraine participants into these two headache frequency subgroups.
Figure 2. Ten-fold cross validation classification accuracies with respect to the different headache frequency thresholds produced by four classification algorithms.
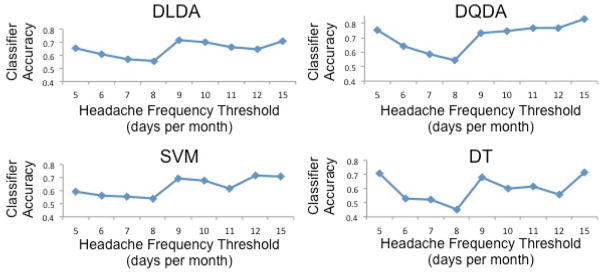
The accuracy of each classifier for identifying individual migraine participants as belonging to a lower-frequency migraine group or a higher-frequency migraine group according to different headache frequency thresholds is demonstrated. These plots show that classifiers based upon DQDA and DT were the most accurate and that the most accurate classification occurred when a headache frequency threshold of 15 headache days per month was used. The plots also indicated that there was a large improvement in classification accuracy when moving from a threshold of 8 headache days per month to 9 headache days per month, suggesting the possible existence of headache frequency subgroups in addition to those based on 15 headache days per month.
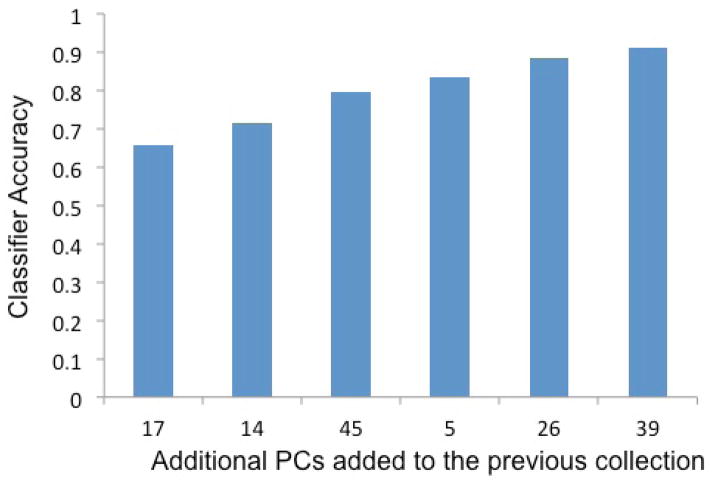
With 15 headache days per month used to divide the migraine group into CM and EM subgroups, we proceeded with CM vs. EM classification. Application of the stepwise search, step (iv) in the analysis pipeline, reduced the number of PCs used in the classification to 1–7 across all four classification algorithms. Figure 3 shows an example of the process of the step-wise search (the example classification algorithm is DQDA). The search first found PC17, which when used alone achieved 65.7% cross validation accuracy. Then, PC14 was identified, which when used together with PC17 achieved an accuracy of 71.6%. The best classification accuracy (91.2%) is achieved with six PCs (PC17, PC14, PC45, PC5, PC26, and PC39). The accuracy improvement from adding more PCs to the model was less than 1% and thus the model consisting of 6 PCs was considered complete. The average overall accuracy of differentiating CM from EM was 84.2% in the DQDA model and 83% in the DT model. Table 3 shows the overall and best accuracies for classifying CM vs. EM.
Figure 3. Step-wise addition of principal components to the chronic migraine vs. episodic migraine classifier.
This figure illustrates the accuracy of the CM vs. EM classifier (this example is based on diagonal quadratic discriminate analysis or DQDA) as individual principal components (PCs) were added to the classifier. For example, a classifier containing PC17 alone had 65.7% accuracy for classifying individuals with migraine as having CM vs. EM. When PC14 was added to the model, the accuracy improved to 71.6% and when all 6 PCs were included in the model, the accuracy improved to 91.2%.
Finally, we looked for the original features that comprised the PCs used in the final classification model. (Table 6, Figure 4) Although several different brain regions were included in these PCs, a few were most frequently present, including the temporal pole, anterior cingulate cortex, superior temporal lobe, medial orbital frontal gyrus, and the pars triangularis.
Table 6.
Principal components of the Chronic Migraine vs. Episodic Migraine classification model.
| DQDA | DT | Brain Hemisphere | MRI Features in PCs | |
|---|---|---|---|---|
| Cortical Surface Area PCs | ||||
| 5 | X | X | R | Superior Temporal |
| L | Superior Temporal | |||
| L | Paracentral | |||
| 12 | X | R | Frontal Pole | |
| L | Medial Orbital Frontal | |||
| L | Postcentral | |||
| L | Posterior Cingulate | |||
| L | Temporal Pole | |||
| 14 | X | X | R | Pars Triangularis |
| L | Frontal Pole | |||
| L | Lingual | |||
| Cortical Thickness PCs | ||||
| 17 | X | X | R | Medial Orbital Frontal |
| L | Medial Orbital Frontal | |||
| L | Rostral Anterior Cingulate | |||
| 26 | X | R | Superior Temporal | |
| R | Insula | |||
| L | Temporal Pole | |||
| 27 | X | X | R | Pericalcarine |
| L | Caudal Anterior Cingulate | |||
| L | Entorhinal | |||
| L | Medial Orbital Frontal | |||
| 29 | X | R | Pars Opercularis | |
| L | Postcentral | |||
| 32 | X | R | Rostral Anterior Cingulate | |
| L | Rostral Anterior Cingulate | |||
| L | Rostral Middle Frontal | |||
| L | Temporal Pole | |||
| Volume PCs | ||||
| 45 | X | R | Caudal Anterior Cingulate | |
| R | Pars Triangularis | |||
| R | Transverse Temporal | |||
| L | Pars Opercularis | |||
| L | Pars Triangularis | |||
| 46 | X | R | Pars Triangularis | |
| R | Precentral | |||
| L | Postcentral | |||
| 47 | X | R | Superior Temporal | |
| R | Temporal Pole | |||
| L | Caudal Anterior Cingulate | |||
| L | Transverse Temporal | |||
The PCs that comprise the CM vs. EM classifiers derived via DQDA and DT are presented. The classifiers contained PCs consisting of cortical surface area, cortical thickness, and regional volume measurements. The left-most column contains the name of the PC. An “X” under “DQDA” or “DT” indicates that the PC was part of the classifier derived using DQDA or DT analyses in at least one of the ten iterations. The right-most column lists the brain regions for which structural measures comprise that PC, named according to FreeSurfer terminology. For example, PC5 is comprised of cortical surface area measurements of the right superior temporal lobe, the left superior temporal lobe and the left paracentral lobule. R = right; L = left.
Figure 4. Regions that comprise the chronic migraine vs. episodic migraine classifiers.
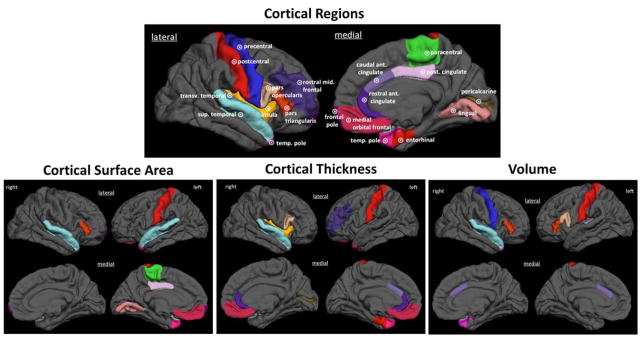
Brain regions for which surface area, thickness or volume measures contributed to a classifier differentiating patients with CM from those with EM are demonstrated on a 3-D rendering of the brain. The principal components to which these regions contribute are listed in Table 6. transv = transverse; sup = superior; temp = temporal; ant = anterior; post = posterior.
Discussion
The main finding of this study is that multivariate models consisting of brain cortical thickness, cortical surface area, and regional volumes were highly accurate for classifying individual people with migraine as having CM vs. EM and for classifying individuals as having CM vs. being a healthy control. Classifiers based upon these structural measures could be of practical use since these structural data can be generated from brain MRI scans typically used in the clinical setting. Exploration of the headache frequency threshold that allowed for the most accurate differentiation of migraine frequency subcohorts showed that 15 days per month was the best threshold, supporting the current ICHD diagnostic criterion of 15 headache days per month to differentiate CM from EM. In our analyses, brain structure differences in participants with migraine (EM + CM) vs. healthy control subjects and EM vs. healthy control subjects did not allow for highly accurate classification of participants as having migraine of any frequency or being a healthy control or as having EM vs. being a healthy control.
This study shows that CM is associated with aberrant brain structure and that the structural differences in CM are of a magnitude that allows for accurate differentiation from the brains of people with EM and from healthy controls. These data suggest that either, 1) more frequent migraine attacks lead to more extensive brain structural change; or 2) more severe brain structural aberrations predispose a migraine patient to a more severe form of migraine (i.e. CM). Longitudinal imaging studies that investigate relationships between changing migraine patterns and brain structure would help to clarify the direction of the relationship between migraine frequency and brain structure.
These study findings support the use of 15 headache days per month as the threshold between CM and EM. Despite testing several different headache frequency thresholds that were less than 15, models of brain structure most accurately differentiated subcohorts of migraineurs based upon headache frequency when 15 headache days per month was used. Although the selection of 15 headache days per month to differentiate CM from EM in the ICHD diagnostic criteria was mostly arbitrary, these study findings suggest that this is likely a good choice, corresponding to significant differences in brain structure. It is possible, and our data suggest, that there may be additional headache frequency thresholds that allow for accurate subclassification of patients with migraine. As illustrated in Figure 2, headache frequency thresholds of 5 days per month (or perhaps less) and 9 days per month might also allow for accurate subclassification based on brain structure. These additional subclassifications could be consistent with and might be helpful to further define the criteria for the “low-frequency EM” and “high-frequency EM” classifications that are often used.12
Structural measures of the temporal pole, anterior cingulate cortex, superior temporal lobe, entorhinal cortex, medial orbital frontal gyrus, and pars triangularis were frequently present within the PCs that comprised the models that classified CM vs. EM and CM vs. healthy control. Each of these structures has previously been shown to be involved in pain processing and several of these regions have previously been identified as having abnormal structure and/or function in patients with migraine. The temporal pole and the anterior cingulate cortex have frequently been identified as regions with atypical structure and function in migraine.
The temporal pole and the superior temporal lobe participate in multisensory integration. The temporal pole is a multisensory region that integrates visual, auditory, olfactory and somatosensory stimuli. 13–15 Several research neuroimaging studies have demonstrated that compared to healthy controls, migraineurs have greater stimulus-induced activation of the temporal pole, atypical resting state functional connectivity of the temporal pole, and atypical structure of the temporal pole.14, 16–20 The superior temporal sulcus participates in multisensory integration and in determining the social salience of someone else’s pain.21, 22 The upper bank of the superior temporal sulcus receives and integrates inputs from somatosensory, visual, and auditory cortices.23 Because of the potentially important role for multisensory integration in production of migraine symptoms, such as worsening headache intensity with exposure to visual and auditory stimuli and triggering of migraine attacks by sensory stimuli, the temporal pole and other regions that mediate multisensory integration might be particularly important in migraine physiology.14, 15
The anterior cingulate cortex, medial orbital frontal gyrus, entorhinal cortex, and pars triangularis participate in affective and cognitive aspects of pain processing. The anterior cingulate cortex is involved in affective and cognitive pain processing, in pain anticipation, and it is a key component of the salience network, a network of functionally connected brain regions that mediates the segregation of important environmental stimuli from those that are less relevant.24–26 Prior studies have demonstrated atypical activation, functional connectivity, and structure of the anterior cingulate cortex in migraineurs.20, 27–35 The orbital frontal cortex participates in the affective response to pleasant and painful stimuli and in emotion-based decision making.36, 37 Brodmann’s area 10 of the orbitofrontal cortex activates during the premonitory and headache phases of a migraine attack and parts of the orbital frontal cortex have been shown to have atypical gray matter volume and atypical functional connectivity in people with migraine compared to healthy controls.27, 34, 35, 38, 39 The entorhinal cortex participates in modulating expectations for pain and in anxiety-driven hyperalgesia.40 The pars triangularis of the inferior frontal gyrus plays a role in determining the empathy for pain in others, an empathy that is likely to be affected by frequently recurring attacks of migraine.41, 42 The inferior frontal gyrus (unclear if specifically the pars triangularis) has been demonstrated as having atypical stimulus-induced activation and atypical functional connectivity in patients with migraine compared to healthy controls.20, 43–45
Few studies have investigated brain structure and function of patients with CM, comparing them to healthy controls or to patients with EM. A small voxel-based morphometry study found that patients with CM have less gray matter in the anterior cingulate cortex than patients with EM and that there were correlations between headache frequency and gray matter volume in anterior cingulate cortex and temporal pole.32 A resting state functional connectivity study of 20 patients with CM vs. 20 healthy controls identified atypical functional connectivity of the anterior cingulate cortex in participants with CM.31 Studies comparing patients who have high frequency EM (i.e. 8–14 headache days per month) to those with lower frequency EM (i.e. 1–2 headache days per month) have found differences in stimulus-induced activations, resting functional connectivity and structure of the temporal pole and anterior cingulate cortex.46–48
There are aspects of the study design and available data that need to be considered when interpreting the results of this study: 1) Headache frequency was determined via patient self-report. Some error in estimation of headache frequency was likely. Future studies should employ prospective headache diary maintenance prior to imaging in order to better determine headache frequency. 2) There were a limited number of patients with headache frequencies less than 5 headache days per month and greater than 15 headache days per month, making it unfeasible to test headache frequency thresholds that were less than 5 and more than 15. Future studies are required to determine if there are additional headache frequency subgroups of migraine patients defined according to headache frequencies of less than 5 or greater than 15 headache days per month. 3) Two MRI scanners were used in this study. As detailed in the Methods section, nearly equal proportions of participants in each subject cohort were imaged on each of the two MRI scanners and thus the use of two scanners likely had little effect on our study results. The use of two MRI machines may in fact make our results more generalizable than if all data were collected from one scanner.
Conclusions
In conclusion, classifiers containing MRI measures of brain cortical thickness, cortical surface area, and regional volumes accurately classified individuals as having CM vs. EM and as having CM vs. being a healthy control. Fifteen headache days per month was the headache frequency threshold that allowed for the most accurate classification of migraine patients into higher and lower frequency headache subgroups according to their brain structure, providing support for the currently used threshold of 15 headache days per month for differentiating CM from EM. Future studies will investigate the utility of other structural measures (e.g. those obtained via diffusion tensor imaging) and the utility of functional MRI data for building classifiers that differentiate migraine from healthy controls and that differentiate EM from CM. It is anticipated that these additional data will enhance the accuracy of such classifiers. Future studies will also construct classifiers that differentiate migraine from other headache disorders. Such classifiers would help to determine brain structural and functional aberrations that are specific to migraine and could eventually be developed into computer aided diagnostic tools that might help to clinically differentiate migraine from other headache disorders when that differentiation is otherwise difficult.
Supplementary Material
Acknowledgments
Funding: NIH K23NS070891 to TJS.
Abbreviations
- BDI
Beck Depression Inventory
- CM
chronic migraine
- DLDA
diagonal linear discriminate analysis
- DQDA
diagonal quadratic discriminate analysis
- DT
decision tree
- EM
episodic migraine
- FOV
field of view
- ICHD
International Classification of Headache Disorders
- MIDAS
Migraine Disability Assessment
- MP-RAGE
magnetization prepared rapid gradient echo
- MRI
magnetic resonance imaging
- PC
principal component
- PCA
principal component analysis
- SMOTE
Synthetic Minority Oversampling Technique
- STAI
State-Trait Anxiety Inventory
- SVM
support vector machine
References
- 1.Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) Pscyhology Corp; San Antonio, TX: 1996. [Google Scholar]
- 3.Spielberger C, Gorsuch R. State trait anxiety inventory for adults: sampler set, manual, test, scoring key. Redwood City, California: Mind Garden; 1983. [Google Scholar]
- 4.Spielberger C. Manual for the state/trait anxiety inventory (form Y): self-evaluation questionnaire. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 5.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 6.Gronenschild EH, Habets P, Jacobs HI, et al. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7:e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 8.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 9.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 10.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321–357. [Google Scholar]
- 12.Lipton RB, Serrano D, Pavlovic JM, et al. Improving the classification of migraine subtypes: an empirical approach based on factor mixture models in the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2014;54:830–849. doi: 10.1111/head.12332. [DOI] [PubMed] [Google Scholar]
- 13.Demarquay G, Royet JP, Mick G, Ryvlin P. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia. 2008;28:1069–1080. doi: 10.1111/j.1468-2982.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 14.Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011;21:435–448. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwedt TJ. Multisensory integration in migraine. Curr Opin Neurol. 2013;26:248–253. doi: 10.1097/WCO.0b013e328360edb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014 doi: 10.1177/0333102414531157. [DOI] [PubMed] [Google Scholar]
- 17.Hadjikhani N, Ward N, Boshyan J, et al. The missing link: Enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33:1264–1268. doi: 10.1177/0333102413490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- 19.Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14:89. doi: 10.1186/1129-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Liu J, Dong X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. 2013;14:85. doi: 10.1186/1129-2377-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senkowski D, Schneider TR, Foxe JJ, Engel AK. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci. 2008;31:401–409. doi: 10.1016/j.tins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl CD, Logothetis NK, Kayser C. Spatial organization of multisensory responses in temporal association cortex. J Neurosci. 2009;29:11924–11932. doi: 10.1523/JNEUROSCI.3437-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- 28.Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012;259:1903–1912. doi: 10.1007/s00415-012-6438-1. [DOI] [PubMed] [Google Scholar]
- 29.Russo A, Tessitore A, Giordano A, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia. 2012;32:1041–1048. doi: 10.1177/0333102412457089. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53:737–751. doi: 10.1111/head.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 33.Xue T, Yuan K, Cheng P, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26:1051–1058. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- 34.Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012;25:806–812. doi: 10.1002/nbm.1796. [DOI] [PubMed] [Google Scholar]
- 35.Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013;14:836–844. doi: 10.1016/j.jpain.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol. 2011;106:2642–2652. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 38.Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232–241. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- 39.Yuan K, Qin W, Liu P, et al. Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS One. 2012;7:e45476. doi: 10.1371/journal.pone.0045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leknes S, Tracey I. Hippocampus and entorhinal complex, functional imaging. In: Gebhardt G, Schmidt R, editors. Encyclopedia of Pain. Springer; Berlin Heidelberg: 2007. [Google Scholar]
- 41.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cereb Cortex. 2007;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- 43.Hougaard A, Amin FM, Hoffmann MB, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Zhao L, Li G, et al. Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One. 2012;7:e51250. doi: 10.1371/journal.pone.0051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012;7:e52927. doi: 10.1371/journal.pone.0052927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32:607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2013;218:903–912. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maleki N, Becerra L, Nutile L, et al. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.