Abstract
Aim
To provide first evidence of construct validity of a semi-quantitative scale for brain structural MRI (sqMRI scale) in children with unilateral cerebral palsy (UCP) secondary to periventricular white matter (PWM) lesions, by examining the relationship with hand sensorimotor function and whole brain structural connectivity.
Methods
Cross-sectional study of 50 children with UCP due to PWM lesions using 3 T (MRI), diffusion MRI and assessment of hand sensorimotor function. We explored the relationship of lobar, hemispheric and global scores on the sqMRI scale, with fractional anisotropy (FA), as a measure of brain white matter microstructure, and with hand sensorimotor measures (Assisting Hand Assessment, AHA; Jebsen–Taylor Test for Hand Function, JTTHF; Melbourne Assessment of Unilateral Upper Limb Function, MUUL; stereognosis; 2-point discrimination).
Results
Lobar and hemispheric scores on the sqMRI scale contralateral to the clinical side of hemiplegia correlated with sensorimotor paretic hand function measures and FA of a number of brain structural connections, including connections of brain areas involved in motor control (postcentral, precentral and paracentral gyri in the parietal lobe). More severe lesions correlated with lower sensorimotor performance, with the posterior limb of internal capsule score being the strongest contributor to impaired hand function.
Conclusion
The sqMRI scale demonstrates first evidence of construct validity against impaired motor and sensory function measures and brain structural connectivity in a cohort of children with UCP due to PWM lesions. More severe lesions correlated with poorer paretic hand sensorimotor function and impaired structural connectivity in the hemisphere contralateral to the clinical side of hemiplegia. The quantitative structural MRI scoring may be a useful clinical tool for studying brain structure–function relationships but requires further validation in other populations of CP.
Keywords: Brain structure, Sensorimotor function, Unilateral cerebral palsy, Magnetic resonance imaging, Diffusion, HARDI
Abbreviations: CP, cerebral palsy; MRI, magnetic resonance imaging; sqMRI, semi-quantitative MRI; PWM, periventricular white matter; AHA, Assisting Hand Assessment; MUUL, Melbourne Assessment of Unilateral Upper Limb function; JTTHF, Jebsen–Taylor test of hand function; GMFCS, Gross Motor Function Classification System; MACS, Manual Ability Classification System; FA, fractional anisotropy
Highlights
-
•
Validity of a novel systematic approach to MR structural imaging
-
•
Relationship between lesion severity on structural MRI, clinical measures and HARDI
-
•
Usefulness of a scale to support brain structure–function relationship research
1. Introduction
Brain structural MRI can provide high-resolution images on cerebral macrostructure, thus improving our capability of investigating anatomy, morphology and volume in normal or pathological conditions. Its use has improved the understanding of the aetiology and pathogenesis of brain injury in cerebral palsy (CP) (Ashwal et al., 2004; Krageloh-Mann et al., 2007). Although essential, qualitative structural imaging approaches do not comprehensively explain the great variability in the clinical phenotype in terms of topography and severity of the brain lesion (Yokochi et al., 1991; Aida et al., 1998; Staudt et al., 2000; Arnfield et al., 2013; Kwon et al., 2014).
Recently our team has developed a new tool for assessing brain damage in children with CP to provide a quantitative analysis of lesion severity on structural MRI, the semi-quantitative MRI (sqMRI) scale (Fiori et al., 2014). The sqMRI is comprised of a global score and a number of subscores specifically assessing the involvement of different brain regions. This modular approach was designed to capture the associations between structural damage and functional impairment within the different clinical subgroups of CP. The scale was developed by a multidisciplinary expert team to ensure good content validity and was proven to be reliable, although requiring further validation (Fiori et al., 2014). Construct validity could be established through comparison with a measure that is believed to reflect the same underlying phenomenon or by using the instrument to test specific hypotheses that support the construct of the test, e.g. that the instrument can distinguish between people with varied clinical conditions (Portney et al., 2009).
The purpose of the current study was therefore to provide first evidence for establishing the construct validity of the sqMRI scale (Portney et al., 2009) in a cohort of children with unilateral CP (UCP) due to periventricular white matter (PWM) lesions. To address this aim, we took advantage of the same cohort of children studied by Pannek et al. (2014), who explored differences in structural connectivity between UCP and typical development, and their relationships with hand function. The semi-quantitative scale was used in Pannek et al. (2014) only to describe the cohort. Here, we expand on previous findings in that we test the relationship between scores on the sqMRI scale with i) measures of impaired hand motor and sensory function and ii) measures of brain microstructure. Our first hypothesis was that sqMRI scores contralateral to the clinical side of hemiplegia correlate with clinical measures of impaired hand motor and sensory function, so that higher lesion scores would correspond to poorer hand function. Our second hypothesis was that sqMRI scores contralateral to the clinical side of hemiplegia correlate with brain MRI FA in connections/tracts, so that higher lesion scores would correspond to more severe disruption of connectivity (Scheck et al., 2012; Pannek et al., 2014).
2. Methods
2.1. Participants
Participants were the same as in Pannek et al. (2014). They were recruited as part of a cohort study of children with congenital UCP at the Queensland Cerebral Palsy and Rehabilitation Research Centre in Brisbane, Australia (Boyd et al., 2013a; Boyd et al., 2013b). Children with mild to moderate congenital spasticity motor type UCP (Gross Motor Function Classification System Score I-II, Manual Ability Classification System Score I-II), aged between 5 and 18 years were considered eligible. They were excluded if they previously had either undergone surgery in the upper limb or received BoNT-A injections within 6 weeks prior to baseline assessments (Boyd et al., 2013a; Boyd et al., 2013b). They were included if they had evidence of PWM on sMRI, no contraindication for MRI and sufficient cooperation and cognitive understanding to participate in the assessment. The University of Queensland and Children's Health Queensland ethics committees granted ethical approval. Informed parental consent was obtained for all participants.
2.2. MRI acquisition
MRI data were acquired at study enrolment, prior to commencing any of the COMBIT or Mitii protocol of rehabilitative intervention (Boyd et al., 2013a; Boyd et al., 2013b) using a 3 T Siemens Tim Trio scanner (Siemens, Erlangen, Germany). A high-resolution structural image was acquired using a 0.9 mm isotropic 3D T1 Magnetisation Prepared Rapid Gradient Echo (MPRAGE) sequence. An axial T2-weighted Fluid Attenuated Inversion Recovery (FLAIR) image was acquired for lesion classification of structural images. FLAIR images were selected over FSE T2 due to its sensitiveness to gliotic white matter lesions (Bastianello et al., 1997) and to match reliability parameters of the semi-quantitative scale (Fiori et al., 2014). Diffusion weighted images were acquired along 64 uniformly distributed diffusion encoding directions (b = 3000 s/mm2) along with one b = 0 image, using a twice-refocussed single-shot echo-planar-imaging sequence to reduce eddy current distortions. An acceleration factor of 2 was employed to reduce susceptibility distortions. A gradient-echo based field map was additionally acquired to assist in the correction of image distortions as described by Pannek et al. (2014).
2.3. Structural image analysis
An MRI-trained child neurologist (SF) assessed all structural images. Each MRI was scored accordingly to the sqMRI scale (Fiori et al., 2014). Briefly, each periventricular, middle and cortico/subcortical layer of the frontal, parietal, temporal and occipital lobes was scored and summarised into a lobar score (LS) ranging from 0 to 3 for each lobe (i.e. a score from 0 to 1 for each of the 3 layers). All lobar scores on each (of the right and left) side were summed to result in the hemispheric score (HS) (range: 0–12). The basal-ganglia-and-brainstem (caudate, lenticular, posterior limb of internal capsule, thalamus and brainstem) corpus callosum and cerebellum scores were also determined. For each side, the hemispheric summary score (HSS) was calculated as the result of the hemispheric and basal-ganglia-and-brainstem scores (range: 0–17). The sum of all the scores of the scale resulted in a global score (GS) (range: 0–40). For all scores, the higher is the score the bigger is the lesion. Good reliability has been confirmed between independent raters for the sqMRI scale (Fiori et al., 2014).
2.4. Clinical testing
The use of the impaired hand as an assisting hand in bimanual tasks was assessed using the school kids version of the Assisting Hand Assessment (AHA) (Krumlinde-Sundholm et al., 2003). Unimanual capacity of the impaired hand was assessed using the Melbourne Assessment of Unilateral Upper Limb Function (MUUL) (Johnson et al., 1994). For both AHA and MUUL, a higher score indicates better function. Speed and dexterity of the impaired upper limb were assessed using the Jebsen–Taylor Test of Hand Function (JTTHF) (Jebsen et al., 1969) where a lower score indicates faster speed. Sensory function was assessed using the stereognosis and 2-point-discrimination (2PD) tests for the impaired hand (Auld et al., 2012). For stereognosis, the number of correct responses out of a possible maximum of nine was recorded (Auld et al., 2012). 2PD was assessed using the Disk-Criminator (MacKinnon et al., 1985; Zalesky et al., 2010) as the smallest separation (in mm) between the two points that could be perceived on at least 7 of 10 trials (Zalesky et al., 2010).
2.5. Diffusion data preprocessing and connectome generation
Cortical reconstruction, volumetric segmentation, and diffusion processing for the generation of connectomes were executed (Pannek et al., 2014). In brief, freesurfer (http://surfer.nmr.mgh.harvard.edu, version 5.0) was used to parcellate the brain into 68 cortical regions, left and right thalami (2 regions), left and right cerebellum (2 regions), and brain stem (1 region). Posterior limb of internal capsule (PLIC) was not included in the connectome because it is not a region where connections should terminate. A total of 73 regions were thus included in the connectome construction.
Following extensive preprocessing of diffusion data to correct for head motion, image distortions and artefacts (Pannek et al., 2014), MRtrix software (http://www.nitrc.org/projects/mrtrix/, version 0.2.11) was used to estimate the fibre orientation distributions using constrained spherical deconvolution. Five million probabilistic streamlines were generated using a whole-brain seeding approach, and employing a termination mask to prevent streamlines from penetrating more than 1 mm into the grey matter (Pannek et al., 2014). Intra- and inter-hemispheric cortico-cortical connections, cortico-thalamic, cortico-cerebellar, and interhemispheric cerebellar connections were extracted from the whole brain tractogram by hit-testing both terminal endpoints of every streamline with every cortical and cerebellar region. For brainstem connections, only one terminal endpoint was required to reside within the cortical, thalamic or cerebellar region, with any part of the streamline passing through the brainstem (Tournier et al., 2012). For every possible link between any pair of nodes, the number of connecting streamlines was noted. Connections were excluded if fewer than 250 streamlines contributed to it. The median FA value was determined from the pooled FA samples obtained from all contributing streamlines. Streamlines were not resampled for this purpose. Median FA values were calculated by sampling the diffusion maps at every step of the selected streamlines. Results were recorded in connectivity matrices (Pannek et al., 2014).
2.6. Statistical analysis
All scores from the sqMRI scale were grouped appropriately to their distribution with respect to the side of clinical phenotype. SqMRI scores were defined “contralateral”, if they refer to the hemisphere opposite to the clinical side of hemiplegia. The LS, HS, HSS contralateral to the side of the hemiplegia and global score were included in the analysis.
To investigate the associations between sqMRI and measures of hand motor (AHA, MUUL, JTTHF) and sensory function (stereognosis, 2PD) Spearman correlation coefficients were used. Our hypothesis was that more severe lesions on the sqMRI scale on the hemisphere contralateral to the paretic hand would result in poorer sensorimotor paretic hand function. Independent contralateral subscores (frontal, parietal, temporal and occipital lobar scores and basal-ganglia-and-brainstem score) and cerebellum and corpus callosum, which were not lateralised, were included in multivariable linear regression models (forward selection method) for each hand motor and sensory outcome measures to estimate the model-explained variability of AHA, MUUL, JTTHF, stereognosis and 2PD and to identify the contribution of individual scores to the model. Statistical analysis was performed on SPSS v20.0.
To investigate whether sqMRI scores contralateral to the clinical side of hemiplegia correlate with FA in connections/tracts, we first grouped children according to the clinical side of the hemiplegia, so that left and right hemiplegia groups could be analysed separately to avoid confounding from normal hemispheric asymmetry. Statistical analysis of the brain networks was performed using the NBS toolbox for Matlab (https://sites.google.com/site/bctnet/comparison/nbs, v1.2). Our hypothesis was that more severe lesions on the sqMRI scale on the hemisphere contralateral to the paretic hand would result in lower FA values in a number of connections. A general linear model was used to assess the relationship between lobar and hemispheric scores on the sqMRI scale and FA. Hypotheses were one-tailed with a significant p-value defined as <0.05 as NBS does not allow two-tailed tests. Instead, two one-tailed tests were performed.
3. Results
3.1. Demographics
Eighty children (38 left, 42 right) with UCP were recruited, of whom 55 (26 left, 29 right) presented with PWM lesions. Data from 5 children (3 left, 2 right) were excluded due to excessive head movement artefacts or signal-to-noise problems on the MRIs. The final sample consisted of 23 left UCP (11 males and 12 females, mean age 10.5 ± 3.0 years, MACS I = 14, II = 9; GMFCS I = 17; II = 6; unilateral lesion = 11, bilateral lesion = 12), and 27 right UCP (15 males and 12 females, mean age 11.4 ± 3.3 years, MACS I = 11, II = 16; GMFCS I = 13, II = 14; unilateral lesion = 19, bilateral lesion = 8) (Table 1). Children were not receiving any intensive therapy before the study, receiving regular weekly or fortnightly physiotherapy and/or occupational therapy.
Table 1.
Demographics.
| Characteristic | Patients (n = 50) |
|---|---|
| Age (years)a | 11.4 ± 3.1 |
| Gender (male/female) | 26/24 |
| Side of UCP (right/left) | 27/23 |
| MACS (I/II) | 25/25 |
| AHAa | 68.6 ± 17.6 |
| MUULa | 86.9 ± 13.8 |
| JTTHFa | 195.27 ± 177.8 |
| Unilateral/bilateral brain lesions | 30/20 |
| Global sqMRI (out of 40)a | 6.4 ± 3.6 |
Abbreviations: UCP, Unilateral Cerebral Palsy; MACS, Manual Ability Classification System; AHA, Assisting Hand Assessment; MUUL, Melbourne Assessment of Unilateral Upper Limb Function; JTTHF, Jebsen–Taylor Test for Hand Function.
Data are expressed in mean ± standard deviation.
3.2. Relationship between sqMRI scores and clinical measures
Lobar, hemispheric and hemispheric-summary scores contralateral to the clinical side of hemiplegia and global score correlated moderately with measures of sensorimotor function (Table 2). In particular, correlations were found among HSS and all hand function measures, with coefficients ranging between 0.434 and 0.287.
Table 2.
Correlation between semi-quantitative MRI scale and clinical hand sensorimotor function measures (Spearman rank correlation coefficients and p-values).
| AHA | JTTHF | MUUL | Stereognosis | 2PD | |
|---|---|---|---|---|---|
| HS | −0.01 (p = 0.473) | 0.03 (p = 0.430) | 0.03 (p = 0.425) | −0.30 (p = 0.024) | 0.31 (p = 0.026) |
| HSS | −0.43 (p = 0.001) | 0.33 (p = 0.010) | −0.28 (p = 0.030) | −0.50 (p < 0.001) | 0.31 (p = 0.026) |
| GS | −0.25 (p = 0.042) | 0.11 (p = 0.231) | −0.02 (p = 0.450) | −0.31 (p = 0.021) | 0.27 (p = 0.048) |
Abbreviations: HS, hemispheric score; HSS, hemispheric summary score; GS, global score; AHA, Assisting Hand Assessment; JTTHF, Jebsen–Taylor Test for Hand Function; MUUL, Melbourne Assessment of Unilateral Upper Limb function; 2PD, 2-point discrimination.
Bold emphasis is given to p< 0.05.
The model relating sqMRI scores with AHA was significant (R2 = 0.42, p < 0.001). The PLIC score (beta = −0.534, p < 0.001) and the middle-corpus-callosum score (beta = −0.241, p = 0.048) made a significant contribution to the model. The model relating sqMRI scores with JTTHF was significant (R2 = 0.20 p = 0.001), with the PLIC score (beta = 0.451, p = 0.001) contributing significantly to the model. The model relating contralateral and not lateralised sqMRI scores with MUUL was significant (p = 0.004 R2 = 0.18). Only the PLIC score (beta = −0.423, p = 0.004) made a significant contribution to the model. The model relating sqMRI scores with stereognosis impairment was significant (p = 0.001, R2 = 0.29). Contributing variables were the brainstem (beta = −0.422, p = 0.04) and temporal lobe (beta = −0.284, p = 0.041). Finally, the model relating sqMRI scores with 2PD impairment was significant (p = 0.000, R2 = 0.45). The middle corpus callosum (beta = 0.537, p = 0.000) and caudate (beta = 0.302, p = 0.023) contributed significantly to the model.
3.3. Relationship between sqMRI scores and FA
For the left hemiplegia group, higher lobar and HSS contralateral to the clinical side of hemiplegia (right hemisphere) correlated with lower FA values in a number of interhemispheric and intrahemispheric connections, including connections of right brain areas involved in motor control (postcentral, precentral and paracentral gyri in the parietal lobe). The relationship between sqMRI scores and FA values is reported in Table 3. Unexpectedly, higher left frontal lobar score (p = 0.0144) and left HSS (p = 0.0292) correlated with higher FA values.
Table 3.
Relationship between semi-quantitative MRI scores (sqMRI) and fractional anisotropy (FA) in left and right unilateral cerebral palsy.
| MRI score | FA | |
|---|---|---|
| Left UCP | R-frontal | p = 0.0374, 7 nodes, 6 edges |
| R-temporal | p = 0.0254, 31 nodes, 40 edges | |
| R-parietal | p = 0.0004, 63 nodes, 165 edges | |
| R-occipital | p = 1.000, none | |
| R-HSS | p < 0.0001, 58 nodes, 166 edges | |
| Right UCP | L-frontal | p = 0.4336, none |
| L-temporal | p = 0.0358, 25 nodes, 45 edges | |
| L-parietal | p = 0.0004, 61 nodes, 131 edges | |
| L-occipital | p = 1.000, none | |
| L-HSS | p = 0.4990, none |
Abbreviations: FA, fractional anisotropy; UCP, unilateral cerebral palsy; HHS, hemispheric summary score; R, right; L, left.
Bold emphasis is given to p< 0.05.
For the right hemiplegia group, higher lobar and HSS contralateral to the clinical side of hemiplegia (left hemisphere) correlated with lower FA values in a number of interhemispheric and intrahemispheric connections, including connections of left brain areas involved in motor control (postcentral, precentral and paracentral gyri in the parietal lobe). The frontal and occipital lobar scores and HSS contralateral to the clinical side of hemiplegia were not correlated to FA. No relationships between higher sqMRI scores and higher FA were found for any score. All relationship and p values are reported in Table 3.
An example of the HARDI connectivity representation of altered connections is reported in Fig. 1.
Fig. 1.
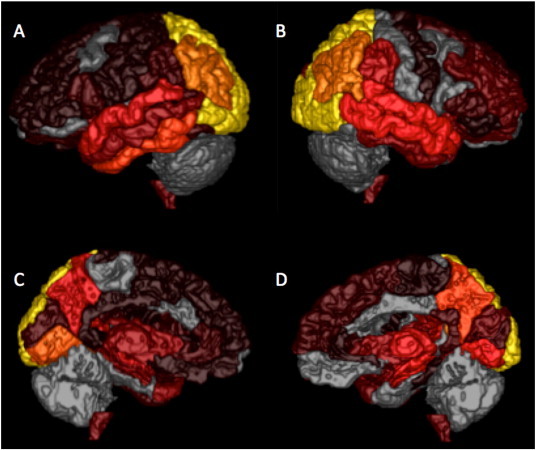
Representation of altered connections correlated with right (A, C) and left (B, D) hemispheric summary scores (lateral view: A, B; medial view C, D). The more bright (yellow) the colour the higher the number of disrupted connections correlated with more severe sqMRI scores.
4. Discussion
The sqMRI scale for classification of brain lesion severity demonstrated a relationship with impaired hand motor function measures and brain microstructure in children with UCP due to PWM lesions. The sqMRI scores contralateral to the clinical side of hemiplegia correlated significantly with the severity of sensori-motor hand function. The hemispheric summary score, expressing severity of brain lesion in both hemisphere and subcortical structures was correlated with the severity of sensory and motor impairment as assessed with all of the five clinical measures tested. Similar findings were obtained when using the global score in four of five clinical measures. It is of interest that the hemispheric score alone showed significant correlations with measures of sensory function but not with motor function, suggesting a differential role of hemispheric and subcortical structures in the functional impairment for these subjects. Overall, these findings are consistent with previous reports showing a specific role of subcortical structures, and in particular the PLIC, as to motor outcome (Staudt et al., 2000; Tsao et al., 2014), and a more distributed role of cortical and subcortical structures, namely the thalamo-cortical connections, as to somato-sensory function (Hoon et al., 2009; Rose et al., 2011; Tsao et al., 2014; Pannek et al., 2014).
In order to shed light on the specific characteristics of these structure–function correlations, a multiple regression analysis was performed. The ability of the impaired hand as an assisting hand (AHA) in bimanual tasks was largely explained (42% explained variability) by subscores of the scale contralateral to the clinical side of hemiplegia. The PLIC was the main structure found to impact on function, in accordance with its fundamental anatomic role as conveying region of all monosynaptic motor projections from sensorimotor cortex to spinal motor neurons. This is in agreement with previous studies that focused on the relationship between selected strategic structures and hemiplegia (De Vries et al., 1999; Tsao et al., 2014; Weinstein et al., 2014). The involvement of middle corpus callosum was the second contributor to explain variability of AHA scores, thus supporting the role of inter-hemispheric connections in hand motor control (Lassonde et al., 1995; Weinstein et al., 2014). The relationship between corpus callosum score and AHA agrees with previous studies demonstrating that reduced white matter integrity of the corpus callosum in children with UCP was associated with reduced motor function (Yokochi et al., 1991). Semi-quantitative MRI scores correlated to a lesser extent with the ability of the impaired hand in unimanual tasks as measured on JTTHF and MUUL compared to AHA, with the PLIC as the main contributor.
The sqMRI scores also correlated with stereognosis impairment (29% of expected variability), with brainstem and temporal lobe as the main contributors to the model. The brainstem is a crucial structure for ascending pathways to the sensorimotor cortex; even if our scale is not intended to discriminate between primary or secondary lesion types (see below), we can speculate that both primary tissue abnormalities in the brainstem or Wallerian degeneration have an impact on ascending input transmission. The temporal lobe has been previously described for its role in tactile object recognition (Reed et al., 2004). SqMRI scores correlated strongly with 2PD (45% explained variance) and the middle corpus callosum and caudate made a significant contribution to the model. The corpus callosum has been reported to be necessary for sensory discrimination (Lassonde et al., 1995), while the caudate was demonstrated to be involved in somatosensory coordination (Nagy et al., 2005).
The described significant and interesting associations with motor and sensory outcomes support first evidence of construct validity of the sqMRI scale. Still the correlations were rather low to moderate, but for this study, only children with PWM lesions, both unilateral and bilateral, have been included. It is known that in these children higher brain reorganisation potential may take place compared, for example, to cortical and deep grey matter lesions. Therefore structural damage may not be the only factor-influencing outcome and explain the modest association. A further point that needs to be considered is that potentially, different mechanism of plasticity can influence recovery of motor function in pure unilateral versus bilateral lesions in UCP. Indeed, the effects of early brain damage are related to a number of factors including the site and laterality (i.e. unilateral/bilateral) of the lesion. This implies a different profile of functional impairment in each subject, with complex and heterogeneous structure–function correlates. Further studies on larger cohorts that allow subgroup analyses are needed to support and clarify our findings and their impact on function.
In both the right and left UCP groups, sqMRI scores correlated with FA contralateral to the clinical side of UCP. Our data demonstrated the relationship between the sqMRI scores and white matter integrity for intra- and inter-hemispheric connections. There were few scores not related to FA. For children with left UCP, the right occipital score was not related to FA values. In children with right UCP, no relationship was found between left frontal, occipital and hemispheric summary scores. The limited number of subjects in the two subgroups could have influenced statistical power to find a difference, in particular for the left HSS, where a trend to significance can be observed. Furthermore, several aspects concerning methods can have an impact on analysis, including on one side the reduced variability of sqMRI scores due to the homogeneity of lesions in terms of type of lesion (Krageloh-Mann et al., 2007), and on the other side factors affecting FA values in the connectome analysis. In particular, in the sqMRI scale loss of tissue and altered tissue (i.e. gliosis) are scored similarly in order to simplify the score calculation. This means that an enlarged ventricle and an area of gliosis can be scored similarly by the sqMRI scale, but they will result in different FA estimation, as FA is influenced by white matter density or integrity. Furthermore, FA estimation can be different in primary or secondary (i.e. Wallerian degeneration) lesions (Pierpaoli et al., 2001). Extremely high or low FA values can therefore be present in areas with compressed white matter (e.g. ventricular dilatation), secondary degeneration, or absent white matter (for loss of tissue). This is a limitation that can impact this analysis. Only two scores were found to be correlated with higher FA values, however the same concerns about FA expected variations can be applied in these cases. Further studies are needed in order to understand the relationship between structural lesion characteristics (primary, secondary, compression or loss), semi-quantitative scores, scalar measures estimations (such as axial diffusivity or radial diffusivity) and neuropathology.
To our knowledge, this is the first study comparing a visual easily accessible structural MRI semi-quantitative scoring to microstructure measures by a quantitative whole brain probabilistic tractography approach in children with UCP due to PWM lesions. In a recent study Shiran et al., compared a novel MR imaging-based scoring system with a diffusion tractography technique (Shiran et al., 2014). Their DTI approach was based on a-priori hypothesis about defined structures to be involved (i.e. corpus callosum and PLIC), while our approach is based on a no a-priori hypothesis on possible connections related to our sqMRI scores.
There are some potential limitations to our study in that our study included only a subgroup of mild or moderately impaired children with CP due to PWM lesions. Firstly, we have to consider that several plasticity mechanisms can influence function recovery after congenital injury as sensorimotor reorganisation of corticospinal system (Staudt et al., 2004), thus limiting the possibility of the full explanation of function based on structural approaches on brain MRI. Despite this, a systematic approach to structural images can improve the understanding of these mechanisms. Moreover, further studies of brain lesion severity in larger cohorts and in children with different type of brain lesions such as cortical and deep grey matter lesions and brain maldevelopments are needed. Also, our scale was developed with no a-priori hypothesis, deciding not to assess only areas involved in motor function, but potentially evaluating all structures involved by the lesion despite their functional role. A further step to optimise the clinical utility of the MRI-based score could be the weighting of individual template scores to provide improved correlation of injury with motor function.
5. Conclusion
The American Academy of Neurology recommends structural MRI of the brain in the evaluation of all children with CP where the aetiology has not been established (Ashwal et al., 2004), however there is a lack of reliable and valid tools for assessing brain lesion severity capable of taking into account different sites and extent of a brain lesion in a systematic approach (Portney et al., 2009; Scheck et al., 2012; Pannek et al., 2014). We propose that structural MRI should be additionally considered to clarify the association between brain structure and function by combining qualitative (Krageloh-Mann et al., 2007) and quantitative (Fiori et al., 2014) approaches. In particular, in children with similar type of lesions (in the present study PWM lesions), the proposed quantitative approach empowers the detection of differences in the clinical phenotype that the qualitative approach cannot detect. Moreover, several outcome measures can be related to sqMRI scale, as the system assesses all brain areas, not specifically those included in motor function. Finally, further studies are needed to validate this approach in different types of brain lesions (e.g. cortical and deep grey matter lesions), thus giving further insights into subject-related mechanisms of plasticity and structure–function relationship in CP.
References
- Aida N., Nishimura G., Hachiya Y., Matsui K., Takeuchi M., Itani Y. MR imaging of perinatal brain damage: comparison of clinical outcome with initial and follow-up MR findings. A.J.N.R. Am. J. Neuroradiol. 1998;19(10):1909–1921. 9874547 [PMC free article] [PubMed] [Google Scholar]
- Arnfield E., Guzzetta A., Boyd R. Relationship between brain structure on magnetic resonance imaging and motor outcomes in children with cerebral palsy: a systematic review. Res. Dev. Disabil. 2013;34(7):2234–2250. doi: 10.1016/j.ridd.2013.03.031. 23643774 [DOI] [PubMed] [Google Scholar]
- Ashwal S., Russman B.S., Blasco P.A. Practice parameter: diagnostic assessment of the child with cerebral palsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurol. 2004;62(6):851–863. doi: 10.1212/01.wnl.0000117981.35364.1b. 15037681 [DOI] [PubMed] [Google Scholar]
- Auld M.L., Ware R.S., Boyd R.N., Moseley G.L., Johnston L.M. Reproducibility of tactile assessments for children with unilateral cerebral palsy. Phys. Occup. Ther. Pediatr. 2012;32(2):151–166. doi: 10.3109/01942638.2011.652804. 22309074 [DOI] [PubMed] [Google Scholar]
- Bastianello S., Bozzao A., Paolillo A., Giugni E., Gasperini C., Koudriavtseva T., Millefiorini E., Horsfield M.A., Colonnese C., Toni D., Fiorelli M., Pozzilli C., Bozzao L. Fast spin-echo and fast fluid-attenuated inversion-recovery versus conventional spin-echo sequences for MR quantification of multiple sclerosis lesions. A.J.N.R. Am. J. Neuroradiol. 1997;18(4):699–704. 9127033 [PMC free article] [PubMed] [Google Scholar]
- Boyd R.N., Mitchell L.E., James S.T., Ziviani J., Sakzewski L., Smith A. Move it to improve it (Mitii): study protocol of a randomised controlled trial of a novel web-based multimodal training program for children and adolescents with cerebral palsy. BMJ Open. 2013;3(4):e002853. doi: 10.1136/bmjopen-2013-002853. 23578686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R.N., Ziviani J., Sakzewski L., Miller L., Bowden J., Cunnington R., Ware R., Guzzetta A., Al Macdonell R., Jackson G.D., Abbott D.F., Rose S. COMBIT: protocol of a randomised comparison trial of COMbined modified constraint induced movement therapy and bimanual intensive training with distributed model of standard upper limb rehabilitation in children with congenital hemiplegia. B.M.C. Neurol. 2013;13:68. doi: 10.1186/1471-2377-13-68. 23809257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries L.S., Groenendaal F., van Haastert I.C., Eken P., Rademaker K.J., Meiners L.C. Asymmetrical myelination of the posterior limb of the internal capsule in infants with periventricular haemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics. 1999;30(6):314–319. doi: 10.1055/s-2007-973511. 10706026 [DOI] [PubMed] [Google Scholar]
- Fiori S., Cioni G., Klingels K., Ortibus E., Van Gestel L., Rose S., Boyd R.N., Feys H., Guzzetta A. Reliability of a novel, semi-quantitative scale for classification of structural brain magnetic resonance imaging in children with cerebral palsy. Dev. Med. Child Neurol. 2014;56(9):839–845. doi: 10.1111/dmcn.12457. 24750109 [DOI] [PubMed] [Google Scholar]
- Hoon A.H., Jr, Stashinko E.E., Nagae L.M., Lin D.D., Keller J., Bastian A., Campbell M.L., Levey E., Mori S., Johnston M.V. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev. Med. Child Neurol. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. 19416315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebsen R.H., Taylor N., Trieschmann R.B., Trotter M.J., Howard L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969;50(6):311–319. 5788487 [PubMed] [Google Scholar]
- Johnson L.M., Randall M.J., Reddihough D.S., Oke L.E., Byrt T.A., Bach T.M. Development of a clinical assessment of quality of movement for unilateral upper-limb function. Dev. Med. Child Neurol. 1994;36(11):965–973. doi: 10.1111/j.1469-8749.1994.tb11792.x. 7958514 [DOI] [PubMed] [Google Scholar]
- Krägeloh-Mann I., Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev. Med. Child Neurol. 2007;49(2):144–151. doi: 10.1111/j.1469-8749.2007.00144.x. 17254004 [DOI] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L., Eliasson A. Development of the Assisting Hand Assessment: a Rasch-built measure intended for children with unilateral upper limb impairments. Scand. J. Occup. Ther. 2003;10(1):16–26. [Google Scholar]
- Kwon S.H., Vasung L., Ment L.R., Huppi P.S. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin. Perinatol. 2014;41(1):257–283. doi: 10.1016/j.clp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Lassonde M., Sauerwein H.C., Lepore F. Extent and limits of callosal plasticity: presence of disconnection symptoms in callosal agenesis. Neuropsychologia. 1995;33(8):989–1007. doi: 10.1016/0028-3932(95)00034-z. 8524457 [DOI] [PubMed] [Google Scholar]
- MacKinnon S.E., Dellon A.L. Two-point discrimination tester. J. Hand Surg. Am. 1985;10(6 Pt 1):906–907. doi: 10.1016/s0363-5023(85)80173-8. 4078279 [DOI] [PubMed] [Google Scholar]
- Nagy A., Paróczy Z., Norita M., Benedek G. Multisensory responses and receptive field properties of neurons in the substantia nigra and in the caudate nucleus. Eur. J. Neurosci. 2005;22(2):419–424. doi: 10.1111/j.1460-9568.2005.04211.x. 16045495 [DOI] [PubMed] [Google Scholar]
- Pannek K., Boyd R.N., Fiori S., Guzzetta A., Rose S.E. Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. Neuroimage Clin. 2014;5:84–92. doi: 10.1016/j.nicl.2014.05.018. 25003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Barnett A., Pajevic S., Chen R., Penix L.R., Virta A., Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6 Pt 1):1174–1185. doi: 10.1006/nimg.2001.0765. 11352623 [DOI] [PubMed] [Google Scholar]
- Portney L., Watkins M. Foundations of Clinical Research: Applications to Practice. third edition. Pearson International; 2009. [Google Scholar]
- Reed C.L., Shoham S., Halgren E. Neural substrates of tactile object recognition: an fMRI study. Hum. Brain Mapp. 2004;21(4):236–246. doi: 10.1002/hbm.10162. 15038005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S., Guzzetta A., Pannek K., Boyd R. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect. 2011;1(4):309–316. doi: 10.1089/brain.2011.0034. 22432420 [DOI] [PubMed] [Google Scholar]
- Scheck S.M., Boyd R.N., Rose S.E. New insights into the pathology of white matter tracts in cerebral palsy from diffusion magnetic resonance imaging: a systematic review. Dev. Med. Child Neurol. 2012;54(8):684–696. doi: 10.1111/j.1469-8749.2012.04332.x. 22646844 [DOI] [PubMed] [Google Scholar]
- Shiran S.I., Weinstein M., Sirota-Cohen C., Myers V., Ben Bashat D., Fattal-Valevski A., Green D., Schertz M. MRI-based radiologic scoring system for extent of brain injury in children with hemiplegia. A.J.N.R. Am. J. Neuroradiol. 2014;35:2388–2396. doi: 10.3174/ajnr.A3950. 24852291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M., Gerloff C., Grodd W., Holthausen H., Niemann G., Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann. Neurol. 2004;56(6):854–863. doi: 10.1002/ana.20297. 15562409 [DOI] [PubMed] [Google Scholar]
- Staudt M., Niemann G., Grodd W., Krägeloh-Mann I. The pyramidal tract in congenital hemiparesis: relationship between morphology and function in periventricular lesions. Neuropediatrics. 2000;31(5):257–264. doi: 10.1055/s-2000-9239. 11204283 [DOI] [PubMed] [Google Scholar]
- Tournier J., Calamante F., Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012;22(1):53–66. [Google Scholar]
- Weinstein M., Green D., Geva R., Schertz M., Fattal-Valevski A., Artzi M., Myers V., Shiran S., Gordon A.M., Gross-Tsur V., Bashat D.B. Interhemispheric and intrahemispheric connectivity and manual skills in children with unilateral cerebral palsy. Brain Struct. Funct. 2014;219:1025–1040. doi: 10.1007/s00429-013-0551-5. 23571779 [DOI] [PubMed] [Google Scholar]
- Tsao H., Pannek K., Fiori S., Boyd R.N., Rose S. Reduced integrity of sensorimotor projections traversing the posterior limb of the internal capsule in children with congenital hemiparesis. Res. Dev. Disabil. 2014;35(2):250–260. doi: 10.1016/j.ridd.2013.11.001. 24291822 [DOI] [PubMed] [Google Scholar]
- Yokochi K., Aiba K., Horie M., Inukai K., Fujimoto S., Kodama M. Magnetic resonance imaging in children with spastic diplegia: correlation with the severity of their motor and mental abnormality. Dev. Med. Child Neurol. 1991;33(1):18–25. doi: 10.1111/j.1469-8749.1991.tb14781.x. 1704863 [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. 20600983 [DOI] [PubMed] [Google Scholar]


