Abstract
Autism Spectrum Disorder (ASD) is a clinically diagnosed, heterogeneous, neurodevelopmental condition, whose underlying causes have yet to be fully determined. A variety of studies have investigated either cortical, subcortical, or cerebellar anatomy in ASD, but none have conducted a complete examination of all neuroanatomical parameters on a single, large cohort. The current study provides a comprehensive examination of brain development of children with ASD between the ages of 4 and 18 years who are carefully matched for age and sex with typically developing controls at a ratio of one-to-two. Two hundred and ten magnetic resonance images were examined from 138 Control (116 males and 22 females) and 72 participants with ASD (61 males and 11 females). Cortical segmentation into 78 brain-regions and 81,924 vertices was conducted with CIVET which facilitated a region-of-interest- (ROI-) and vertex-based analysis, respectively. Volumes for the cerebellum, hippocampus, striatum, pallidum, and thalamus and many associated subregions were derived using the MAGeT Brain algorithm. The study reveals cortical, subcortical and cerebellar differences between ASD and Control group participants. Diagnosis, diagnosis-by-age, and diagnosis-by-sex interaction effects were found to significantly impact total brain volume but not total surface area or mean cortical thickness of the ASD participants. Localized (vertex-based) analysis of cortical thickness revealed no significant group differences, even when age, age-range, and sex were used as covariates. Nonetheless, the region-based cortical thickness analysis did reveal regional changes in the left orbitofrontal cortex and left posterior cingulate gyrus, both of which showed reduced age-related cortical thinning in ASD. Our finding of region-based differences without significant vertex-based results likely indicates non-focal effects spanning the entirety of these regions. The hippocampi, thalamus, and globus pallidus, were smaller in volume relative to total cerebrum in the ASD participants. Various sub-structures showed an interaction of diagnosis-by-age, diagnosis-by-sex, and diagnosis-by-age-range, in the case where age was divided into childhood (age < 12) and adolescence (12 < age < 18). This is the most comprehensive imaging-based neuro-anatomical pediatric and adolescent ASD study to date. These data highlight the neurodevelopmental differences between typically developing children and those with ASD, and support aspects of the hypothesis of abnormal neuro-developmental trajectory of the brain in ASD.
Abbreviations: ASD, Autism Spectrum Disorder; CT, cortical thickness; CV, cortical volume; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; FDR, False Discovery Rate; GP, globus pallidus; SA, surface area; Stdv, standard deviation
Keywords: Autism Spectrum Disorder (ASD), Magnetic resonance imaging (MRI), Pediatric neuroanatomical development, Cerebellar hippocampal and basal ganglia volume, Cortical anatomy
Highlights
-
•
Thorough analysis of neuroanatomical patterns in children and adolescents with ASD
-
•
Cortical, subcortical, and cerebellar development is assessed.
-
•
Total brain volume is smaller in males with ASD compared with Controls.
-
•
Cortical thickness in ASD does not decrease between childhood and adulthood.
-
•
Hippocampi, thalamus, globus pallidus and cerebellum are relatively smaller in ASD.
1. Introduction
Autism Spectrum Disorder (ASD), a life-long neurodevelopmental condition, is clinically diagnosed based on its socio-behavioral characteristics which include impaired social communication and interaction, and repetitive behavior and restricted interests. Efforts to understand this developmental condition have focused on characterizing any accompanying age-related neuro-pathophysiology. Clinical pathophysiological investigations have utilized neuroimaging techniques, in particular magnetic resonance imaging (MRI), to examine neurodevelopmental parameters (Stigler et al., 2011). Some of the parameters previously investigated with MRI include total brain volume, gray and white matter volume, cortical thickness, cortical surface area, cortical volume, as well as structure and volume of specific cerebral regions of interest in ASD, including those of the cerebellum and hippocampi (Brambilla et al., 2003; Courchesne et al., 2001; Scott et al., 2009). However, no single study has investigated all of these neuroanatomical parameters in a single ASD cohort. Additionally, studies that investigated multiple parameters are often limited by a small sample size or a poorly matched group of typically-developing participants.
Studies investigating brain volume have reported that in ASD the brain undergoes an abnormal short overgrowth in early postnatal life, which is followed by growth arrest in later childhood (Redcay and Courchesne, 2005; Courchesne et al., 2011; Hazlett et al., 2011a). More recently, this model has been challenged, as it appears that this is a finding due to only a small percentage of children who later develop autism (Amaral et al., 2008; Raznahan et al., 2013). Nevertheless, since age-related differences in brain volume could be attributed to abnormal maturation of the cerebral cortex (Raznahan et al., 2009), specific effort has been directed toward understanding cortical anatomy and development. Cortical development can be assessed via three inter-related morphometric components: cortical volume (CV), cortical thickness (CT), and surface area (SA). Since CV, by definition, is a product of CT and SA, it does not inherently provide a distinct piece of information about neuronal organization (Ecker et al., 2014). On the other hand, CT and SA are each believed to result from different progenitor cells and, therefore, reflect distinct neuro-organizational processes. Namely, CT is a product of intermediate progenitor cells, which divide in the subventricular zone and produce neurons (Chenn and Walsh, 2002; Pontious et al., 2008); whereas SA is shaped by radial-unit progenitor cells, which divide in the periventricular area and dictate the number of radial units (Chenn and Walsh, 2002; Pontious et al., 2008).
In addition to cortical structural differences, cerebellar abnormality is frequently seen including an increase in white matter in young children with ASD, and decrease in gray matter in mid-to-late adolescents with ASD (Allen, 2005).
Cerebral anatomical changes have elicited interest in functionally-connected subcortical structures in the limbic system, and particularly in the hippocampi, caudate, putamen, and thalamus. Inconsistency is apparent in the literature on hippocampal volume in children with ASD and youth. While some studies have reported an increase in volume (Schumann et al., 2004; Groen et al., 2010), others have found no change (Palmen et al., 2006) or even a decrease in volume (Aylward et al., 1999). Alterations have also been found in the caudate, which has been reported to be increased in volume in adolescents and adults with ASD (Rojas et al., 2006; Brambilla et al., 2003; Nickl-Jockschat et al., 2012; Sears et al., 1999). The putamen, has been reported to be decreased in volume in ASD independently of age (Nickl-Jockschat et al., 2012). Finally, the thalamus, has been found to be volumetrically decreased in ASD children (Stigler et al., 2011; Estes et al., 2011).
The current study provides a comprehensive examination across age of the neurodevelopment in children with ASD between the ages of 4 and 18 years by examining the structural changes of the cortex, cerebellum, hippocampi, thalamus, striatum, pallidum, and thalamus using MRI. The results from the ASD cohort are compared with those obtained from typically-developing (Control) children who were carefully matched for age and sex (using propensity score matching), to provide a more complete and accurate picture of the neurodevelopmental patterns in ASD and how they may differ from Control children.
2. Materials and methods
This study was approved by The Hospital for Sick Children Research Ethics Board and conducted in accordance with its guidelines. Written informed consent was obtained from all participants and/or their parent(s).
2.1. Participants
Three-hundred and seventy eight child participants between the ages of 4 and 18 were initially recruited to the study through Autism Ontario, recruitment in the local community, flyers posted at the hospital, as well as through the Province of Ontario Neurodevelopmental Disorders Network (POND). Of these, 280 were typically developing controls and 194 participants fulfilled the DSM-IV clinical diagnostic criteria for Autism Spectrum Disorder (ASD). The ASD diagnosis was supported by the Autism Diagnostic Observation Schedule — General (ADOS-G) (Lord et al., 2000) and/or the Autism Diagnosis Interview — Revised (ADI-R) (Lord et al., 1994). Control participants were excluded if they were born preterm, or had a history of psychiatric illness, concussion or learning disabilities. The Wechsler Abbreviated Scale of Intelligence (WASI) was also administered to 96% of the Control and 93% of the ASD group participants. Only children with ASD who could cooperate and lie still for the MRI session could be included (we use no sedation in our studies); thus this resulted in the inclusion of only high-functioning children with ASD.
Nearest-neighbor matching of remaining (117) ASD to (261) Control participants was then carried out using Propensity Score Matching technique (Ho et al., 2011). Propensity score was calculated as the probability of a subject being assigned to a particular group, given their age and sex (i.e. their observed covariates). Such matching mimics randomization by creating a sample of ASD subjects comparable on observed covariates to a sample of Control subjects. One-to-two matching of ASD-to-Control participants was conducted, yielding a smaller but carefully matched group of 138 Control participants (116 males and 22 females) and 72 ASD participants (61 males and 11 females), i.e. 210 children in total for which the age and sex covariate values were the closest. Statistical analysis was then carried out on this group of matched participants.
2.2. MRI acquisition
Participants were scanned on a 3 T Siemens Trio MRI scanner (MAGNETOM Tim Trio, Siemens AG, Erlangen, Germany) with a 12-channel head coil, using a T1-weighted 3D sagittal magnetization-prepared rapid gradient echo (MP-RAGE) sequence. This sequence provided 1 mm isotropic voxels, a FOV of 192 × 240 × 256 mm with a 240 × 256 matrix, 192 slices, and TR/TE/TI/FA = 2300 ms/2.96 ms/900 ms/9°. During image acquisition, children watched a movie of their choice using MR compatible goggles and earphones, and their heads were stabilized with foam padding to restrict motion.
2.3. Image processing
2.3.1. Cortical analysis
All MR images were processed with the CIVET pipeline (Zijdenbos et al., 2002), which linearly registers MRIs into a common 3-dimensional space, corrects for RF inhomogeneity artifacts (Collins et al., 1995; Sled et al., 1998), and classifies cortical regions according to their physiological category; gray matter, white matter, and cerebrospinal fluid (Zijdenbos et al., 2002). This classification is initially conducted using discrete tag points, and is then improved upon by use of partial volume information for the different tissue classes (Kim et al., 2005). The surfaces of the gray and white matter are then produced through the Constrained Laplacian Anatomical Segmentation using Proximities (CLASP) method, as explained by Kim et al. (2005). These are used in computing the cortical surface area (SA). Expanding the white matter surfaces until they reach the gray matter or cerebrospinal fluid surface boundary then allows for a more accurate identification of gray and white matter surface boundary (Kim et al., 2005), and creates 4 surfaces (2 per hemisphere) of 40,962 vertices each. These surfaces are registered to the MNI ICBM152 surface template, which allows for a group-wise statistical comparison. The distance between surface boundaries is thereafter used to compute the cortical thickness (CT), and together with SA, it is utilized in computing the cortical volume (CV) (Lerch and Evans, 2005; Kabani et al., 2001). An analysis of CT was conducted both through a vertex-based analysis of all 81,924 vertices, as well as with a region-based analysis of 78 brain regions, as segmented using the AAL atlas (Tzourio-Mazoyer et al., 2002). Mean CT was calculated by summing CTs of all AAL regions and dividing by the number of regions.
2.3.2. Cerebellar and subcortical analysis of the hippocampus, striatum, pallidum, and thalamus
Segmentation of the cerebellum, hippocampi, striatum, pallidum, and thalamus in all subjects was carried out using the Multiple Automatically Generated Templates (MAGeT) algorithm (Chakravarty et al., 2013). This algorithm uses five accurately and manually segmented atlases for the cerebellum and the hippocampi, and one accurately manually segmented atlas for the striatum, pallidum, and thalamus (Park et al., 2014; Winterburn et al., 2013; Chakravarty et al., 2013). It then generates multiple anatomical segmentations of representative “template” images; i.e. 11 MR images that were selected such that together they spanned the entire age-range. This is attained through pair-wise registration of each template MR image to each of the atlases, which yields a template library consisting of five labeled candidate segmentations per structure. Each subject brain is then registered to these templates. The most frequently occurring segmentation label at each voxel is retained, yielding a more accurate final anatomical segmentation. This is known as a “voxel voting” procedure (Collins and Pruessner, 2010). The relative volume of cerebellar structures was calculated by dividing their absolute volumes by the total cerebellar volume. For all cerebrum structures, the relative volume was calculated by dividing their volumes by the total cerebrum volume, as computed through the sum of the gray matter, white matter and cerebrospinal fluid volumes in CIVET.
Each atlas identified subregions within the anatomical structure of interest and in each brain hemisphere. The cerebellar atlas segmented out the following 13 cerebellar regions: lobules 1–2, lobule 3, lobule 4, lobule 5, lobule 6, Crus 1, Crus 2, lobule 7b, lobule 8a, lobule 8b, lobule 9, lobule 10, and white matter (Park et al., 2014). In the case of the hippocampi, the following 5 subfields were identified: CA1, subiculum, CA4/dentate gyrus, CA2/3, and stratum radiatum/lacunosum/moleculare (Winterburn et al., 2013). Finally, the atlas for the basal ganglia divided the region into the thalamus, globus pallidus, caudate, and putamen. Subregions in the right and left hemisphere were summed, and the combined volume was used in the data analysis.
2.3.3. Statistical analysis
Analysis of variance (ANOVA) was used to test the ROI-wise dependence of CT, SA, CV, cerebellar and subcortical volumes on sex, group, age, and age-range, along with group-by-age and group-by-age-range interactions. Age-range parameter was set to either “childhood” or “adolescence”, roughly corresponding to 4–11 and 12–18 years, respectively. Age was used as a continuous parameter to which a natural spline was applied with varying degrees of freedom. This spine fit yielded a linear, quadratic, or cubic fit with age. These three fits were then compared with an ANOVA and the one that resulted in a significant improvement in fit (p < 0.05) was used for that imaging metric ANOVA analysis along with the other demographic parameters in the model.
In the analysis, multiple comparisons were accounted for through the False Discovery Rate (FDR) technique applied to each ANOVA comparison (Benjamini and Yekutieli, 2001). This technique was also used to correct the results of the vertex-based CT analysis. FDR values are quoted along with the results. Only FDR values equal to or less than 10% were considered significant. The exact FDR values are also included with each result. T-statistics maps of vertex-based cortical thickness were generated by centering age 4 years apart, at 8, 12, and 16 years. The maps showing the most change are included in the results for a visual illustration of the group and group-by-age effects.
Data values are reported in the form mean ± standard deviation. Bar plots show the mean with an error bar corresponding to the 95% confidence interval. Histograms are included to show the distribution of the data and contain a cross (+) with a vertical line corresponding to the mean, and a horizontal line corresponding to the standard error of the mean.
3. Results
3.1. Demographic parameters
Age groups and IQ data are summarized in Table 1 below. A detailed breakdown of ADOS total scores and IQ by age-range is included in Table 2 in the Supplementary Material section.
Table 1.
Number of male and female participants per group and age range.
| Age Range (years) | Control males | Control females | ASD males | ASD females | IQ (mean ± stdv) |
|---|---|---|---|---|---|
| 4 - 11 | 46 | 12 | 34 | 6 | 107.3 ± 16.5 |
| 12 - 18 | 70 | 10 | 27 | 5 | 107.8 ± 13.0 |
| Total | 116 | 22 | 61 | 11 |
Age groups and IQ data are summarized in Table 1 below. A detailed breakdown of ADOS total scores and IQ by age-range is included in Table 2 in the Supplementary Material section.
3.2. Cortical parameters
Analysis of whole-brain volume revealed both a group effect, and a group-by-age effect. As depicted in Fig. 1, males with ASD have a significantly smaller brain volume compared with the Control males (FDR = 1%); a difference that was not observed between the females in the ASD and Control groups. While brain volume appeared to increase linearly with age for the Control participants, it followed a parabolic trend with a peak around 12 years of age for the ASD participants. Separation of males and females in this analysis revealed that this trend was mainly dictated by the males with ASD. White matter volume was found to be slightly increased (FDR = 10%) in males with ASD compared with Control males, while the opposite trend was found for the females with ASD compared with the Control females. Gray matter volume was also found to be slightly increased in participants with ASD compared with Controls, independently of sex (FDR = 10%). Neither mean cortical thickness nor total surface area was significantly different between the two groups, as depicted in Fig. 1. No group-by-sex interaction effect was found in any of the cortical parameters.
Fig. 1.
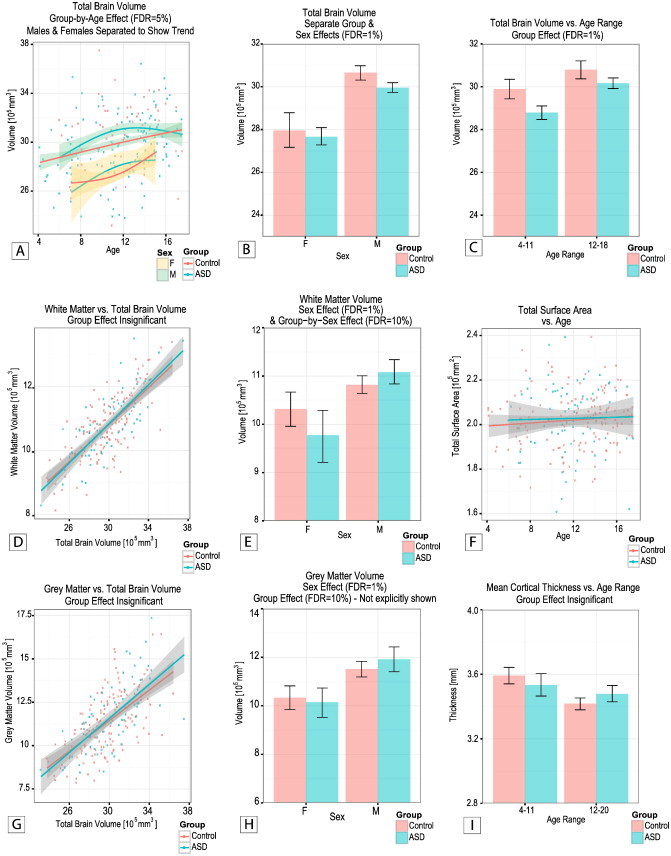
(A) Group-by-age, (b) separate group and sex, and (C) group by age-range effects on total brain volume. (D) White matter volume as a function of total brain volume and (E) showing a sex and a group-by-sex effect. (F) Total cortical surface area versus age. (G) Gray matter volume as a function of total brain volume and (H) showing a sex effect (group effect not shown). (I) Mean cortical thickness versus age-range. Note the parabolic trend of brain volume versus age in the ASD, the overall decrease in total brain volume in ASD group, and the decrease in white matter volume in the females with ASD.
A significant group-by-age-range difference in thickness was found in two cortical regions: the left orbitofrontal cortex and left posterior cingulate gyrus. These results are depicted in the F-statistics map shown in Fig. 2, along with corresponding graphs of cortical thickness as a function of age-range and study-group. In both cases the cortical thickness of Control participants significantly decreased between childhood and adolescence period. However, this trend was not observed in the participants with ASD, whose cortical thickness remained roughly unaltered with age.
Fig. 2.
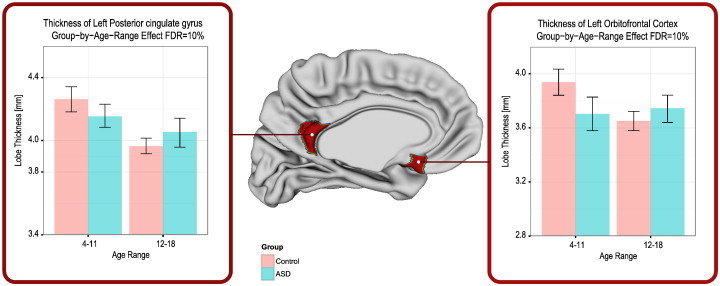
Group differences in lobe-based cortical thickness analysis: highlighted areas indicate atlas regions which are statistically different with an FDR = 10%. Group-by-age-range differences in cortical thickness for statistically-significant cortical regions: left posterior cingulate gyrus, and left orbitofrontal cortex. Note that the thicknesses of these regions substantially decrease from childhood to adolescence in the Control, but not in the ASD group.
None of the cortical regions significantly differed by group, group-by-age, and group-by-sex in the vertex-based analysis of cortical thickness.
3.3. Subcortical volumes
3.3.1. Hippocampi
Volumetric analysis of the hippocampi revealed group differences in the left hippocampus. These differences were significant only when volume was computed as percentage of total brain volume and not as absolute volume. Specifically, in absolute volume the left hippocampus was found to occupy 2418 ± 252 mm3 in the Control group, and a statistically similar volume of 2384 ± 248 mm3 in the ASD group. However, in relative volume it occupied 0.082 ± 0.007% in Control children but 0.079 ± 0.007% in children with ASD (FDR = 10%). Group differences were also found for the relative volume of the stratum radiatum, which was found to be significantly larger for the Control children 0.038 ± 0.004% compared with the children with ASD 0.036 ± 0.004% (FDR = 1%). When computed as absolute volume, there was no significant group difference: the stratum radiatum occupied 1115 ± 133 mm3 in the average Control brain, and 1083 ± 131 mm3 in the average ASD brain.
Both the right (FDR = 15%) and left (FDR = 10%) hippocampi had a group-by-sex effect caused by the females with ASD having a smaller relative volume (R: 0.078 ± 0.003%, L: 0.077 ± 0.005%) compared with males with ASD (R: 0.082 ± 0.007%, L: 0.080 ± 0.007%) and compared with the Control females (R: 0.083 ± 0.006%, L: 0.083 ± 0.004%) (Fig. 3). In addition, there was a group-by-sex difference in the relative volume of the subiculum, which occupied 0.023 ± 0.002% of the total brain of males with ASD, but only 0.022 ± 0.002% of the total brain of females with ASD (FDR = 10%). A similar drop in relative volume of females with ASD was noted in hippocampal region CA1; while it occupied 0.045 ± 0.003% in females with ASD, it occupied a significantly larger relative volume of 0.048 ± 0.004% in the Control females. These results are summarized in Fig. 3.
Fig. 3.
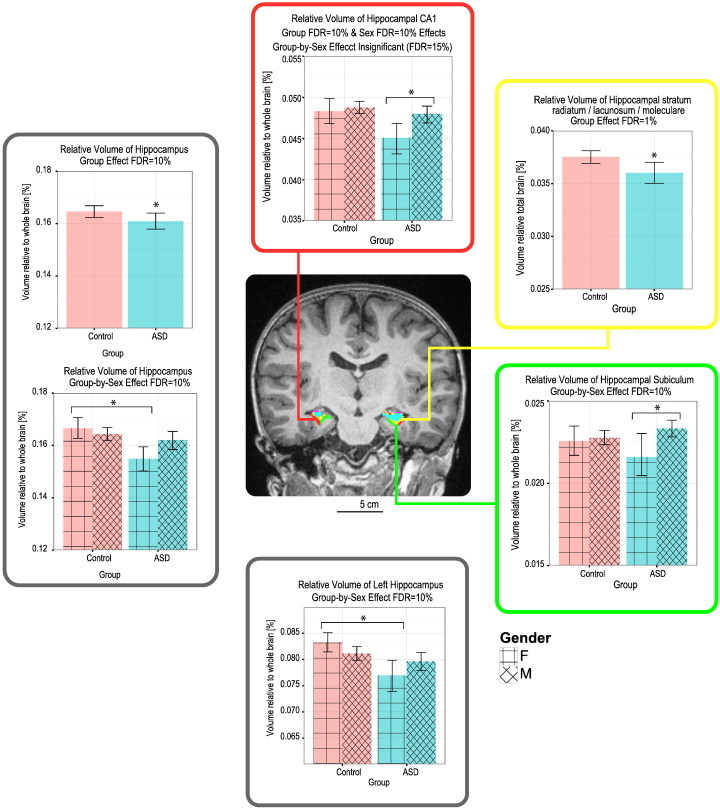
Group and sex effects on relative volume of hippocampal sub-structures. Note the decrease in relative volumes of most structures in the females with ASD compared with males with ASD and Control females (FDR = 10–15%), as well as the decrease in relative volume of the stratum radiatum in the ASD compared with Control group, independently of Sex (FDR = 1%).
3.3.2. Thalamus and basal ganglia
The relative volumes of both the thalamus and globus pallidus (GP) were significantly smaller in participants with ASD (thalamus: 0.443 ± 0.030%; GP: 0.104 ± 0.008%) compared with Controls (thalamus: 0.456 ± 0.034%; GP: 0.108 ± 0.009%), independent of sex or age effects (FDR = 1–5%). Group-by-sex effects were only found for the absolute volumes of the GP; however, those differences lost significance when total brain volume was taken into account. No other structure showed statistical significance in relative or absolute volume, and no group-by-age effect existed for any of the basal ganglia structures. These results are summarized in Fig. 4.
Fig. 4.
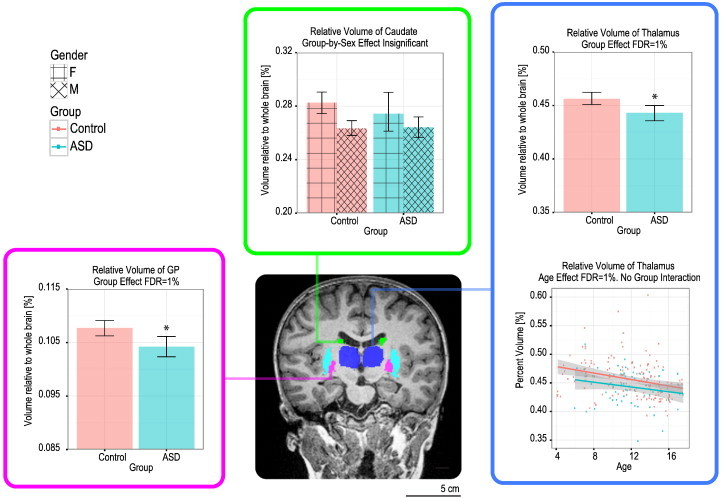
Group differences in relative volume of basal ganglia substructures. The thalamus and globus pallidus are decreased in relative volume in the ASD group (FDR = 5%). The thalamus also decreases in relative volume as a function of age.
3.3.3. Cerebellum
A group effect was only noted in the relative volume of Crus I which was significantly larger in the ASD (19.59 ± 1.40%) than Control participants (19.15 ± 1.43%, FDR = 5%). Group-by-age interaction effects were found for the relative volumes of lobules 1–2, and 3, and the right and left cerebellum. The age-trend of lobule 1–2 was best described by a quadratic fit and shows a drop in relative volume at 11 years of age in the ASD group. A similar trend was found for the left cerebellum, but only for the males with ASD compared with the Control males. The right cerebellum also followed a quadratic trend, but opposite in shape and having a peak in relative volume at 11 years of age. In all of these cases, the Control group trend described a linear curve without a prominent peak or drop at any specific age. The relative volume of lobule 3 followed a linear trend which showed a lower relative volume for the ASD group in childhood until 11 years of age, followed by a larger relative volume in adolescence (Fig. 5).
Fig. 5.
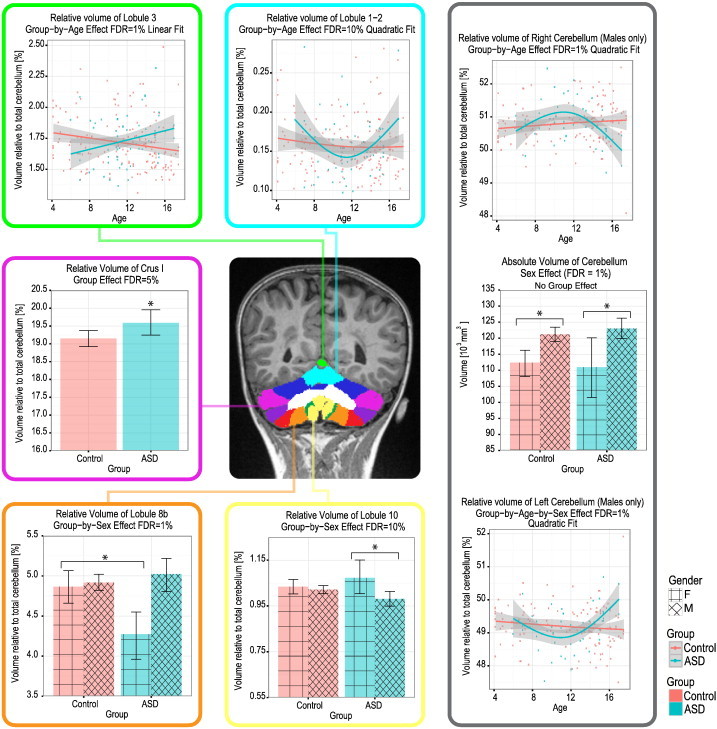
Group differences in relative volume of cerebellar subregions. Note the increase in relative volume of Crus I in the ASD group (FDR = 5%), the reduced relative volume of lobule 8b in females with ASD compared with Control females (FDR = 1%), and the increased relative volume of lobule 10 in females with ASD compared with males with ASD (FDR = 10%).
A group-by-sex interaction was found to affect the relative volumes of lobules 8b and 10. In both cases the difference was caused by the females with ASD who exhibited a significantly smaller relative volume in lobule 8b (4.27 ± 0.54%), but a significantly larger relative volume in lobule 10 (1.07 ± 0.14%) compared with the males with ASD (lobule 8: 5.02 ± 0.74%, lobule 10: 0.98 ± 0.11%) and Control females (lobule 8: 4.87 ± 0.54%, lobule 10: 1.03 ± 0.08% — Fig. 5).
Finally, a sex effect was found for the absolute volume of the cerebellum, which was smaller in females (111, 952 ± 12, 828 mm3) compared with males (121, 668 ± 12, 562 mm3), independently of group (Fig. 5).
4. Discussion
The currently available literature lacks a comprehensive investigation of the cortical, subcortical, and cerebellar anatomy of children and adolescents with ASD. The present study provides an extensive investigation of neurodevelopmental patterns by analyzing total brain volume, cortical surface area, cortical thickness, volume of the hippocampi, cerebellum, and basal ganglia, as well as their sub-structures in a single ASD cohort from early childhood through adolescent years (ages 4–18 years). We found a number of atypical brain changes with age in the participants with ASD compared with the matched Controls. Brain volume, lobe-based cortical thickness, the hippocampi, cerebellum, thalamus, and globus pallidus were found to be different between the two groups.
In the case of total brain volume, while that of the Control participants gradually increased between the ages of 4 and 18 years, those of the participants with ASD showed early overgrowth: in childhood volume increased at a faster rate such that volume peaked in early adolescence (age ~ 11 years). This peak was followed by a gradual decrease in volume in adolescence, which is similar to reports from other studies of teenage (Mak-Fan et al., 2013; Stigler et al., 2011) and of adult participants with ASD (Courchesne et al., 2001). When the data were separated by sex, it became evident that this trend was driven by the males with ASD. Despite following a similar trend, the females with ASD do not differ significantly from the Control females in total cerebral volume. This is a novel finding which should draw attention to the differences between brain development in males and females with ASD. However, since the majority of previous ASD studies focused exclusively on the developmental trajectories of ASD males, there is limited data to support whether the lack of difference between ASD and Control females is real, or whether it is due to the small number of female participants. Future studies focusing on females with ASD may elucidate this ambiguity. Independent of age, males with ASD have an enlarged total cerebral volume compared with their Control counterparts. No difference was observed between the females in the ASD and Control groups in terms of total cerebral volume with or without age as a covariate. However, the significant volumetric difference in males stresses the need for reporting cerebral volumes relative to total brain volume when comparing the average brain in the ASD and Control study groups.
Lobe-based cortical thickness also differed between the groups; unlike the Control group which showed a significant thickness decrease between childhood and adolescence, the ASD group maintained approximately the same thickness throughout these two developmental periods. A similar trend was observed in specific cortical structures: the left orbitofrontal cortex and left posterior cingulate. This reduced age-related cortical thinning was also reported by Doyle-Thomas et al. (2013). Similarly, Raznahan et al. (2010) found that in regions showing a group-by-age interaction, there was no decrease in thickness with age in the participants with ASD, as that seen in the Controls (Raznahan et al., 2010). The use of a broader age range with many more adult participants, particularly in Raznahan's study (age range: 10–65 years) may hinder a proper comparison with our findings. A contradicting observation was made by Hardan et al. who found an overall increase in cortical thickness across lobes when using data from ASD- and age-matched Control-group children aged 8–12 years (Hardan et al., 2006). Nonetheless, in that study thickness was averaged across all lobes and age was not used as a covariate, rendering it difficult to infer whether their data contained any age-related variations. By carrying out an analysis of mean cortical thickness as well as lobe-specific cortical thickness as a function of age throughout childhood and adolescence periods we were able to more accurately capture age-related variations.
Our analysis showed no group difference in surface area. This is consistent with results from previous studies on ASD and age-matched Control participants between the ages of 12–64 years (mean age = 34) (Raznahan et al., 2010), 6–15 years (mean age = 11) (Mak-Fan et al., 2013), and 12–23 years (Wallace et al., 2013). Only a few contradicting observations have been reported. For example, a study comparing participants with ASD between the ages of 7 and 25 years with age- and IQ-matched Controls found a reduction in surface area in the right anterior cingulate gyrus, right medial orbitofrontal and rostral middle frontal lobe, as well as the left temporal pole (Ecker et al., 2014). However, these results were obtained with a vertex-based approach, which yields localized detail that is not easily comparable to the averaging method we used in the lobule-based approach to surface area analysis. In our analysis we also found no significant group-by-age interaction effect on surface area, in agreement with other lobule-based studies on participants with ASD ranging from 10 to 60 years of age (Raznahan et al., 2010) and those ranging from 2–5 years of age (Raznahan et al., 2013) and their age-matched Control participants. Contradicting observations of positive group-by-age differences have been reported primarily by studies using a vertex-based analysis, such as by Ecker et al. (2014) and Mak-Fan et al. (2013). However, the latter study was limited in terms of the number of participants and compared only 25 participants with ASD with 63 Controls.
In our subcortical analysis we found that independent of age and sex, the ASD group had reduced relative volume of the left hippocampus, in hippocampal stratum radiatum. This result is consistent with the role that the hippocampus plays in associative memory and information integration; functions that are known to be impaired in ASD. Impact to the left hippocampus, in particular, implies alteration to verbal memory function (Richardson et al., 2003), as is the case in patients with ASD (Barendse et al., 2013). The volumetric decrease could be due to a decline in the number of synaptic receptors (Nagy et al., 2004), a structural abnormality that was found to occur in the stratum radiatum of a mouse model of ASD (Bozdagi et al., 2010) that relies on the SHANK3-gene deficiency associated with this disorder (Boccuto et al., 2013). Our observations also correlate with a study by Aylward et al. who noted a total reduction in relative hippocampal volume in 11–37 year-old autistic subjects compared with their age-, gender-, and IQ-matched controls (Aylward et al., 1999). Another report of decreased hippocampal volume is available in the literature (Howard et al., 2000); however, it is unclear whether this study corrected for total brain volume. Nicolson et al. evaluated hippocampal volume in 6–16 year-old ASD and age-matched Control participants, and also confirmed a relative reduction in volume (Nicolson, 2006). Yet, this reduction only existed in the right hippocampus, while no significant differences were noted in the left hippocampus. Additional divergent findings are in the literature, with some reporting an increase in hippocampal volume (Barnea-Goraly et al., 2014; Schumann et al., 2004; Groen et al., 2010), while others reporting no difference compared with controls (Piven et al., 1998; Haznedar et al., 2000; Palmen et al., 2006). The lack of consistent results could be due to heterogeneous diagnosis criteria, inclusion and exclusion criteria, age distribution of the tested cohort, and imaging techniques in various studies.
Our observation of reduced relative volume of the thalamus and globus pallidus in the participants with ASD could contribute to disturbed regulation of the sensory and motor systems (Bear and Connors, 2006), which are known to be impaired in ASD (Nair et al., 2013). A similar observation of reduced thalamic volume in ASD was reported by Tamura et al. (2010), using an adult cohort (Tamura et al., 2010). A later study by Estes et al. also demonstrated a volumetric decrease in the thalamus and globus pallidus of 4-year-old children with ASD (Estes et al., 2011). These results indicate a potential association between the morphological findings and the behavioral correlates in ASD.
In the case of the cerebellum, we found a significant group effect only for Crus I, which was significantly larger in ASD. Crus I activity has been associated with biological motion perception, which is known to be altered in ASD (Jack and Morris, 2014). It is, therefore, plausible that the different relative volume of Crus I is indicative of underlying functional alterations. Additionally, a group-by-age effect was found for the relative volume of the right and left cerebellum, as well as for cerebellar lobules 1–2, and 3. These regions, with the exception of lobule 3, showed a quadratic effect with age, with either a peak (right cerebellum) or a drop (left cerebellum and lobules 1–2) in the ASD, but not in the Control group. Lobule 3 was the only cerebellar region following a linear trend indicating delayed growth of this lobule in ASD in childhood, followed by over-corrected growth in adolescence. Together these altered age-trends imply that these cerebellar differences are caused by an underlying difference in developmental regulation in the ASD population. Given the important functional role of the cerebellum in cognitive, emotional, motor, and social functions, atypicalities could certainly have an impact on the observed functional and behavioral deficits in ASD population (Allen, 2005).
A significant group-by-sex effect was found for the relative volume of cerebellar lobules 8b and 10, the total hippocampus, left hippocampus, and hippocampal subiculum. Compared with Control girls, girls with ASD had a significantly decreased relative volume of cerebellar lobules 8b and of the total as well as left hippocampus. Compared with boys with ASD, girls with ASD had a reduction in relative volume in hippocampal subiculum, but an increase in relative volume in cerebellar lobule 10. These results, in conjunction with the unique trends in total brain volume outlined above, stress the differences between males and females with ASD, and possible differences in developmental mechanisms affecting males and females with this disorder (Lai et al., 2011; Baron-Cohen, 2002).
One of the study's main limitations is the small number of female participants with ASD. This, may also reflect the present issue with late diagnosis of ASD in females. Nonetheless, we carried out careful 2-to-1 matching of Control-to-ASD participants using a propensity matching technique which mimics randomization by creating samples that are comparable on age and sex covariates. The present report is one of the very few studies which assessed neurodevelopmental patterns in both males and females with ASD. It also provides much more rigorous matching than what is typically carried out in the literature, where simply mean age of the two groups do not differ significantly. Another limitation of the study is that it did not include participants with ASD who had a low IQ score.
In conclusion, to better understand ASD, it is imperative to carry out an extensive neuroanatomical investigation to best characterize its neuro-anatomical features. Our extensive cross-sectional study attempted to do that by analyzing the neurostructure of a large number of subjects in carefully-matched groups. The study revealed several cortical, subcortical, and cerebellar differences between the ASD- and Control-group participants. While mean cortical thickness and total surface area were not found to be significantly different, brain volume was different between the groups as a function of age-range. The only cortical regions that showed group differences were the left posterior cingulate gyrus and left orbitofrontal cortex. A closer look at localized cortical thickness changes using a vertex-based analysis revealed no significant group difference. This likely indicates that the effects within these regions were diffuse and spanned the entire region, as opposed to more focal effects that would have been detected by vertex-based analyses. The cortical thickness versus age trends in the regions with the extreme t-statistics value were best described by a quadratic fit. Only one other study has carried out a similar vertex-based analysis of cortical thickness in pediatric participants with ASD. The hippocampus, cerebellum, and several subfields and lobules were found to have a significant group, group-by-age, and group-by-sex differences between the ASD- and Control-group participants. Given these broad developmental features, one could speculate that an underlying cause of ASD is a prenatal insult. Such an early insult to the developing brain could theoretically trigger a multi-region response impacting multiple pathways, as that seen in this study. Future studies focusing on prenatal brain development of fetuses of high-risk pregnancies may allow us to better understand the circumstances that could produce this range of neuroanatomical features.
To the best of our knowledge, this is the most comprehensive imaging-based neuro-anatomical pediatric and adolescent ASD study to date. A future longitudinal study of the same anatomical measures will enable us to better characterize atypical structural development that occurs in participants with ASD from early childhood.
The following is the supplementary data related to this article.
IQ and total ADOS (mean ± standard deviation) of male and female participants per group and age range.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.04.008.
Funding
This study was supported by grant IDS-11-02 from the Ontario Brain institute and by grants MOP-119541 and MOP-106582 from the Canadian Institutes of Health Research.
Acknowledgments
The authors thank the MRI technicians Ruth Weiss and Tammy Rayner, for all their support with data acquisition. Many thanks to Matt Park for his help with MAGeT registration, Tamara Powell for her help with subject recruitment and MRI testing, George Ibrahim for input on propensity matching, and Joel Pollock for assistance with graphic design.
References
- Allen Greg. The cerebellum in autism. Clin. Neuropsychiatr. 2005;2(6):321–337. [Google Scholar]
- Amaral David G., Schumann Cynthia Mills, Nordahl Christine Wu. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. (March) [DOI] [PubMed] [Google Scholar]
- Aylward E.H., Minshew N.J., Goldstein G., Honeycutt N.A., Augustine A.M., Yates K.O., Barta P.E., Pearlson G.D. Mri volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. (December ) [DOI] [PubMed] [Google Scholar]
- Barendse Evelien M., Hendriks Marc P.H., Jansen Jacobus F.A., Backes Walter H., Hofman Paul A.M., Thoonen Geert, Kessels Roy P.C., Aldenkamp Albert P. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J. Neurodev. Disord. 2013;5(1):14. doi: 10.1186/1866-1955-5-14. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly Naama, Frazier Thomas W., Piacenza Lucia, Minshew Nancy J., Keshavan Matcheri S., Reiss Allan L., Hardan Antonio Y. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:124–128. doi: 10.1016/j.pnpbp.2013.09.010. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Bear M.F., Connors B.W. 3rd edition. Williams & Wilkins; Baltimore: 2006. Neuroscience: Exploring the Brain. [Google Scholar]
- Benjamini Yoav, Yekutieli Daniel. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29(4):1165–1188. (August, Mathematical Reviews number (MathSciNet) MR1869245, Zentralblatt MATH identifier01829051) [Google Scholar]
- Boccuto Luigi, Lauri Maria, Sarasua Sara M., Skinner Cindy D., Buccella Daniela, Dwivedi Alka, Orteschi Daniela, Collins Julianne S., Zollino Marcella, Visconti Paola, Dupont Barb, Tiziano Danilo, Schroer Richard J., Neri Giovanni, Stevenson Roger E., Gurrieri Fiorella, Schwartz Charles E. Prevalence of shank3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet. 2013;21(3):310–316. doi: 10.1038/ejhg.2012.175. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi Ozlem, Sakurai Takeshi, Papapetrou Danae, Wang Xiaobin, Dickstein Dara L., Takahashi Nagahide, Kajiwara Yuji, Yang Mu, Katz Adam M., Luisa Scattoni Maria, Harris Mark J., Saxena Roheeni, Silverman Jill L., Crawley Jacqueline N., Zhou Qiang, Hof Patrick R., Buxbaum Joseph D. Haploinsufficiency of the autism-associated shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism. 2010;1(1):15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla Paolo, Hardan Antonio, Ucelli di Nemi Stefania, Perez Jorge, Soares Jair C., Barale Francesco. Brain anatomy and development in autism: review of structural MRI studies. Brain Res. Bull. 2003;61(6):557–569. doi: 10.1016/j.brainresbull.2003.06.001. (October) [DOI] [PubMed] [Google Scholar]
- Chakravarty M. Mallar, Steadman Patrick, van Eede Matthijs C., Calcott Rebecca D., Gu Victoria, Shaw Philip, Raznahan Armin, Collins D. Louis, Lerch Jason P. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum. Brain Mapp. 2013;34(10):2635–2654. doi: 10.1002/hbm.22092. (October, 00011 PMID: 22611030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn Anjen, Walsh Christopher A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science (New York, N.Y.) 2002;297(5580):365–369. doi: 10.1126/science.1074192. (July) [DOI] [PubMed] [Google Scholar]
- Collins D. Louis, Pruessner Jens C. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting animal with a template library and label fusion. Neuroimage. 2010;52(4):1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. (October) [DOI] [PubMed] [Google Scholar]
- Collins D. Louis, Holmes C.J., Peters T.M., Evans A.C. Automatic 3-D model-based neuroanatomical segmentation. Hum. Brain Mapp. 1995;3(3):190–208. (January, 00677) [Google Scholar]
- Courchesne E., Karns C.M., Davis H.R., Ziccardi R., Carper R.A., Tigue Z.D., Chisum H.J., Moses P., Pierce K., Lord C., Lincoln A.J., Pizzo S., Schreibman L., Haas R.H., Akshoomoff N.A., Courchesne R.Y. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. (July) [DOI] [PubMed] [Google Scholar]
- Courchesne Eric, Campbell Kathleen, Solso Stephanie. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Thomas Krissy A.R., Duerden Emma G., Taylor Margot J., Lerch Jason P., Soorya Latha V., Wang A. Ting, Fan Jin, Hollander Eric, Anagnostou Evdokia. Effects of age and symptomatology on cortical thickness in autism spectrum disorders. Res. Autism Spectr. Disord. 2013;7(1):141–150. doi: 10.1016/j.rasd.2012.08.004. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C., Shahidiani A., Feng Y., Daly E., Murphy C., D'Almeida V., Deoni S., Williams S.C., Gillan N., Gudbrandsen M., Wichers R., Andrews D., Van Hemert L., Murphy D.G.M. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. J. Neural Transm. 2014;121(9):1157–1170. doi: 10.1007/s00702-014-1207-1. [DOI] [PubMed] [Google Scholar]
- Estes Annette, Shaw Dennis W.W., Sparks Bobbi F., Friedman Seth, Giedd Jay N., Dawson Geraldine, Bryan Matthew, Dager Stephen R. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res. 2011;4(3):212–220. doi: 10.1002/aur.193. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen Wouter, Teluij Michelle, Buitelaar Jan, Tendolkar Indira. Amygdala and hippocampus enlargement during adolescence in autism. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(6):552–560. doi: 10.1016/j.jaac.2009.12.023. (June) [DOI] [PubMed] [Google Scholar]
- Hardan Antonio Y., Muddasani Sri, Vemulapalli Madhuri, Keshavan Matcheri S., Minshew Nancy J. An MRI study of increased cortical thickness in autism. Am. J. Psychiatry. 2006;163(7):1290–1292. doi: 10.1176/appi.ajp.163.7.1290. (July) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett Heather Cody, Poe Michele, Gerig Guido, Styner Martin, Chappell Chad, Gimpel Smith Rachel, Vachet Clement, Piven Joseph. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2. Arch. Gen. Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar M.M., Buchsbaum M.S., Wei T.C., Hof P.R., Cartwright C., Bienstock C.A., Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am. J. Psychiatry. 2000;157(12):1994–2001. doi: 10.1176/appi.ajp.157.12.1994. (December) [DOI] [PubMed] [Google Scholar]
- Ho Daniel, Imai Kosuke, King Gary, Stuart Elizabeth. MatchIt: nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42(8) [Google Scholar]
- Howard M.A., Cowell P.E., Boucher J., Broks P., Mayes A., Farrant A., Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11(13):2931–2935. doi: 10.1097/00001756-200009110-00020. (September) [DOI] [PubMed] [Google Scholar]
- Jack Allison, Morris James P. Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev. Cogn. Neurosci. 2014;10C:77–92. doi: 10.1016/j.dcn.2014.08.001. (August) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani N., Le Goualher G., MacDonald D., Evans A.C. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13(2):375–380. doi: 10.1006/nimg.2000.0652. (February) [DOI] [PubMed] [Google Scholar]
- Kim June Sic, Singh Vivek, Ki Lee Jun, Lerch Jason, Ad-Dab'bagh Yasser, MacDonald David, Min Lee Jong, Kim Sun I., Evans Alan C. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. (August, 00340 PMID: 15896981) [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Pasco G., Ruigrok A.N.V., Wheelwright S.J., Sadek S.A., Chakrabati B., MRC AIMS Consortium, Baron-Cohen S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One. 2011;6(6):1–10. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch Jason P., Evans Alan C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. (January) [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. (October) [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. (June) [PubMed] [Google Scholar]
- Mak-Fan Kathleen M., Morris Drew, Vidal Julie, Anagnostou Evdokia, Roberts Wendy, Taylor Margot J. White matter and development in children with an autism spectrum disorder. Autism. 2013;17(5):541–557. doi: 10.1177/1362361312442596. (September) [DOI] [PubMed] [Google Scholar]
- Nagy Gergely G., Al-Ayyan Muna, Andrew David, Fukaya Masahiro, Watanabe Masahiko, Todd Andrew J. Widespread expression of the AMPA receptor glur2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of glur1 in response to noxious stimulation revealed with an antigen-unmasking method. J. Neurosci. 2004;24(25):5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Treiber J.M., Shukla D.K., Shih P., Muller R.-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136(6):1942–1955. doi: 10.1093/brain/awt079. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat Thomas, Habel Ute, Michel Tanja Maria, Manning Janessa, Laird Angela R., Fox Peter T., Schneider Frank, Eickhoff Simon B. Brain structure anomalies in autism spectrum disorder—a meta-analysis of vbm studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012;33(6):1470–1489. doi: 10.1002/hbm.21299. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Res. Neuroimaging. 2006;148(1):11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Palmen Saskia J.M.C., Durston Sarah, Nederveen Hilde, Van Engeland Herman. No evidence for preferential involvement of medial temporal lobe structures in high-functioning autism. Psychol. Med. 2006;36(6):827–834. doi: 10.1017/S0033291706007215. (June) [DOI] [PubMed] [Google Scholar]
- Park Min Tae M., Pipitone Jon, Baer Lawrence H., Winterburn Julie L., Shah Yashvi, Chavez Sofia, Schira Mark M., Lobaugh Nancy J., Lerch Jason P., Voineskos Aristotle N., Chakravarty M. Mallar. Derivation of high-resolution MRI atlases of the human cerebellum at 3 T and segmentation using multiple automatically generated templates. Neuroimage. 2014;95:217–231. doi: 10.1016/j.neuroimage.2014.03.037. (July) [DOI] [PubMed] [Google Scholar]
- Piven J., Bailey J., Ranson B.J., Arndt S. No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J. Autism Dev. Disord. 1998;28(2):105–110. doi: 10.1023/a:1026084430649. (April) [DOI] [PubMed] [Google Scholar]
- Pontious Adria, Kowalczyk Tom, Englund Chris, Hevner Robert F. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2008;30(1–3):24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Raznahan Armin, Toro Roberto, Daly Eileen, Robertson Dene, Murphy Clodagh, Deeley Quinton, Bolton Patrick F., Paus Tomas, Murphy Declan G.M. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb. Cortex. 2010 doi: 10.1093/cercor/bhp198. (page bhp198, January) [DOI] [PubMed] [Google Scholar]
- Raznahan Armin, Toro Roberto, Daly Eileen, Robertson Dene, Murphy Clodagh, Deeley Quinton, Bolton Patrick F., Paus Tomas, Murphy Declan G.M. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb. Cortex. 2010;20(6):1332–1340. doi: 10.1093/cercor/bhp198. (June) [DOI] [PubMed] [Google Scholar]
- Raznahan Armin, Wallace Gregory L., Antezana Ligia, Greenstein Dede, Lenroot Rhoshel, Thurm Audrey, Gozzi Marta, Spence Sarah, Martin Alex, Swedo Susan E., Giedd Jay N. Compared to what? Early brain overgrowth in autism and the perils of population norms. Biol. Psychiatry. 2013;74(8):563–575. doi: 10.1016/j.biopsych.2013.03.022. (October) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay Elizabeth, Courchesne Eric. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. (July) [DOI] [PubMed] [Google Scholar]
- Richardson Mark P., Strange Bryan A., Duncan John S., Dolan Raymond J. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003;20(Suppl. 1):S112–119. doi: 10.1016/j.neuroimage.2003.09.008. (November) [DOI] [PubMed] [Google Scholar]
- Rojas Donald C., Peterson Eric, Winterrowd Erin, Reite Martin L., Rogers Sally J., Tregellas Jason R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann Cynthia Mills, Hamstra Julia, Goodlin-Jones Beth L., Lotspeich Linda J., Kwon Hower, Buonocore Michael H., Lammers Cathy R., Reiss Allan L., Amaral David G. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. (July) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Julia A., Mills Schumann Cynthia, Goodlin-Jones Beth L., Amaral David G. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2009;2(5):246–257. doi: 10.1002/aur.97. (October) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears L.L., Vest C., Mohamed S., Bailey J., Ranson B.J., Piven J. An MRI study of the basal ganglia in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1999;23(4):613–624. doi: 10.1016/s0278-5846(99)00020-2. (May) [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. (February, 02140 PMID: 9617910) [DOI] [PubMed] [Google Scholar]
- Stigler Kimberly A., McDonald Brenna C., Anand Amit, Saykin Andrew J., McDougle Christopher J. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146–161. doi: 10.1016/j.brainres.2010.11.076. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Ryu, Kitamura Hideaki, Endo Taro, Hasegawa Naoya, Someya Toshiyuki. Reduced thalamic volume observed across different subgroups of autism spectrum disorders. Psychiatry Res. 2010;184(3):186–188. doi: 10.1016/j.pscychresns.2010.07.001. (December) [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. (January) [DOI] [PubMed] [Google Scholar]
- Wallace Gregory L., Robustelli Briana, Dankner Nathan, Kenworthy Lauren, Giedd Jay N., Martin Alex. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136(Pt 6):1956–1967. doi: 10.1093/brain/awt106. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn Julie L., Pruessner Jens C., Chavez Sofia, Schira Mark M., Lobaugh Nancy J., Voineskos Aristotle N., Chakravarty M. Mallar. A novel in vivo atlas of human hippocampal subfields using high-resolution 3 T magnetic resonance imaging. Neuroimage. 2013;74:254–265. doi: 10.1016/j.neuroimage.2013.02.003. (July) [DOI] [PubMed] [Google Scholar]
- Zijdenbos Alex P., Forghani Reza, Evans Alan C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. (October) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IQ and total ADOS (mean ± standard deviation) of male and female participants per group and age range.


