Abstract
Background
Individuals with at-risk mental state for psychosis (ARMS) often suffer from depressive and anxiety symptoms, which are clinically similar to the negative symptomatology described for psychosis. Thus, many ARMS individuals are already being treated with antidepressant medication.
Objectives
To investigate clinical and structural differences between psychosis high-risk individuals with or without antidepressants.
Methods
We compared ARMS individuals currently receiving antidepressants (ARMS-AD; n = 18), ARMS individuals not receiving antidepressants (ARMS-nonAD; n = 31) and healthy subjects (HC; n = 24), in terms of brain structure abnormalities, using voxel-based morphometry. We also performed region of interest analysis for the hippocampus, anterior cingulate cortex, amygdala and precuneus.
Results
The ARMS-AD had higher ‘depression’ and lower ‘motor hyperactivity’ scores than the ARMS-nonAD. Compared to HC, there was significantly less GMV in the middle frontal gyrus in the whole ARMS cohort and in the superior frontal gyrus in the ARMS-AD subgroup. Compared to ARMS-nonAD, the ARMS-AD group showed more gray matter volume (GMV) in the left superior parietal lobe, but less GMV in the left hippocampus and the right precuneus. We found a significant negative correlation between attenuated negative symptoms and hippocampal volume in the whole ARMS cohort.
Conclusion
Reduced GMV in the hippocampus and precuneus is associated with short-term antidepressant medication and more severe depressive symptoms. Hippocampal volume is further negatively correlated with attenuated negative psychotic symptoms. Longitudinal studies are needed to distinguish whether hippocampal volume deficits in the ARMS are related to attenuated negative psychotic symptoms or to antidepressant action.
Keywords: Structural MRI, Antidepressant medication, Hippocampus, Precuneus, Negative psychotic symptoms
Highlights
-
•
We compared brain structure in high-risk patients with/without antidepressants (AD).
-
•
We found attenuated negative psychotic symptoms (ANS) irrespective of AD.
-
•
We found a significant correlation between ANS and hippocampal volume.
-
•
Results indicate relevance of ANS for clinical high-risk studies.
1. Introduction
The clinical high-risk state of psychosis (at-risk mental state, hereafter ARMS) is defined by attenuated positive psychotic symptoms, genetic liability and functional deterioration or brief and self-remitting psychotic symptoms (Fusar-Poli et al., 2013; Yung et al., 1998). However, affective symptoms, including depressive and anxiety symptoms, are also highly prevalent in these individuals (Salokangas et al., 2012). A recent meta-analysis, conducted in 1683 high-risk subjects, confirmed that the baseline prevalence of comorbid depressive and anxiety disorder is 41% and 15%, respectively (Fusar-Poli et al., 2014a). Depressive and anxiety symptoms can precede the onset of attenuated positive psychotic symptoms (Fusar-Poli et al., 2013). Some studies indicate that co-occurrence of depressive disorders can predict subsequent transition to psychosis in ARMS individuals (Salokangas et al., 2012). However, other studies have not confirmed this finding (Fusar-Poli et al., 2014a). Additionally, a large study on 3349 twins suggests an association between depressive and/or anxiety symptoms and psychosis-like traits (schizotypy) and emphasizes a major role for genetics, especially as regards positive symptoms (Macare et al., 2012). The comorbidity of psychotic and depressive disorders in the ARMS population is associated with specific psychopathological features at the time of the presentation to high risk services and with low functional level (Fusar-Poli et al., 2013). Because of these problems, clinical high-risk individuals often receive antidepressant medication (e.g. 42% of ARMS individuals in our previous study (Smieskova et al., 2012a)).
Negative psychotic symptoms are a major source of disability in the psychosis spectrum and are refractory to any effective treatment (Fusar-Poli et al., 2014b). Negative symptoms group into two factors, one involving diminished expression of affect and alogia and the second involving avolition, including anhedonia and asociality (Fusar-Poli et al., 2014b). Antidepressants may have a potential benefit for ARMS individuals, as they may target their negative attenuated psychotic symptoms (Cornblatt et al., 2007; Fusar-Poli et al., 2007). These studies indicate that antidepressant treatments in ARMS individuals can impact their longitudinal outcomes. However, it is not clear if these improvements are associated with underlying neurobiological changes (Wood et al., 2011).
Neuroimaging studies using magnetic resonance imaging (MRI) have indicated that ARMS showed brain alterations in the prefrontal (Borgwardt et al., 2006; Borgwardt et al., 2008; Koutsouleris et al., 2009; Mechelli et al., 2011; Wood et al., 2010), cingulate (Fornito et al., 2008; Koutsouleris et al., 2009), superior (Takahashi et al., 2009; Takahashi et al., 2010) and medial temporal (Borgwardt et al., 2007b; Tognin et al., 2014), insular (Smieskova et al., 2010) and cerebellar regions when compared to healthy controls. Furthermore, ARMS individuals with subsequent transition to psychosis showed volumetric reductions in the prefrontal, insular and cingulate cortex compared to those without transition (Smieskova et al., 2010).
Similar alterations were found in depressive disorders. Reductions in gray matter volume (GMV) in the anterior cingulate gyrus, hippocampus, amygdala (Koolschijn et al., 2009) and prefrontal cortex (Lorenzetti et al., 2009) were associated with major depression. The only available study directly testing the effect of comorbid depressive disorders on the neurobiology of ARMS uncovered a significant impact on the anterior cingulate region (Modinos et al., 2014). On the other hand, long-term antidepressant medication can be neuroprotective and some studies have linked the use of antidepressants to an increase in hippocampal volume in patients with major depressive disorder (Amico et al., 2011; Malykhin et al., 2010). It has been shown that antidepressants increase hippocampal neurogenesis (Anacker et al., 2011). Thus, both affective symptoms (Baynes et al., 2000) and antidepressant medication (Kraus et al., 2014) are known to impact brain structure.
In the present study, we addressed for the first time the effect of antidepressant treatment and attenuated negative psychotic symptoms on the neurobiology of ARMS. Firstly, we hypothesized that ARMS individuals without current antidepressant treatment (ARMS-nonAD) would manifest more severe attenuated negative symptoms than ARMS subjects currently receiving antidepressants (ARMS-AD). Secondly, we hypothesized that ARMS-AD individuals would have increased GMV in regions associated with depressive symptoms and/or antidepressant medication (hippocampus, anterior cingulate gyrus, amygdala and precuneus) compared to the ARMS-nonAD individuals. Thirdly, we hypothesized that the volumetric abnormalities in gray matter between ARMS-AD and ARMS-nonAD would be associated with attenuated negative symptoms.
2. Materials and methods
2.1. Subjects
MRI data were collected within the framework of a research program on the early detection of psychosis. The subjects were recruited in our specialized clinic for the early detection of psychosis (FEPSY) at the Psychiatric Outpatient Department, Psychiatric University Clinics Basel, Switzerland (Riecher-Rössler et al., 2006).
The entire group of ARMS individuals (n = 49) conforms to Yung's criteria (Yung et al., 1998) and overlaps with previously published data (Borgwardt et al., 2007a; Borgwardt et al., 2007b; Smieskova et al., 2012a; Smieskova et al., 2012b). All the ARMS individuals were antipsychotic-free and were assessed prior to the neuroimaging session. ARMS inclusion required one or more of the following:
-
(a)
attenuated psychotic symptoms that do not reach full psychosis threshold
-
(b)
brief limited intermittent psychotic symptoms (lasting less than a week with spontaneous remission)
-
(c)
a first degree relative with a psychotic disorder plus at least two indicators of a clinical change, such as a marked decline in social or occupational functioning.
We assessed the subjects using the Basel Screening Instrument for Psychosis (BSIP) (Riecher-Rössler et al., 2008), the Brief Psychiatric Rating Scale (BPRS) (Lukoff et al., 1986), the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989) and the Global Assessment of Functioning (GAF) (Endicott et al., 1976). Attenuated negative psychotic symptom severity was investigated with the cluster ‘negative symptoms’, calculated from the BPRS as a sum of blunted affect, emotional withdrawal, and motor retardation (BPRS16, BPRS17 and BPRS18) (Fusar-Poli et al., 2014b; Velligan et al., 2005). Additionally, we calculated ‘mood disturbance’ BPRS cluster as a sum of anxiety, depression, suicidality and guilt (BPRS02, BPRS03, BPRS04 and BPRS05) (Thomas et al., 2004), as well as depression (BPRS03) and motor retardation (BPRS18) scores alone. We used these scores for stepwise regression analysis with backward elimination.
In a second step, we divided the ARMS individuals into two subgroups, based on whether they were currently being treated with antidepressants (ARMS-AD, n = 18) or not (ARMS-nonAD, n = 31) (Table 1). Antidepressant medication within the AD subgroup included: fluoxetine (SSRI; n = 2), escitalopram (SSRI; n = 5), sertraline (SSRI; n = 1), mirtazapine [NaSSA (noradrenergic and specific serotonergic antidepressant); n = 4], venlafaxine [SSNRI (selective serotonin–norepinephrine reuptake inhibitor), n = 2], duloxetine (SSNRI; n = 2), fluoxetine plus trazodone [SSRI, SARI (serotonin antagonist and reuptake inhibitor); n = 1] and St. John's Wort (n = 1). Antidepressant therapy had a mean duration of 50 ± 47 days (range 4–170 days). In order to exclude possible biases through antipsychotic therapy, we confirmed that all individuals were antipsychotic-free. In addition, current and previous psychotropic medication, alcohol, nicotine, cannabis, and other illegal drug consumption were assessed using a semi-structured interview, as adapted from the Drug and Alcohol Assessment Schedule of the Early Psychosis Prevention and Intervention Centre (EPPIC).
Table 1.
Demographic and clinical data.
| Characteristic | ARMS-AD (n = 18) | ARMS-nonAD (n = 31) | HC (n = 24) | Statistic | Post hoc* |
|---|---|---|---|---|---|
| Gender M/F | 14/4 | 24/7 | 10/14 | χ2 = 9.231 p = 0.010 | p = 0.977 |
| Mean age (SD) | 24.9 (5.0) | 23.9 (4.9) | 27.6 (4.6) | F = 4.140 p = 0.020 | p = 1.000 |
| Handedness (left) | 1 (5.6%) | 2 (6.5%) | 2 (8.3%) | χ2 = 0.138 p = 0.933 | |
| Years of education (SD) | 14.11 (3.45) | 13.05 (2.59) | 16.00 (2.80) | F = 7.123 p = 0.002 | p = 0.654 |
| BPRS total score (SD) | 41.56 (8.00) | 39.04 (9.37) | 24.54 (1.10) | F = 36.971 p < 0.0001 |
p = 0.807 |
| BPRS mood disturbance (SD) | 11.22 (3.34) | 9.35 (3.64) | 4.50 (0.98) | F = 30.746 p < 0.0001 |
p = 0.108 |
| BPRS negative symptoms (SD) | 6.33 (2.43) | 5.36 (2.78) | 3.00 (0.00) | F = 13.699 p < 0.0001 |
p = 0.400 |
| BPRS depression (SD) | 4.17 (1.15) | 3.23 (1.33) | 1.13 (0.34) | F = 47.456 p < 0.0001 |
p = 0.011** |
| BPRS motor hyperactivity (SD) | 1.00 (0.00) | 1.48 (0.89) | 1.00 (0.00) |
F = 5.856 p = 0.005 |
p = 0.029** |
| SANS total score (SD) | 22.00 (11.55) | 17.88 (14.26) | 0.00 (0.00) | F = 25.721 p < 0.0001 |
p = 0.674 |
| GAF (SD) | 62.11 (10.92) | 66.0 (12.38) | 88.08 (4.15) | F = 45.236 p < 0.0001 |
p = 0.583 |
| Cigarettes smoked per day (SD) | 12.83 (8.92) | 5.31 (8.32) | 3.25 (6.52) | F = 8.105 p = 0.001 |
p = 0.006** |
| Current cannabis use | 11 | 8 | 4 | χ2 = 10.226 p = 0.006 |
p = 0.014** |
| Current alcohol use No/moderate/ uncontrolled |
3/10/5 | 7/21/3 | 1/21/2 | χ2 = 8.125 p = 0.087 |
p = 0.253 |
| Days on antidepressants (SD) |
50 (47) |
None | None | ||
| Range days | 4–170 |
Abbreviations: ARMS, at-risk mental state individuals; ARMS-AD, ARMS with antidepressants; ARMS-nonAD, ARMS without antidepressants; BPRS, Brief Psychiatric Rating Scale; ‘BPRS depression’, BPRS 3; ‘BPRS motor hyperactivity’, BPRS 23; ‘BPRS mood disturbance’, as a sum of anxiety, depression, suicidality and guilt (BPRS02, BPRS03, BPRS04 and BPRS05); ‘BPRS negative symptoms’ as a sum of blunted affect, emotional withdrawal, and motor retardation (BPRS16, BPRS17 and BPRS18); GAF, Global Assessment of Functioning; HC, healthy controls; SANS, Scale for the Assessment of Negative Symptoms.
Bonferroni correction (at p = 0.05) was calculated for post hoc analysis in SPSS 22.0. Only post hoc results between ARMS-AD and ARMS-nonAD groups are presented.
Significant results in post hoc analysis between ARMS-AD and ARMS-nonAD.
Participants were excluded from the study if they presented with a history of previous psychotic disorder, psychotic symptomatology secondary to an organic disorder, substance abuse, affective psychosis, borderline personality disorder, age under 18 or over 40, inadequate knowledge of the German language or IQ less than 70 (assessed by multiple-choice vocabulary intelligence test) (Lehrl et al., 1995).
Healthy controls (n = 24) were from the same geographical area as the other groups (Table 1). All participants provided written informed consent. The study was approved by the local ethics committee.
2.2. Magnetic resonance imaging acquisition
For structural imaging, a whole brain 3D T1-weighted MPRAGE (magnetization prepared rapid acquisition gradient) sequence was applied using a 3 T magnetic resonance imaging scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) and a 12-channel radio frequency head coil. The acquisition was based on a sagittal matrix of 256 × 256 × 176 and 1 × 1 × 1 mm3 isotropic spatial resolution, with an inversion time of 1000 ms, repetition time of 2 s, echo time of 3.4 ms, flip angle of 8° and bandwidth of 200 Hz/pixel. All images were reviewed by trained neuroradiologists for radiological abnormalities.
2.3. Image analysis
Structural MRI data were analyzed using the voxel-based morphometry toolbox (VBM8, http://dbm.neuro.uni-jena.de/vbm8/), implemented within SPM8 (Wellcome Department of Cognitive Neurology, London, UK) and running on Matlab 7.11 (MathWorks, USA). All T1-weighted images were first checked for scanner artifacts and anatomical abnormalities. Images were then segmented into gray matter, white matter and cerebrospinal fluid using the adaptive maximum a posteriori technique (in contrast to the classical use of a priori Tissue Probability Maps), where local variations in the parameters are modeled by means of slowly varying spatial functions (Rajapakse et al., 1997). More accurate segmentation can be achieved with partial volume estimation of additional mixed tissue classes in every voxel. All images were DARTEL-normalized (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra; Ashburner, 2007). The DARTEL template was derived from 550 healthy controls as provided in MNI space. This method produces more accurate results for registration and additional registration in MNI space was unnecessary (Ashburner, 2007). Finally, sample homogeneity was reviewed and all images were smoothed using an isotropic 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel (Shen and Sterr, 2013).
Group differences were explored using a one-way ANOVA. Since our groups differed significantly in gender and age, we introduced these two variables as additional covariates of interest. Group comparisons included ARMS-AD versus ARMS-nonAD versus healthy controls. Brain region labeling was achieved using the Harvard–Oxford structural atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases (Desikan et al., 2006)), incorporated within the FMRIB Software Library (FSL).
Statistical significance was assessed at a cluster level using a threshold of p < 0.005 uncorrected (cluster-forming threshold); statistical inference was then made at p < 0.05, adjusted to provide a family-wise error (FWE) correction at the peak and cluster levels.
2.4. ROI analysis
On the basis of the previous evidence, we defined 4 specific regions of interest (ROIs) to test for differences in GMV between our two ARMS groups: the bilateral hippocampus, (associated with greater risk of depressive disorder; Amico et al., 2011; Vasconcelos et al., 2011), the anterior cingulate gyrus (reported to be reduced during ongoing depression; Amico et al., 2011; Koolschijn et al., 2009) and in co-morbid depression in ARMS (Modinos et al., 2014); the amygdala (inconsistent changes in depression; Koolschijn et al., 2009) and the precuneus (associated with increased gray matter density after short antidepressant application in HC; Kraus et al., 2014).
All the regions were individually defined using the Wake Forest University PickAtlas Toolbox (http://fmri.wfubmc.edu/software/PickAtlas). For each region, a small volume correction was conducted using a 5 mm radius for the hippocampus (Amico et al., 2011; Vasconcelos et al., 2011) and amygdala or a 10 mm radius for the precuneus and anterior cingulate cortex (ACC) (Abutalebi et al., 2012; Amico et al., 2011).
Mean gray matter volume indices were extracted from these regions using the Rex Toolbox (http://web.mit.edu/swg/software.htm) implemented in Matlab 7.11. The analysis was performed on region of interest basis, with no conjunction mask, no scaling and extraction of the mean within the predefined ROI. The extracted values were used for a stepwise backward regression analysis (see Supplementary table). Only corrected family-wise error values were taken into consideration, in order to avoid a type I error.
Mean gray matter volume indices were extracted from these regions using the Rex Toolbox (http://web.mit.edu/swg/software.htm) implemented in Matlab 7.11. The analysis was performed on region of interest basis, with no conjunction mask, no scaling and extraction of the mean within the predefined ROI. The extracted values were used for a stepwise backward regression analysis (see Supplementary table). Only corrected family-wise error values were taken into consideration, in order to avoid a type I error.
2.5. Statistical analysis of clinical variables
Clinical and socio-demographic differences were assessed using one-way ANOVA and χ2-test. For post hoc analysis, the Bonferroni correction was conducted. In addition, a stepwise regression analysis with backward elimination was applied, to restrict correlating variables. We included the BPRS total score and SANS total score; as well as the BPRS clusters for ‘negative symptoms’ as a sum of blunted affect, emotional withdrawal, and motor retardation (BPRS16, BPRS17 and BPRS18); and ‘mood disturbance’ as a sum of anxiety, depression, suicidality and guilt (BPRS02, BPRS03, BPRS04 and BPRS05); and the single scores ‘depression’ (BPRS 3) and ‘motor hyperactivity’ (BPRS 23) (see Supplementary table). We applied outlier detection using Cook's Distance Test and no subject had to be excluded from regression analysis. Still, we excluded one ARMS-nonAD individual due to missing data in the BPRS 16, 17, and 18 necessary for calculating the cluster ‘negative symptoms’. We then performed a correlation with our significant clinical parameters from the stepwise regression. From the regions of interest with significant differences between ARMS-AD and ARMS-nonAD (hippocampus and precuneus), we extracted the volumes and used them in our in correlation calculations. The data were normally distributed and we performed a series of two-tailed Pearson's correction analyses with statistical threshold set at p < 0.05. All analyses were performed using the Statistical Package for the Social Science (SPSS, Version 22).
Clinical and socio-demographic differences were assessed using one-way ANOVA and χ2-test. For post hoc analysis, the Bonferroni correction was conducted. In addition, a stepwise regression analysis with backward elimination was applied, to restrict correlating variables. We included the BPRS total score and SANS total score; as well as the BPRS clusters for ‘negative symptoms’ as a sum of blunted affect, emotional withdrawal, and motor retardation (BPRS16, BPRS17 and BPRS18); and ‘mood disturbance’ as a sum of anxiety, depression, suicidality and guilt (BPRS02, BPRS03, BPRS04 and BPRS05); and the single scores ‘depression’ (BPRS 3) and ‘motor hyperactivity’ (BPRS 23) (see Supplementary table). We applied outlier detection using Cook's Distance Test and no subject had to be excluded from regression analysis. Still, we excluded one ARMS-nonAD individual due to missing data in the BPRS 16, 17, and 18 necessary for calculating the cluster ‘negative symptoms’. We then performed a correlation with our significant clinical parameters from the stepwise regression. From the regions of interest with significant differences between ARMS-AD and ARMS-nonAD (hippocampus and precuneus), we extracted the volumes and used them in our in correlation calculations. The data were normally distributed and we performed a series of two-tailed Pearson's correction analyses with statistical threshold set at p < 0.05. All analyses were performed using the Statistical Package for the Social Science (SPSS, Version 22).
3. Results
3.1. Clinical and demographic characteristics
The ARMS-AD, ARMS-nonAD and HC showed significant differences in age at MRI scan (p = 0.02), gender (p = 0.01), years of education (p = 0.002), smoking (p = 0.001), and cannabis (p = 0.006); but no differences in alcohol consumption or handedness. Post hoc analysis showed that smoking (p = 0.006) and cannabis consumption (p = 0.014) were significantly more common in the ARMS-AD group than in the ARMS-nonAD group (Table 1).
We observed significant clinical differences between ARMS-AD, ARMS-nonAD and HC in the total BPRS score (p < 0.0001), total SANS score (p < 0.0001), GAF total score (p < 0.0001), BPRS cluster for ‘negative symptoms’ (p < 0.0001), BPRS ‘mood disturbance’ (p < 0.0001), BPRS ‘depression’ score (p < 0.0001), and BPRS ‘motor hyperactivity’ score (p = 0.005). Post hoc analysis showed that the ARMS-AD had a higher BPRS ‘depression’ score (p = 0.011) and less ‘motor hyperactivity’ (p = 0.029) than the ARMS-nonAD (Table 1). The test of our a priori defined contrast in attenuated negative symptoms (BPRS cluster for ‘negative symptoms’) between ARMS-nonAD and ARMS-AD found no significant difference (p = 0.220).
3.2. Whole brain analysis
Compared to the HC, there was significantly less GMV in the middle frontal gyrus in the whole ARMS cohort, and in the superior frontal gyrus in the ARMS-AD group (puncorr. < 0.05, Table 2).
Table 2.
Between group differences in gray matter volume.
| Contrast | Region | p value | KE/radius | z value | x | y | z |
|---|---|---|---|---|---|---|---|
| 1. Whole brain analysis | |||||||
| p uncorr. | |||||||
| ARMS < HC | Middle frontal gyrus | 0.035 | 790 | 3.59 | −33 | 57 | 16 |
| ARMS-AD < HC | Superior frontal gyrus | 0.028 | 874 | 3.98 | −33 | 57 | 18 |
| ARMS-nonAD < ARMS-AD | Left superior parietal lobe | 0.023 | 944 | 4.33 | −32 | −40 | 45 |
| 2. Region of interest | |||||||
| p FWE-corr* | |||||||
| ARMS-AD < ARMS-nonAD | Left hippocampus | 0.029 | 5 mm | 2.86 | −18 | −19 | −17 |
| ARMS-AD < ARMS-nonAD | Right precuneus | 0.042 | 10 mm | 3.23 | 10 | −78 | 51 |
The data presented here are from ANOVA of 3 included groups (ARMS-AD, ARMS-nonAD, HC) at a threshold of p < 0.005 uncorrected across the whole brain.
There were no significant differences in the following contrasts: ARMS > HC, ARMS-AD > HC, ARMS-nonAD vs. HC, and ARMS-nonAD > ARMS-AD.
Abbreviations: ARMS, at risk mental state; ARMS-AD, ARMS subjects currently receiving antidepressants; ARMS-nonAD, ARMS individuals without current antidepressant medication; HC, healthy controls; KE, voxels per cluster; x y z, coordinates according to the Montreal Neurological Institute.
Abbreviations: ARMS — at-risk mental state; ARMS-AD — ARMS with antidepressants; ARMS-nonAD — ARMS without antidepressants; KE — voxels per cluster.
p < 0.0125 required.
The ARMS-nonAD group showed reduced GMV in the left superior parietal lobe compared with the ARMS-AD group (puncorr. < 0.05, Table 2).
3.3. Region of interest analyses
The ARMS-AD group had less GMV in the left hippocampus (Fig. 1) and right precuneus than the ARMS-nonAD (pFWE-corr. < 0.05 after small volume correction, Table 2). However, these results did not survive correction for multiple comparisons (p < 0.0125). No significant differences were found for the ACC and amygdala.
Fig. 1.
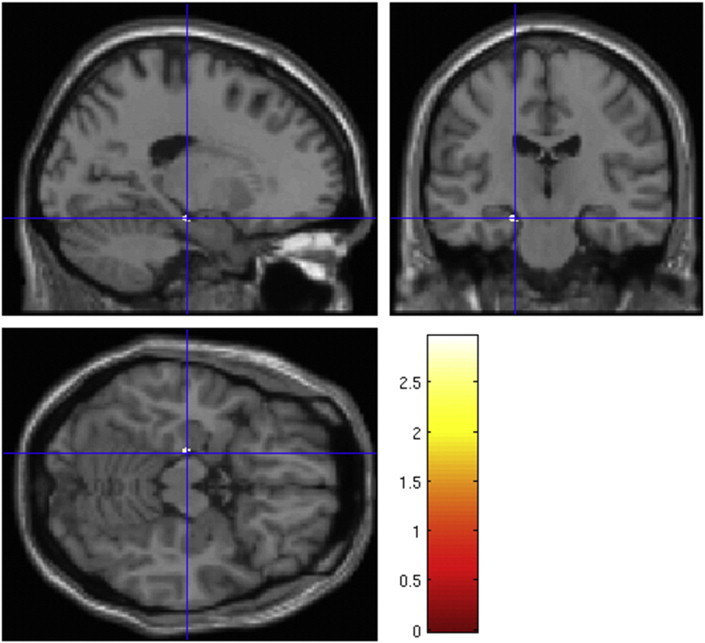
Reductions in gray matter volume are associated with more severe depression. ARMS-AD individuals show less gray matter volume in the left hippocampus than ARMS-nonAD.
3.4. Correlation between ROI volumes and clinical parameters
We found a negative correlation between the hippocampal volume and the BPRS ‘negative symptoms’ cluster, both in ARMS subjects (r = −0.314, p = 0.030, Fig. 2) and in all our subjects, including the HC (r = −0.293, p = 0.013).
Fig. 2.
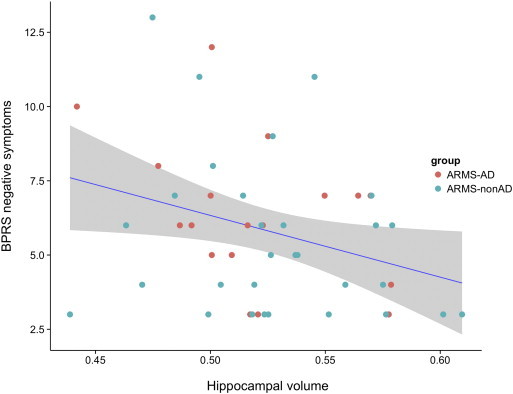
Attenuated negative psychotic symptoms are associated with hippocampal volume. ‘BPRS negative symptoms’ correlate negatively with the bilateral hippocampus volume in all included ARMS subjects. The shaded area indicates the 95% CI of the fitted regression line. ‘BPRS negative symptoms’ score is a sum of blunted affect, emotional withdrawal, and motor retardation (BPRS16, BPRS17 and BPRS18). Hippocampal volume was extracted as mean gray matter volume in arbitrary units using Rex Toolbox.
There was no significant correlation between the BPRS ‘negative symptoms’ and the precuneus volume.
4. Discussion
In the present study, we addressed for the first time the effect on psychosis of antidepressant treatment and attenuated negative psychotic symptoms in ARMS individuals.
Firstly, we have found no evidence for our first hypothesis, that ARMS individuals suffer more pronounced attenuated negative psychotic symptoms if they have not been treated with antidepressants.
We found that ARMS-AD individuals had a higher depression score, lower motor hyperactivity and smoked more cigarettes and marijuana than the ARMS-nonAD individuals. The antidepressants that ARMS-AD individuals were receiving differed in their mode of action – some inhibited the reuptake of serotonin and/or noradrenaline, while others enhanced the release of these monoamines (Andrade and Rao, 2010). Moreover, the antidepressants may take weeks or longer to take effect after dosage (Penn and Tracy, 2012). The duration of antidepressant therapy in the ARMS-AD group varies from 4 to 170 days and thus the observed effect could be indicative of both the predominant depressive and/or attenuated negative psychotic symptoms or of the already developed antidepressant effect of the medication. Hence, we cannot clearly distinguish the extent to which each of the two components contributes to the current clinical state.
Secondly, we did not find increased GMV in the regions associated with depressive symptoms in those ARMS who were receiving antidepressants, compared to those without this medication. Thus we could not confirm our second hypothesis, but found GMV deficits in the hippocampus and precuneus only in the ARMS individuals currently receiving antidepressant medication.
Our third hypothesis is related to the volumetric abnormalities in ARMS and their association with the attenuated negative symptoms; we confirmed this relationship in the hippocampus. We found a clear negative correlation between the bilateral hippocampal volume and attenuated negative symptoms in all ARMS individuals.
This corresponds to studies linking the hippocampus with psychosis (Jun et al., 2012). Previous studies similarly either found negative correlations between the left hippocampal volume and negative symptoms in schizophrenics (Rajarethinam et al., 2001), or at least a strong trend in this direction (Brambilla et al., 2013). These findings underline the role of the hippocampus in the pathophysiology of schizophrenia and suggest specific associations between individual structures and both the positive and negative symptoms of the illness (Kühn et al., 2012; Rajarethinam et al., 2001). Thus, our findings support the importance of hippocampal structures as a region of interest in the early stage of psychosis (Benetti et al., 2009; Fusar-Poli et al., 2012; Walter et al., 2012).
Our ROI analysis demonstrated smaller GMV in the left hippocampus in the ARMS-AD group than in the ARMS-nonAD group. The hippocampus is involved in various psychiatric conditions, including major depression and psychosis (Videbech and Ravnkilde, 2004; Walter et al., 2012). Three meta-analyses have confirmed significant reductions in the hippocampal volume in depression (Campbell et al., 2004; Cole et al., 2011; Videbech and Ravnkilde, 2004). Furthermore, the total number of depressive episodes was significantly correlated to the reduction in the right hippocampal volume (Videbech and Ravnkilde, 2004). The left-hemispheric deficits in hippocampal volume may reflect brain degeneration, as a consequence of chronic stress (Schmidt and Duman, 2010). Recent data on antipsychotic-free ARMS have confirmed that vulnerability to psychosis may be associated with a significant decrease in hippocampal volume (Fusar-Poli et al., 2012; Wood et al., 2010).
In the right precuneus, we found less GMV in the ARMS-AD group than in the ARMS-nonAD group. This is consistent with Grieve et al. (Grieve et al., 2013), who found significant reductions in the precuneus volumes, along with several other structural changes in depression. However, the direction of the effect is controversial. For example, a positive association was described between the volume of the precuneus and the severity of depression (Kroes et al., 2011). It is well established that intrusive imagery and increased self-focus, common in patients suffering from depression, are regularly associated with higher depression scores (Kroes et al., 2011). Since the precuneus is involved in visuospatial processing, imagery and self-related processing (Kjaer et al., 2002; Wenderoth et al., 2005), depression could in principle enhance its GMV.
We acknowledge the limitations of a cross-sectional design, which precludes studying clinical and structural abnormalities within the same group of ARMS before and after antidepressant medication. In order to decide whether brain volumetric deficits are related to distinct depressive symptoms or to the antidepressant effect, longitudinal study designs are needed. Secondly, other confounders, such as nicotine and cannabis consumption, may have influenced our findings (higher consumption in ARMS-AD individuals). Furthermore, the ARMS-nonAD group shows more motor hyperactivity than the ARMS-AD group. This could result from the early effect of the antidepressant medication or from the sedative effect of cannabis or nicotine self-medication, which was higher in the ARMS-AD group (Warburton, 1985). Cigarette use may serve as an instrument to alleviate depressive symptoms, although the role of cannabis consumption is unclear. We can only speculate that the attenuated negative symptoms may drive cannabis consumption, although this abuse may exacerbate the positive symptoms observed (Gill et al., 2013). We are also aware that not all negative symptoms are of hippocampal origin. In addition, the prescribed antidepressant drugs have different affinities to various synaptic receptors and therefore their effects on macroscopic structures and neurogenesis may vary and also be associated with other brain regions. Likewise, differences in the duration of antidepressant therapy may affect their impact on brain structure. Finally, relatively small sample groups are included, which reduces the statistical power to detect any significant effects.
5. Conclusion
Hippocampal volume was negatively associated with attenuated negative psychotic symptoms in ARMS individuals. Surprisingly, ARMS individuals without antidepressant medication did not suffer more pronounced attenuated negative psychotic symptoms. The short-term antidepressant medication in this study is more likely to be an indicator of a more serious depressed state than to have a direct effect on attenuated negative psychotic symptoms. These findings emphasize the importance of comorbidity issues, especially in the context of depressive and attenuated negative psychotic symptoms in clinical high-risk individuals and their functional outcomes.
The following are the supplementary data related to this article.
Stepwise regression analysis with backward elimination.
Supplementary material for this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.04.016.
References
- Abutalebi J., Della Rosa P.A., Green D.W., Hernandez M., Scifo P., Keim R., Cappa S.F., Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb. Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. 22038906 [DOI] [PubMed] [Google Scholar]
- Amico F., Meisenzahl E., Koutsouleris N., Reiser M., Möller H.J., Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J. Psychiatry Neurosci. 2011;36(1):15–22. doi: 10.1503/jpn.090186. 20964952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Zunszain P.A., Carvalho L.A., Pariante C.M. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. 20399565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C., Rao N.S. How antidepressant drugs act: a primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J. Psychiatry. 2010;52(4):378–386. doi: 10.4103/0019-5545.74318. 21267376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989:49–58. 2695141 [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- Baynes D., Mulholland C., Cooper S.J., Montgomery R.C., MacFlynn G., Lynch G., Kelly C., King D.J. Depressive symptoms in stable chronic schizophrenia: prevalence and relationship to psychopathology and treatment. Schizophr. Res. 2000;45(1–2):47–56. doi: 10.1016/s0920-9964(99)00205-4. 10978872 [DOI] [PubMed] [Google Scholar]
- Benetti S., Mechelli A., Picchioni M., Broome M., Williams S., McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132(9):2426–2436. doi: 10.1093/brain/awp098. 19420091 [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., Radue E.-W., Götz K., Aston J., Drewe M., Gschwandtner U., Haller S., Pflüger M., Stieglitz R.-D., McGuire P.K., Riecher-Rössler A. Radiological findings in individuals at high risk of psychosis. J. Neurol. Neurosurg. Psychiatry. 2006;77(2):229–233. doi: 10.1136/jnnp.2005.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt S.J., McGuire P.K., Aston J., Berger G., Dazzan P., Gschwandtner U., Pflüger M., D'Souza M., Radue E.W., Riecher-Rössler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br. J. Psychiatry Suppl. 2007;51:s69–s75. doi: 10.1192/bjp.191.51.s69. 18055941 [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., McGuire P.K., Aston J., Gschwandtner U., Pflüger M.O., Stieglitz R.D., Radue E.W., Riecher-Rössler A. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr. Res. 2008;106(2–3):108–114. doi: 10.1016/j.schres.2008.08.007. 18789654 [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., Riecher-Rössler A., Dazzan P., Chitnis X., Aston J., Drewe M., Gschwandtner U., Haller S., Pflüger M., Rechsteiner E., D'Souza M., Stieglitz R.D., Radü E.W., McGuire P.K. Regional gray matter volume abnormalities in the at risk mental state. Biol. Psychiatry. 2007;61(10):1148–1156. doi: 10.1016/j.biopsych.2006.08.009. 17098213 [DOI] [PubMed] [Google Scholar]
- Brambilla P., Perlini C., Rajagopalan P., Saharan P., Rambaldelli G., Bellani M., Dusi N., Cerini R., Pozzi Mucelli R., Tansella M., Thompson P.M. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br. J. Psychiatry. 2013;202(1):50–55. doi: 10.1192/bjp.bp.111.105700. 23284150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., Marriott M., Nahmias C., MacQueen G.M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. 15056502 [DOI] [PubMed] [Google Scholar]
- Cole J., Costafreda S.G., McGuffin P., Fu C.H. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011;134(1–3):483–487. doi: 10.1016/j.jad.2011.05.057. 21745692 [DOI] [PubMed] [Google Scholar]
- Cornblatt B.A., Lencz T., Smith C.W., Olsen R., Auther A.M., Nakayama E., Lesser M.L., Tai J.Y., Shah M.R., Foley C.A., Kane J.M., Correll C.U. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J. Clin. Psychiatry. 2007;68(4):546–557. doi: 10.4088/jcp.v68n0410. 17474810 [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. 16530430 [DOI] [PubMed] [Google Scholar]
- Endicott J., Spitzer R.L., Fleiss J.L., Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. 938196 [DOI] [PubMed] [Google Scholar]
- Fornito A., Yung A.R., Wood S.J., Phillips L.J., Nelson B., Cotton S., Velakoulis D., McGorry P.D., Pantelis C., Yücel M. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry. 2008;64(9):758–765. doi: 10.1016/j.biopsych.2008.05.032. 18639238 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Bechdolf A., Addington J., Riecher-Rössler A., Schultze-Lutter F., Keshavan M., Wood S., Ruhrmann S., Seidman L.J., Valmaggia L., Cannon T., Velthorst E., De Haan L., Cornblatt B., Bonoldi I., Birchwood M., McGlashan T., Carpenter W., McGorry P., Klosterkötter J., McGuire P., Yung A. The psychosis high-risk state: a comprehensive state-of-the-art review. J.A.M.A. Psychiatry. 2013;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. 23165428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Nelson B., Valmaggia L., Yung A.R., McGuire P.K. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr. Bull. 2014;40(1):120–131. doi: 10.1093/schbul/sbs136. 23180756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Papanastasiou E., Stahl D., Rocchetti M., Carpenter W., Shergill S., McGuire P. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull. 2014 doi: 10.1093/schbul/sbu170. 25528757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Radua J., McGuire P., Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr. Bull. 2012;38(6):1297–1307. doi: 10.1093/schbul/sbr134. 22080494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Valmaggia L., McGuire P. Can antidepressants prevent psychosis? Lancet. 2007;370(9601):1746–1748. doi: 10.1016/S0140-6736(07)61732-2. 18037073 [DOI] [PubMed] [Google Scholar]
- Gill K.E., Poe L., Azimov N., Ben-David S., Vadhan N.P., Girgis R., Moore H., Cressman V., Corcoran C.M. Reasons for cannabis use among youths at ultra high risk for psychosis. Early Interv. Psychiatry. 2013 doi: 10.1111/eip.12112. 24274357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Koslow S.H., Gordon E., Williams L.M. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. 24273717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H., Mohammed Qasim Hussaini S.M., Rigby M.J., Jang M.H. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012;2012:854285. doi: 10.1155/2012/854285. 23346419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T.W., Nowak M., Lou H.C. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17(2):1080–1086. 12377180 [PubMed] [Google Scholar]
- Koolschijn P.C., van Haren N.E., Lensvelt-Mulders G.J., Hulshoff Pol H.E., Kahn R.S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. 19441021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Schmitt G.J., Gaser C., Bottlender R., Scheuerecker J., McGuire P., Burgermeister B., Born C., Reiser M., Möller H.J., Meisenzahl E.M. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br. J. Psychiatry. 2009;195(3):218–226. doi: 10.1192/bjp.bp.108.052068. 19721111 [DOI] [PubMed] [Google Scholar]
- Kraus C., Ganger S., Losak J., Hahn A., Savli M., Kranz G.S., Baldinger P., Windischberger C., Kasper S., Lanzenberger R. Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. Neuroimage. 2014;84:236–244. doi: 10.1016/j.neuroimage.2013.08.036. 23988273 [DOI] [PubMed] [Google Scholar]
- Kroes M.C., Rugg M.D., Whalley M.G., Brewin C.R. Structural brain abnormalities common to posttraumatic stress disorder and depression. J. Psychiatry Neurosci. 2011;36(4):256–265. doi: 10.1503/jpn.100077. 21418787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Musso F., Mobascher A., Warbrick T., Winterer G., Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl. Psychiatry. 2012;2:e127. doi: 10.1038/tp.2012.51. 22692142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S., Triebig G., Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol. Scand. 1995;91(5):335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. 7639062 [DOI] [PubMed] [Google Scholar]
- Lorenzetti V., Allen N.B., Fornito A., Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 2009;117(1–2):1–17. doi: 10.1016/j.jad.2008.11.021. 19237202 [DOI] [PubMed] [Google Scholar]
- Lukoff D., Liberman R.P., Nuechterlein K.H. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophr. Bull. 1986;12(4):578–602. doi: 10.1093/schbul/12.4.578. 3810065 [DOI] [PubMed] [Google Scholar]
- Macare C., Bates T.C., Heath A.C., Martin N.G., Ettinger U. Substantial genetic overlap between schizotypy and neuroticism: a twin study. Behav. Genet. 2012;42(5):732–742. doi: 10.1007/s10519-012-9558-6. 22955548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N.V., Carter R., Seres P., Coupland N.J. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J. Psychiatry Neurosci. 2010;35(5):337–343. doi: 10.1503/jpn.100002. 20731966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Riecher-Rössler A., Meisenzahl E.M., Tognin S., Wood S.J., Borgwardt S.J., Koutsouleris N., Yung A.R., Stone J.M., Phillips L.J., McGorry P.D., Valli I., Velakoulis D., Woolley J., Pantelis C., McGuire P. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch. Gen. Psychiatry. 2011;68(5):489–495. doi: 10.1001/archgenpsychiatry.2011.42. 21536978 [DOI] [PubMed] [Google Scholar]
- Modinos G., Allen P., Frascarelli M., Tognin S., Valmaggia L., Xenaki L., Keedwell P., Broome M., Valli I., Woolley J., Stone J.M., Mechelli A., Phillips M.L., McGuire P., Fusar-Poli P. Are we really mapping psychosis risk? Neuroanatomical signature of affective disorders in subjects at ultra high risk. Psychol. Med. 2014;44(16):3491–3501. doi: 10.1017/S0033291714000865. 25066827 [DOI] [PubMed] [Google Scholar]
- Penn E., Tracy D.K. The drugs don't work? Antidepressants and the current and future pharmacological management of depression. Ther. Adv. Psychopharmacol. 2012;2(5):179–188. doi: 10.1177/2045125312445469. 23983973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse J.C., Giedd J.N., Rapoport J.L. Statistical approach to segmentation of single-channel cerebral MR images. I. E.E.E. Trans. Med. Imaging. 1997;16(2):176–186. doi: 10.1109/42.563663. 9101327 [DOI] [PubMed] [Google Scholar]
- Rajarethinam R., DeQuardo J.R., Miedler J., Arndt S., Kirbat R., Brunberg J.A., Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. 2001;108(2):79–87. doi: 10.1016/s0925-4927(01)00120-2. 11738542 [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Aston J., Ventura J., Merlo M., Borgwardt S., Gschwandtner U., Stieglitz R.D. The Basel Screening Instrument for Psychosis (BSIP): development, structure, reliability and validity. Fortschr. Neurol. Psychiatr. 2008;76(4):207–216. doi: 10.1055/s-2008-1038155. 18393134 [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Gschwandtner U., Borgwardt S., Aston J., Pflüger M., Rössler W. Early detection and treatment of schizophrenia: how early? Acta Psychiatr. Scand. Suppl. 2006:73–80. doi: 10.1111/j.1600-0447.2005.00722.x. 16445487 [DOI] [PubMed] [Google Scholar]
- Salokangas R.K., Ruhrmann S., von Reventlow H.G., Heinimaa M., Svirskis T., From T., Luutonen S., Juckel G., Linszen D., Dingemans P., Birchwood M., Patterson P., Schultze-Lutter F., Klosterkötter J., EPOS group Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr. Res. 2012;138(2–3):192–197. doi: 10.1016/j.schres.2012.03.008. 22464922 [DOI] [PubMed] [Google Scholar]
- Schmidt H.D., Duman R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. 20686454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Sterr A. Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. J. Magn. Reson. Imaging. 2013;37(6):1468–1475. doi: 10.1002/jmri.23927. 23172789 [DOI] [PubMed] [Google Scholar]
- Smieskova R., Allen P., Simon A., Aston J., Bendfeldt K., Drewe J., Gruber K., Gschwandtner U., Klarhoefer M., Lenz C., Scheffler K., Stieglitz R.D., Radue E.W., McGuire P., Riecher-Rössler A., Borgwardt S.J. Different duration of at-risk mental state associated with neurofunctional abnormalities. A multimodal imaging study. Hum. Brain Mapp. 2012;33(10):2281–2294. doi: 10.1002/hbm.21360. 21922599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieskova R., Fusar-Poli P., Allen P., Bendfeldt K., Stieglitz R.D., Drewe J., Radue E.W., McGuire P.K., Riecher-Rössler A., Borgwardt S.J. Neuroimaging predictors of transition to psychosis — a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2010;34(8):1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. 20144653 [DOI] [PubMed] [Google Scholar]
- Smieskova R., Fusar-Poli P., Aston J., Simon A., Bendfeldt K., Lenz C., Stieglitz R.D., McGuire P., Riecher-Rössler A., Borgwardt S.J. Insular volume abnormalities associated with different transition probabilities to psychosis. Psychol. Med. 2012;42(8):1613–1625. doi: 10.1017/S0033291711002716. 22126702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Wood S.J., Yung A.R., Soulsby B., McGorry P.D., Suzuki M., Kawasaki Y., Phillips L.J., Velakoulis D., Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66(4):366–376. doi: 10.1001/archgenpsychiatry.2009.12. 19349306 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Wood S.J., Yung A.R., Walterfang M., Phillips L.J., Soulsby B., Kawasaki Y., McGorry P.D., Suzuki M., Velakoulis D., Pantelis C. Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. Br. J. Psychiatry. 2010;196(3):206–211. doi: 10.1192/bjp.bp.109.069732. 20194543 [DOI] [PubMed] [Google Scholar]
- Thomas A., Donnell A.J., Young T.R. Factor structure and differential validity of the expanded Brief Psychiatric Rating Scale. Assessment. 2004;11(2):177–187. doi: 10.1177/1073191103262893. 15171466 [DOI] [PubMed] [Google Scholar]
- Tognin S., Riecher-Rössler A., Meisenzahl E.M., Wood S.J., Hutton C., Borgwardt S.J., Koutsouleris N., Yung A.R., Allen P., Phillips L.J., McGorry P.D., Valli I., Velakoulis D., Nelson B., Woolley J., Pantelis C., McGuire P., Mechelli A. Reduced parahippocampal cortical thickness in subjects at ultra-high risk for psychosis. Psychol. Med. 2014;44(3):489–498. doi: 10.1017/S0033291713000998. 23659473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos L.de G., Jackowski A.P., Oliveira M.O., Flor Y.M., Bueno O.F., Brucki S.M. Voxel-based morphometry findings in Alzheimer's disease: neuropsychiatric symptoms and disability correlations — preliminary results. Clinics (Sao Paulo) 2011;66(6):1045–1050. doi: 10.1590/S1807-59322011000600021. 21808873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan D., Prihoda T., Dennehy E., Biggs M., Shores-Wilson K., Crismon M.L., Rush A.J., Miller A., Suppes T., Trivedi M., Kashner T.M., Witte B., Toprac M., Carmody T., Chiles J., Shon S. Brief Psychiatric Rating Scale expanded version: how do new items affect factor structure? Psychiatry Res. 2005;135(3):217–228. doi: 10.1016/j.psychres.2005.05.001. 15993949 [DOI] [PubMed] [Google Scholar]
- Videbech P., Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. 15514393 [DOI] [PubMed] [Google Scholar]
- Walter A., Studerus E., Smieskova R., Kuster P., Aston J., Lang U.E., Radue E.W., Riecher-Rössler A., Borgwardt S. Hippocampal volume in subjects at high risk of psychosis: a longitudinal MRI study. Schizophr. Res. 2012;142(1–3):217–222. doi: 10.1016/j.schres.2012.10.013. 23123134 [DOI] [PubMed] [Google Scholar]
- Warburton D.M. Nicotine and the smoker. Rev. Environ. Health. 1985;5(4):343–390. 3916378 [PubMed] [Google Scholar]
- Wenderoth N., Debaere F., Sunaert S., Swinnen S.P. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosci. 2005;22(1):235–246. doi: 10.1111/j.1460-9568.2005.04176.x. 16029213 [DOI] [PubMed] [Google Scholar]
- Wood S.J., Kennedy D., Phillips L.J., Seal M.L., Yücel M., Nelson B., Yung A.R., Jackson G., McGorry P.D., Velakoulis D., Pantelis C. Hippocampal pathology in individuals at ultra-high risk for psychosis: a multi-modal magnetic resonance study. Neuroimage. 2010;52(1):62–68. doi: 10.1016/j.neuroimage.2010.04.012. 20399273 [DOI] [PubMed] [Google Scholar]
- Wood S.J., Yung A.R., McGorry P.D., Pantelis C. Neuroimaging and treatment evidence for clinical staging in psychotic disorders: from the at-risk mental state to chronic schizophrenia. Biol. Psychiatry. 2011;70(7):619–625. doi: 10.1016/j.biopsych.2011.05.034. 21762875 [DOI] [PubMed] [Google Scholar]
- Yung A.R., Phillips L.J., McGorry P.D., McFarlane C.A., Francey S., Harrigan S., Patton G.C., Jackson H.J. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br. J. Psychiatry Suppl. 1998;172(33):14–20. 9764121 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stepwise regression analysis with backward elimination.


