Abstract
Introdcution
Although nipple sparing mastectomy (NSM) has attracted increased recognition as an alternative to traditional mastectomy approaches, its oncological safety is unclear. The purpose of this study was to compare the local recurrence rate between NSM and total mastectomy (TM).
Methods
Between 2003 and 2013, 121 and 557 patients with stage 0–III breast cancer underwent NSM and TM respectively. Multivariate Cox regression and propensity score models were used to compare the two groups.
Results
There was no significant difference in the five-year local recurrence rate between the NSM and TM groups (7.6% vs 4.9%, p=0.398). In multivariate analysis, NSM was not a risk factor for local recurrence (hazard ratio: 1.653, 95% confidence interval: 0.586–4.663, p=0.343). Propensity score matching found similar five-year local recurrence free survival rates between the two groups (92.3% vs 93.7%, p=0.655).
Conclusions
Our results suggest that NSM may provide oncological safety comparable with mastectomy for carefully selected patients.
Keywords: Breast cancer, Nipple sparing mastectomy, Propensity score matching
Since the advent of Halsted’s radical mastectomy in the 1800s, there have been significant advances in breast cancer surgery concerning oncological safety and cosmetic outcome. The modified radical mastectomy commonly performed today was first described by Madden in 1965. 1 The local recurrence rate after mastectomy was roughly 10% at 10 years. 2–4 Toth and Lappert first described skin sparing mastectomy (SSM) in 1991. 5 SSM removes the entire breast and nipple–areola complex (NAC) while preserving the skin envelope and the natural inframammary fold. A meta-analysis revealed that the local recurrence rates after SSM are equivalent to those after modified radical mastectomy. 6
Surgery for breast cancer has traditionally included resection of the NAC over concerns that this area may harbour occult tumour cells. Large trials have reported NAC involvement in 5–12% of cases. 7,8 As early as 1984, Hinton et al reported that nipple sparing mastectomy (NSM) achieved local recurrence and survival rates equivalent to those for modified radical mastectomy. 9 Nevertheless, prominent surgeons concluded that NSM may carry an unacceptably high risk for local recurrence and that this procedure should therefore not be advocated. 10,11
The controversy over the safety of NSM may be similar to the early controversy over the safety of breast conserving surgery. In 2002 Fisher et al reported 20-year follow-up data comparing lumpectomy, lumpectomy followed by radiation and mastectomy. 12 They included two treatment arms in which the nipple was preserved and one treatment arm in which the nipple was removed. The fact that the prospective randomised trial identified no treatment related difference in long-term survival is evidence that removal of the nipple is not associated with a survival advantage in the initial treatment of breast cancer.
In observational studies, treatment selection is often influenced by subject characteristics and baseline characteristics of treated subjects often differ systematically from those of untreated subjects. These differences in baseline characteristics between treated and untreated subjects must therefore be taken into account when estimating the effect of treatment on outcomes. As a result, there has been increasing interest in methods based on propensity score matching to reduce the effects of confounding variables when using observational data.
Although its oncological safety is still controversial, NSM has attracted increased recognition as an alternative to traditional mastectomy approaches. In this study, we investigated the local recurrence rate after NSM, comparing it with that of total mastectomy (TM) using propensity score matching.
Methods
A prospective database of stage 0–III breast cancer patients who received NSM or TM between 1 January 2003 and 30 October 2013 at Keio University Hospital was analysed retrospectively. Patients without indication for breast conserving surgery by preoperative imaging including magnetic resonance imaging (MRI), ultrasonography and mammography were eligible for NSM. Those with suspicion of tumour involvement in the NAC or skin according to preoperative imaging were ineligible for NSM. Patients who received neoadjuvant therapy were included in the study.
Frozen section analysis of subareolar tissue was performed during the surgery. Appropriate adjuvant therapy was administered based on the postoperative pathological examination.
Statistical analysis
The t-test and chi-squared test were used for continuous and categorical data respectively. Survival was calculated from the time of surgical resection of the primary tumour to the last follow-up visit or to the death of the patient. Local recurrence was defined as tumour relapse in the ipsilateral nipple, skin flap or chest wall. The probabilities of overall survival and of recurrence free survival were estimated using the Kaplan–Meier method. The logrank test was used to compare survival between the groups.
Propensity score matching was used to reduce selection bias. Binary logistic regression was used to calculate a propensity score for each patient who underwent NSM or TM. The covariates entered in the propensity model were age, distance between the tumour and the nipple, clinical tumour stage and multicentricity/multifocality. The discrimination of the propensity model was assessed with calculation of the area under the receiver operating characteristic curve (AUC). After estimating the propensity score, matched pairs were created between patients who underwent NSM and TM using a one-to-one nearest-neighbour caliper matching method, which pairs patients who have the closest propensity scores. A caliper of 1.0 (maximum allowable difference in propensity scores between the two patients being matched) was defined. Only patients whose propensity scores were matched were included in the study.
Subsequently, the balance of all observed covariates, interactions among all covariates and quadratic terms of all covariates were examined. The Kaplan–Meier method was used to compare the prognosis of each group after propensity score matching. The hazard ratio (HR) for local recurrence was examined using the Cox proportional hazards model. Variables found to be statistically significant in univariate analysis were included in multivariate analysis.
Statistical analysis of the data was performed using SPSS® version 20 (IBM, New York, US) and Stata® version 12 (StataCorp, College Station, TX, US). A p-value of <0.05 was considered statistically significant and all tests were two-tailed.
Results
Between January 2003 and October 2013, NSM and TM was performed in 121 and 557 patients respectively. The baseline characteristics of the patients are summarised in Table 1. Significant differences between the NSM and TM groups were observed in terms of age, tumour–nipple distance, multicentricity/multifocality, clinical tumour stage, histological type, pathological tumour size, nuclear grade, hormone receptor status and number of pathologically positive lymph nodes. The proportion of patients who had endocrine therapy and radiotherapy was significantly different between the two groups.
Table 1.
Patient characteristics
| Variable | NSM (n=121) | TM (n=557) | p-value |
| Mean age (years) | 48 (SD: 8.9) | 61 (SD: 12.8) | <0.001 |
| Mean tumour–nipple distance (cm) | 2.3 (SD: 1.6) | 1.5 (SD: 1.7) | <0.001 |
| Preoperative imaging | <0.001 | ||
| Solitary | 20 (16.5%) | 212 (38.6%) | |
| Multicentric/multifocal | 101 (83.5%) | 337 (60.4%) | |
| T stage | 0.001 | ||
| Tis | 38 (31.4%) | 86 (15.4%) | |
| T1 | 45 (37.1%) | 179 (32.1%) | |
| T2 | 37 (30.6%) | 239 (42.9%) | |
| T3 | 2 (1.7%) | 26 (4.7%) | |
| T4 | 0 (0.0%) | 27 (4.9%) | |
| Clinical nodal status | 0.039 | ||
| Negative | 109 (90.1%) | 444 (79.7%) | |
| Positive | 12 (9.9%) | 114 (20.3%) | |
| Histological type | <0.001 | ||
| Ductal carcinoma in situ | 38 (31.4%) | 86 (15.4%) | |
| Invasive ductal carcinoma | 73 (60.3%) | 418 (75.0%) | |
| Invasive lobular carcinoma | 8 (6.6%) | 19 (3.4%) | |
| Other | 2 (1.7%) | 34 (6.1%) | |
| Mean pathological tumour size (cm) | 1.0 (SD: 1.3) | 1.7 (SD: 1.7) | <0.001 |
| Nuclear grade | 0.004 | ||
| 1 | 51 (42.1%) | 199 (35.7%) | |
| 2 | 15 (12.3%) | 137 (24.6%) | |
| 3 | 14 (11.6%) | 122 (21.9%) | |
| Unknown | 41 (33.9%) | 99 (17.8%) | |
| Lymphovascular invasion | 0.455 | ||
| Positive | 32 (26.5%) | 170 (30.5%) | |
| Negative | 89 (73.5%) | 387 (69.5%) | |
| Hormone receptor status | 0.034 | ||
| Positive | 105 (86.8%) | 436 (78.3%) | |
| Negative | 16 (13.2%) | 121 (21.7%) | |
| HER2 status | 0.088 | ||
| Positive | 15 (12.4%) | 142 (25.5%) | |
| Negative | 66 (54.5%) | 328 (58.9%) | |
| Unknown / not evaluated | 40 (33.1%) | 87 (15.6%) | |
| Number of pathological positive nodes | <0.001 | ||
| 0 | 102 (84.3%) | 353 (63.4%) | |
| 1 | 8 (6.6%) | 76 (13.6%) | |
| Variable | NSM (n=121) | TM (n=557) | p-value |
| 2 | 5 (4.1%) | 38 (6.8%) | |
| 3 | 3 (2.5%) | 22 (3.9%) | |
| 4+ | 3 (2.5%) | 68 (12.2%) | |
| Adjuvant therapy | |||
| Endocrine therapy | 92 (77.3%) | 366 (65.7%) | 0.017 |
| Chemotherapy | 48 (40.3%) | 224 (40.1%) | 0.964 |
| Radiotherapy | 7 (5.9%) | 70 (12.9%) | 0.030 |
NSM = nipple sparing mastectomy; TM = total mastectomy; SD = standard deviation; HER2 = human epidermal growth factor receptor 2
Tumour involvement in the nipple–areola complex
In the NSM group, frozen section analysis during surgery demonstrated tumour involvement of the NAC in five patients (4.1%) and the NAC was removed immediately in all five patients. Permanent section analysis identified another two cases (1.7%) of NAC involvement. Among those with NAC involvement determined after surgery, one patient underwent removal of the NAC one month following primary surgery. The other patient received radiotherapy without removal of the NAC in accordance with the patient’s preference.
Reconstruction
Forty-seven patients (38.8%) in the NSM group underwent two-stage reconstruction with a tissue expander and four (3.3%) underwent immediate autologous reconstruction, including one patient with a deep inferior epigastric perforator flap and three with a latissimus dorsi flap.
Complications
Nipple epidermal necrosis occurred in three patients (2.5%). There were no patients with a haematoma needing surgical intervention or infection in the NSM group.
Oncological outcomes
The median follow-up durations in the NSM and TM groups were 28.0 months (range: 1–115 months) and 43.0 months (range: 1–121 months) respectively. Local recurrence was observed in 5 patients (4.1%) in the NSM group and 13 patients (2.3%) in the TM group. There was no significant difference in the five-year local recurrence rates for the NSM and TM groups (7.6% vs 4.9%, p=0.398) (Fig 1). Among the five patients with local recurrence in the NSM group, NAC and skin flap recurrence was observed in two (1.7%) and three patients (2.5%), respectively (Table 2). In all 5 patients, locally recurrent lesions were excised completely and all of these patients were disease free at a median follow-up period of 20.6 months.
Figure 1.
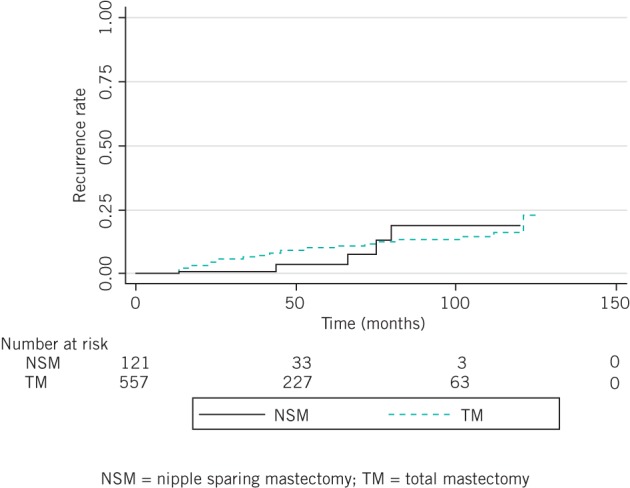
Local recurrence rate
Table 2.
Patients with local recurrence after nipple sparing mastectomy
| Age | TNM classification | Preoperative imaging | Tumour size | Tumour–nipple distance | Histological type | Pathological tumour size | Nuclear grade | Lympho-vascular invasion | Oestrogen receptor | Progesterone receptor | HER2 status | Number of pathologically positive lymph nodes | Adjuvant therapy | Site of recurrence | Duration to recurrence |
| TNM = tumour, lymph nodes, metastasis; HER2 = human epidermal growth factor receptor 2; MC = multicentric; MF = multifocal; IDC = invasive ductal carcinoma; NAC = nipple–areola complex; NE = not evaluated; DCIS = ductal carcinoma in situ | |||||||||||||||
| 58 | T2 N0 M0 | MC/MF | 3.5cm | 1.3cm | IDC | 0.4cm | 2 | - | - | - | - | 0 | Chemotherapy | NAC | 42 mths |
| 64 | T1 N0 M0 | MC/MF | 1.2cm | 3.5cm | IDC | 0.4cm | 2 | - | - | - | + | 0 | Chemotherapy | NAC | 18 mths |
| 46 | Tis N0 M0 | MC/MF | NE | NE | DCIS | 0cm | NE | - | + | + | NE | 0 | No treatment | Skin flap | 42 mths |
| 43 | Tis N0 M0 | MC/MF | NE | NE | DCIS | 0cm | NE | - | + | + | NE | 0 | Endocrine therapy | Skin flap | 65 mths |
| 50 | Tis N0 M0 | MC/MF | NE | NE | DCIS | 0cm | NE | - | + | + | NE | 0 | Endocrine therapy | Skin flap | 18 mths |
Distant metastases occurred in 6 patients (5.0%) in the NSM group and 61 patients (11.0%) in the TM group. There was no significant difference in the five-year distant recurrence rates for the two groups (7.4% vs 3.9%, p=0.235) (Fig 2). Simultaneous local and distant recurrence was not observed in the NSM group whereas it occurred in six cases (1.1%) in the TM group. Five-year overall survival was similar for the two groups (98.4% vs 95.2%, p=0.143) (Fig 3).
Figure 2.
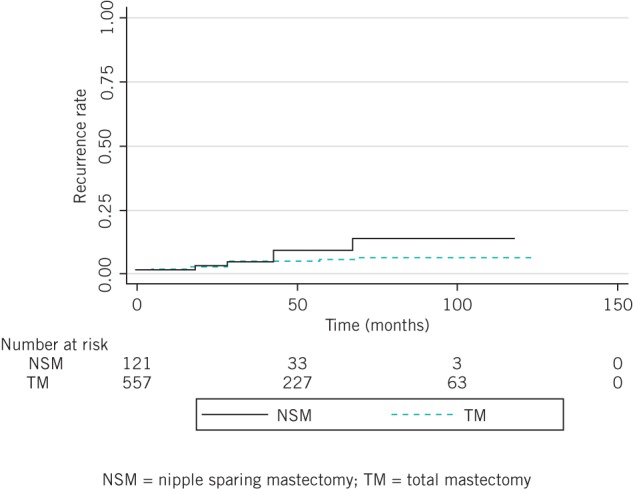
Distant recurrence rate
Figure 3.
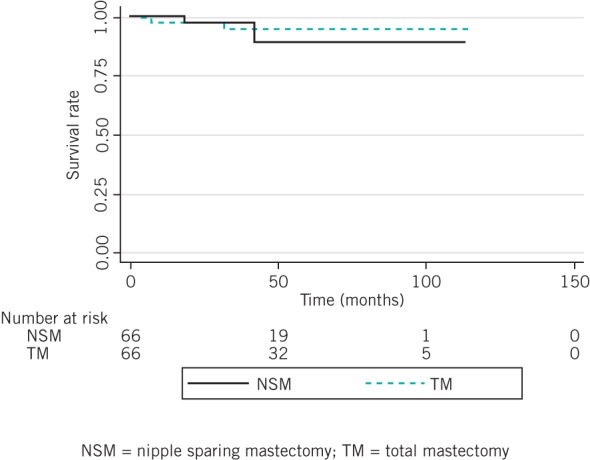
Overall survival
In univariate analysis, the risk factors for local recurrence after NSM and TM were lymphovascular invasion and the number of pathologically positive lymph nodes. The surgical procedure was not a significant risk factor for local recurrence (HR: 1.557, 95% confidence interval [CI]: 0.554–4.381, p=0.401). In multivariate analysis, the only significant risk factor for local recurrence was lymphovascular invasion (HR: 2.995, 95% CI: 1.071–8.375, p=0.037).
After nearest-neighbour matching using the propensity scores, there were 66 cases in each group that could be matched while there were 55 and 491 unmatched cases in the NSM and TM groups respectively. The significant baseline differences between the NSM and TM groups in terms of age, tumour–nipple distance, multicentricity/multifocality and clinical tumour stage disappeared (Table 3). The AUC was 0.853, indicating satisfactory discrimination. The five-year local recurrence free survival was similar for the NSM and TM groups (92.3% vs 93.7%, p=0.655) (Fig 4).
Table 3.
Clinical characteristics after adjustment of propensity scores
| Variable | NSM (n=66) | TM (n=66) | p-value |
| NSM = nipple sparing mastectomy; TM = total mastectomy; SD = standard deviation | |||
| Mean age | 48 yrs (SD: 9.1 yrs) | 46 yrs (SD: 8.7 yrs) | 0.256 |
| Mean tumour–nipple distance | 2.2cm (SD: 1.5cm) | 1.9cm (SD: 1.8cm) | 0.235 |
| Preoperative imaging | 1.000 | ||
| Solitary | 16 (24.2%) | 16 (24.2%) | |
| Multicentric/multifocal | 50 (75.8%) | 50 (75.8%) | |
| T stage | 0.920 | ||
| Tis | 5 (7.6%) | 6 (19.2%) | |
| T1 | 30 (45.5%) | 30 (45.5%) | |
| T2 | 30 (45.5%) | 28 (42.4%) | |
| T3 | 1 (1.4%) | 2 (3.0%) | |
| T4 | 0 (0.0%) | 27 (4.9%) | |
| Clinical nodal status | 0.627 | ||
| Negative | 55 (83.3%) | 57 (86.4%) | |
| Positive | 11 (16.7%) | 9 (13.6%) | |
| Histological type | 0.753 | ||
| Ductal carcinoma in situ | 5 (7.6%) | 6 (9.1%) | |
| Invasive carcinoma | 61 (92.4%) | 60 (90.9%) | |
| Mean pathological tumour size | 1.4cm (SD: 1.2cm) | 1.4cm (SD: 1.2cm) | 1.000 |
| Nuclear grade | 0.592 | ||
| 1/2 | 47 (78.3%) | 43 (74.1%) | |
| 3 | 14 (21.7%) | 15 (25.9%) | |
| Lymphovascular invasion | 0.845 | ||
| Negative | 22 (33.3%) | 23 (34.8%) | |
| Positive | 44 (66.7%) | 43 (65.2%) | |
| Hormone receptor status | 1.000 | ||
| Negative | 56 (84.9%) | 55 (83.3%) | |
| Positive | 10 (15.1%) | 11 (16.7%) | |
| Number of pathological positive nodes | 1.000 | ||
| 0 | 53 (80.3%) | 41 (62.1%) | |
| 1 | 5 (7.6%) | 10 (15.1%) | |
| 2 | 3 (4.5%) | 10 (15.1%) | |
| 3 | 2 (3.0%) | 1 (1.5%) | |
| 4+ | 3 (4.5%) | 4 (6.1%) | |
| Adjuvant therapy | |||
| Endocrine therapy | 35 (53.0%) | 39 (59.1%) | 0.590 |
| Chemotherapy | 51 (77.3%) | 50 (75.8%) | 1.000 |
| Radiotherapy | 6 (9.2%) | 7 (10.8%) | 1.000 |
Figure 4.
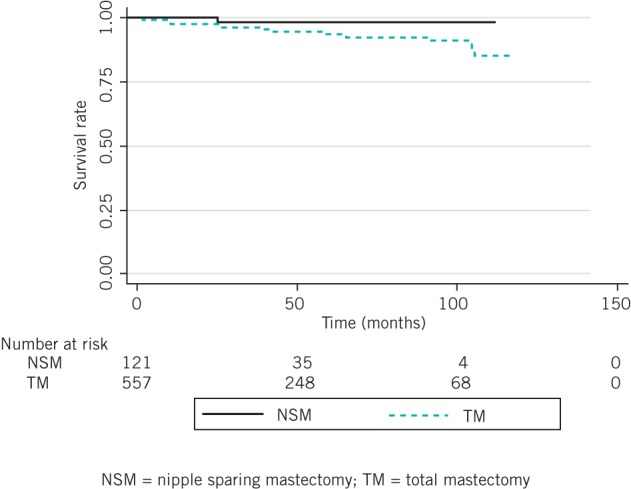
Local recurrence free survival after propensity score adjustment
Discussion
Although NSM has been considered useful for risk reduction or small, peripherally located tumours without multicentricity, use of this technique has recently been extending to larger tumours and cases with locally advanced disease. Much of the reluctance to use NSM arises from concern for potentially higher local recurrence rates of the nipple sparing technique. In our study, local recurrence was observed in five patients in the NSM group (4.1%), including two NAC and three skin flap recurrences, and there was no significant difference in the five-year local recurrence rate between the NSM and TM groups (7.6% vs 4.9%, p=0.398).
Our local recurrence rates were consistent with previous studies. Varying widely in sample size, inclusion criteria and follow-up duration, prospective and retrospective studies have found local recurrence rates of 0–24% after NSM. 13–25 Although Benediktsson and Perbeck reported a relatively high local recurrence rate of 24%, the NAC recurrence rate was 4%. 15 Comparison of NSM, SSM and modified mastectomy revealed local recurrence rates of 10.4%, 11.5% and 11.7% respectively. 17 Petit et al found a local recurrence rate of 5.2% after a median follow-up period of 50 months for 934 patients who underwent NSM. 23 A meta-analysis of 23 studies assessed incidence of local recurrence following NSM. 26 The mean follow-up duration was 38.4 months (range: 7.9–101 months) and the overall incidence of NAC recurrence was 0.9%, compared with 4.2% for of skin flap recurrence.
In our study, locally recurrent lesions were excised completely in all 5 patients with local recurrences and all of them were disease free after a median follow-up period of 20.6 months. Owing to the long natural course of breast cancer, our follow-up duration was not sufficient to allow us to evaluate the prognosis of local recurrence after NSM.
This is the first study to compare the oncological outcome of NSM and TM using propensity score matching. Most previous studies and our study were retrospective, and the findings were subject to selection bias. Propensity score matching was used to minimise this bias. Previous research suggests that propensity score matching eliminates a greater proportion of baseline differences between any two treatment groups than stratification or covariates adjustment. 27
Our one-to-one propensity score matching provided a robust evaluation of NSM for breast cancer. After nearest-neighbour matching using the propensity scores, there were 66 cases in each group that could be matched. The distribution of propensity scores in this cohort was varied. In order to ensure good matches, a caliper of 1.0 was defined. As a result, a relatively large number of cases were eliminated from our study. The two groups were matched for baseline clinicopathological characteristics and there was no significant difference in the local recurrence rate between the groups.
Our study identified lymphovascular invasion as a predictive factor of local recurrence after NSM and TM. Benediktsson and Perbeck found that local recurrence was dependent on the pathological lymph node status and clinical stage. 15 Petit et al reported that number of positive lymph nodes, histological type and Ki-67 index were significant predictive factors of local recurrence in multivariate analysis. 23
Retrospective studies have evaluated the incidence of NAC involvement using sample NAC tissue preserved as mastectomy specimens; the reported rate of NAC involvement in mastectomy specimens ranges from 0% to 58%. 7,8,26 Brachtel et al found that tumour–nipple distance, pathological lymph node status, lymphovascular invasion, human epidermal growth factor receptor 2 status, nuclear grade and multicentricity were correlated with the incidence of occult nipple malignancy, and concluded that a retroareolar en face margin could be used for assessment of NAC involvement in NSM. 8 Although patients in our study had risk factors such as a tumour–nipple distance of <2cm (40.9%) and multicentric/multifocal disease (83.5%), the NAC involvement rate (5.8%) was relatively low compared with previous reports.
In addition to oncological safety, complication rates have been another concern for NSM. A complication common to NSM is nipple necrosis. In our study, nipple necrosis was observed in three patients (2.5%) and no other complications such as haematoma requiring surgical intervention or infection occurred. Large studies have reported an incidence of nipple necrosis ranging from 0.3% to 10%. 13–26
Study limitations
Our study had its limitations. First, this was a retrospective study with a small number of patients so various biases should be considered. Selection bias, especially the tendency to perform NSM for patients with low grade tumours as well as for younger patients, is a particular problem. Despite the fact that propensity score matching was used, it is not possible to eliminate all biases and uncertainty still remains. Unfortunately, a randomised study would be difficult to conduct because of the number of patients and long follow-up duration needed as well as the high financial costs.
Second, the follow-up period of this study was relatively short compared with the long natural course of breast cancer. Longer follow-up might show an increase in the recurrence rate owing to late recurrence in patients with a good prognosis. Moreover, the median duration of follow-up in the NSM group (28.0 months) was significantly shorter than in the TM group (43.0 months). An analysis of multi-institutional studies with longer follow-up periods is needed.
Conclusions
This study demonstrates the usefulness of NSM for carefully selected patients with breast cancer. The complication rates were low and local recurrence rates were comparable with those for total mastectomy. NSM has a positive impact on patient satisfaction and body image, 28 and patients should be counselled about the controversies and complications prior to performing this procedure.
References
- 1. Madden JL. Modified radical mastectomy. Surg Gynecol Obstet 1965; 121: 1,221–1,230. [PubMed] [Google Scholar]
- 2. Fisher B, Anderson S, Redmond CK et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333: 1,456–1,461. [DOI] [PubMed] [Google Scholar]
- 3. Voogd AC, Nielsen M, Peterse JL et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 2001; 19: 1,688–1,697. [DOI] [PubMed] [Google Scholar]
- 4. Clarke M, Collins R, Darby S et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2,087–2,106. [DOI] [PubMed] [Google Scholar]
- 5. Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991; 87: 1,048–1,053. [PubMed] [Google Scholar]
- 6. Lanitis S, Tekkis PP, Sgourakis G et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 2010; 251: 632–639. [DOI] [PubMed] [Google Scholar]
- 7. Laronga C, Kemp B, Johnston D et al. The incidence of occult nipple–areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol 1999; 6: 609–613. [DOI] [PubMed] [Google Scholar]
- 8. Brachtel EF, Rusby JE, Michaelson JS et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 2009; 27: 4,948–4,954. [DOI] [PubMed] [Google Scholar]
- 9. Hinton CP, Doyle PJ, Blamey RW et al. Subcutaneous mastectomy for primary operable breast cancer. Br J Surg 1984; 71: 469–472. [DOI] [PubMed] [Google Scholar]
- 10. Cense HA, Rutgers EJ, Lopes Cardozo M, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol 2001; 27: 521–526. [DOI] [PubMed] [Google Scholar]
- 11. Simmons RM, Brennan M, Christos P et al. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol 2002; 9: 165–168. [DOI] [PubMed] [Google Scholar]
- 12. Fisher B, Anderson S, Bryant J et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1,233–1,241. [DOI] [PubMed] [Google Scholar]
- 13. Margulies AG, Hochberg J, Kepple J et al. Total skin-sparing mastectomy without preservation of the nipple–areola complex. Am J Surg 2005; 190: 907–912. [DOI] [PubMed] [Google Scholar]
- 14. Sacchini V, Pinotti JA, Barros AC et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006; 203: 704–714. [DOI] [PubMed] [Google Scholar]
- 15. Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008; 34: 143–148. [DOI] [PubMed] [Google Scholar]
- 16. Crowe JP, Patrick RJ, Yetman RJ, Djohan R. Nipple-sparing mastectomy update. Arch Surg 2008; 143: 1,106–1,110. [DOI] [PubMed] [Google Scholar]
- 17. Gerber B, Krause A, Dieterich M et al. The oncological safety of skin sparing mastectomy with conservation of the nipple–areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009; 249: 461–468. [DOI] [PubMed] [Google Scholar]
- 18. Kim HJ, Park EH, Lim WS et al. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Ann Surg 2010; 251: 493–498. [DOI] [PubMed] [Google Scholar]
- 19. Niemeyera M, Ettla J, Plattnera B et al. Nipple-sparing mastectomy – extended indications and limitations. Breast Care 2010; 5: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boneti C, Yuen J, Santiago C et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg 2011; 212: 686–693. [DOI] [PubMed] [Google Scholar]
- 21. de Alcantara Filho P, Capko D, Barry JM et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011; 18: 3,117–3,122. [DOI] [PubMed] [Google Scholar]
- 22. Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011; 18: 1,665–1,670. [DOI] [PubMed] [Google Scholar]
- 23. Petit JY, Veronesi U, Orecchia R et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012; 23: 2,053–2,058. [DOI] [PubMed] [Google Scholar]
- 24. Burdge EC, Yuen J, Hardee M et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 2013; 20: 3,294–3,302. [DOI] [PubMed] [Google Scholar]
- 25. Peled AW, Duralde E, Foster RD et al. Patient-reported outcomes and satisfaction after total skin-sparing mastectomy and immediate expander-implant reconstruction. Ann Plast Surg 2014; 72: S48–S52. [DOI] [PubMed] [Google Scholar]
- 26. Mallon P, Feron JG, Couturaud B et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131: 969–684. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med 2006; 25: 2,084–2,106. [DOI] [PubMed] [Google Scholar]
- 28. Yueh JH, Houlihan MJ, Slavin SA et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009; 62: 586–590. [DOI] [PubMed] [Google Scholar]


