Abstract
We present a case of necrotising pancreatitis following ampullary biopsy in a patient with Barrett’s oesophagus. The patient needed multiple necrosectomies and several admissions to the intensive care unit. This report is only the third and most severe case of pancreatitis following ampullary biopsy, highlighting its importance as a complication.
Keywords: Pancreatitis, Ampulla of Vater, Ampullary biopsy
Acute pancreatitis is inflammation of the pancreas, diagnosed by elevated levels of pancreatic enzymes in serum and/or radiographic evidence of inflammation. 1 It is a serious condition with a high rate of associated morbidity and mortality. 2 In its most severe form, the inflamed tissue can become necrotic and require surgical drainage. Necrotising pancreatitis carries a mortality rate of approximately 30%. 2 The most common causes are gallstones and alcohol. 2
Surveillance oesophagogastroduodenoscopy (OGD) is an important part in the management of Barrett’s oesophagus, a premalignant condition, and biopsy of suspicious lesions is recommended. 3 Multiple biopsies are preferred, 3 and recognised risks of biopsy include haemorrhage, ampullary obstruction and jaundice. 4 This report details a case of severe necrotising pancreatitis following ampullary biopsy. Only two previous cases of pancreatitis following ampullary biopsy have been described in the literature and in only one of these did the patient develop necrosis of the pancreatic tissue. 4,5
Case History
A 69-year-old man with a past medical history of hypertension and osteoarthritis was admitted for a routine OGD as part of ongoing surveillance for Barrett’s oesophagus. Endoscopy revealed a prominent ampulla, a duodenal polyp at D2 and a short segment of Barrett’s oesophagus. Biopsies were taken from duodenal and oesophageal mucosa as well as from the duodenal polyp and the prominent, suspicious looking ampulla. The procedure was uneventful.
Later that day, the patient developed severe epigastric pain that radiated to his back. He was tender on palpation but had no signs of peritonism. Initial blood tests showed a mild leucocytosis while an erect chest x-ray showed no evidence of pneumoperitoneum. However, his clinical condition deteriorated with evidence of respiratory failure and a systemic inflammatory response syndrome. Repeat blood tests showed worsening leucocytosis (16.5 × 109 cells/l), raised amylase (897u/l), raised lactate dehydrogenase (295iu/l) and glucose of 9.6mmol/l. A diagnosis of acute pancreatitis was made and he was managed supportively. Despite this, his respiratory failure worsened and he required intensive care unit (ICU) admission.
The patient underwent computed tomography (CT), which showed fat stranding and interstitial oedema consistent with pancreatitis and early signs of necrosis. He had a thrombosed superior mesenteric vein, pleural effusions and consolidation (Fig 1).
Figure 1.
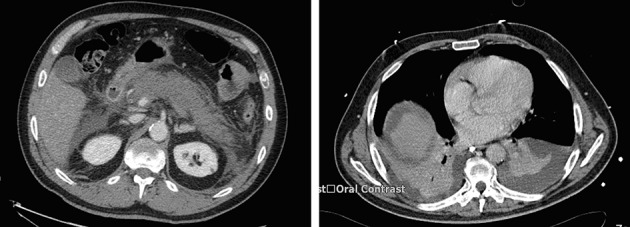
Initial computed tomography confirming pancreatitis and consolidation/effusions in the chest
CT the following day showed diffuse pancreatic parenchymal change with failure to enhance in all areas except for the uncinate process. No collections were suitable for drainage. Subsequent CT imaging (Fig 2) confirmed severe necrotising pancreatitis and a large collection in the lesser sac. The patient was managed supportively on the ICU with regular advice from the regional specialist pancreatic centre before transfer for further intervention 45 days after his initial procedure.
Figure 2.
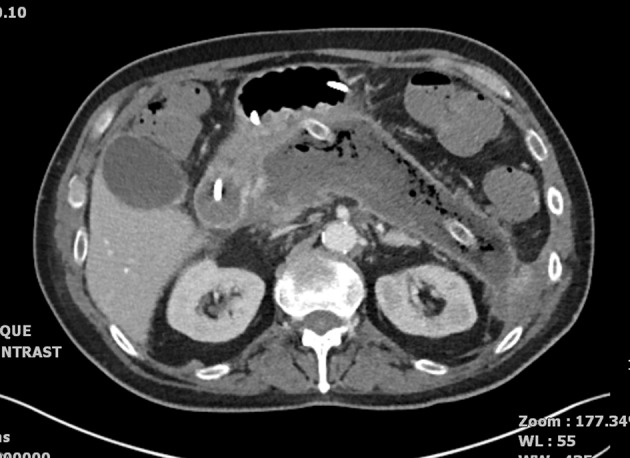
Severe necrotising pancreatitis: left flank drain in situ
Specialist management
On transfer, the patient was ventilated via a tracheostomy, but was requiring no vasoactive support or renal replacement therapy. A nasojejunal tube was inserted for enteral feeding. His blood glucose level was labile and managed with bolus doses of short acting insulin. On arrival at the specialist centre, a percutaneous drain was inserted into the pancreatic bed, under CT guidance (Fig 3), followed by upsizing and a percutaneous necrosectomy. He spent a further ten days in the ICU and was transferred to a specialist surgical ward.
Figure 3.
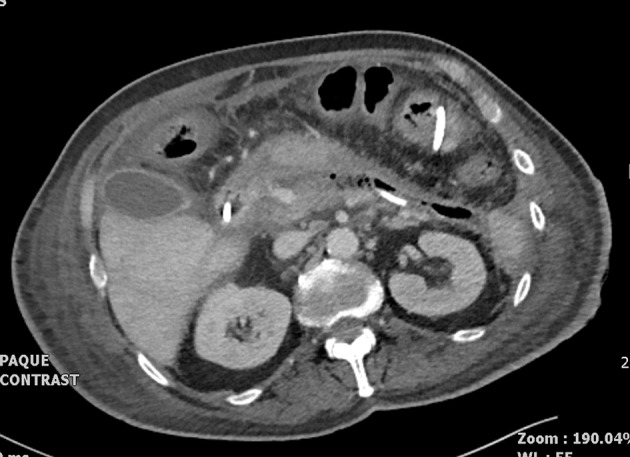
Pancreatic drain in situ 46 days after initial gastroscopy and ampullary biopsy
The patient stayed in hospital for another 113 days, during which there were three readmissions to the ICU for respiratory failure/cardiovascular collapse, multiple courses of antibiotics for chest sepsis and several blood transfusions. A second drain was inserted under CT guidance 84 days after the initial gastroscopy. Seven further percutaneous necrosectomies were performed over a period of 74 days, including one that was complicated with severe bleeding. This required angiography and stenting of a splenic artery pseudoaneurysm. Nutrition was managed using a combination of parenteral, nasojejunal and oral supplementation products.
The patient was discharged home 168 days after his initial gastroscopy with ampullary biopsy (Fig 4). He required regular gliclazide and close follow-up review. At the last clinic review (11 months after the initial procedure), he was pain free and well nourished. He is currently requiring pancrelipase supplementation three times daily.
Figure 4.
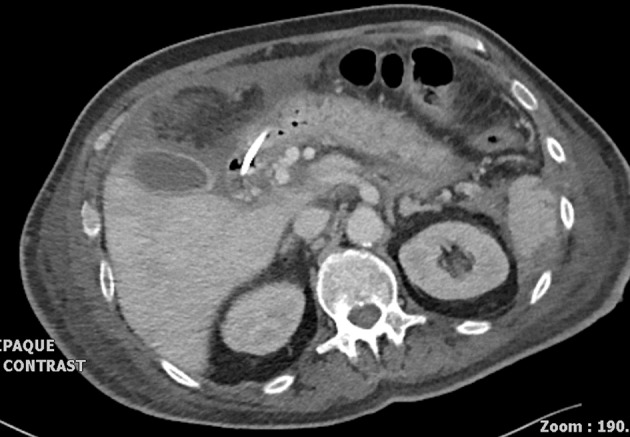
Improved appearances of pancreatic bed 116 days after initial gastroscopy
Biopsy results
Histology confirmed fibrotic small bowel mucosa, fibrosis and gastric metaplasia consistent with previous duodenitis. The biopsy of the duodenal polyp showed no significant inflammation, features of dysplasia or malignancy. Oesophageal biopsy results confirmed gastric cardiac-type mucosa, in keeping with Barrett’s oesophagus.
Discussion
OGD is a routine investigation in the surveillance of conditions such as Barrett’s oesophagus and biopsy of suspicious lesions is recommended. Acute pancreatitis following such a procedure represents a rare but significant complication. Only two previous cases of pancreatitis following ampullary biopsy exist in the literature; in only one did the patient develop pancreatic necrosis. 4,5
In previous cases, the proposed pathophysiological basis for the development of pancreatitis after biopsy was pressure on the pancreas due to intramural or submucosal oedema/haematoma following the biopsy although this was not confirmed on imaging. This is also the likely mechanism in our case.
Conclusions
We believe this report represents the third and most severe published case of severe pancreatitis as a direct result of ampullary biopsy. The patient required several ICU admissions, multiple necrosectomies and two CT guided drains. He suffered recurrent chest sepsis and developed diabetes mellitus.
The following learning points can be gained from this case:
-
>
Gastroscopy and biopsy is a routine part of management for conditions such as Barrett’s oesophagus.
-
>
Pancreatitis is a rare but serious complication of ampullary biopsy.
-
>
Having a high index of suspicion should lead to prompt recognition of pancreatitis and early supportive care.
-
>
Early liaison and timely transfer to specialist centres is crucial.
It is important for clinicians and patients to be aware of this potential complication when deciding whether an ampullary biopsy would be helpful in the management of the condition.
References
- 1. Freeny PC. Classification of pancreatitis. Radiol Clin North Am 1989; 27: 1–3. [PubMed] [Google Scholar]
- 2. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144: 1,252–1,261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]
- 4. Morales TG, Hixson LJ. Acute pancreatitis following endoscopic biopsy of the ampulla in a patient with Gardner’s syndrome. Gastrointest Endosc 1994; 40: 367–369. [DOI] [PubMed] [Google Scholar]
- 5. Ishida Y, Okabe Y, Tokuyasu H et al. A case of acute pancreatitis following endoscopic biopsy of the ampulla of Vater. Kurume Med J 2013; 60: 67–70. [DOI] [PubMed] [Google Scholar]


