Abstract
Introduction
Necrotising soft tissue infection (NSTI) is a rare but life threatening diagnosis. Geographic, economic and social variances influence presentation and prognosis. As the current literature does not reflect a UK metropolitan population, we conducted a retrospective chart review to establish pertinent features relevant to our practice.
Methods
Patients with histologically confirmed diagnoses of NSTI presenting to two London teaching hospitals between January 2007 and July 2013 were included in the study. Features of presentation, surgical and medical management, microbiological findings and outcome were evaluated.
Results
Twenty-four patients with histologically confirmed NSTI were included. Two age clusters were identified, with means of 46 years (standard deviation [SD]: 10 years) and 80 years (SD: 6 years). Pain, erythema and sepsis were common findings. Hypertension, hypercholesterolaemia and type II diabetes mellitus were common co-morbidities. A third of younger patients had human immunodeficiency virus or hepatitis C, with a quarter dependent on drugs and/or alcohol. The mean Laboratory Risk Indicator for Necrotising Fasciitis (LRINEC) score was 5.8 (SD: 3.3). The lower extremities, groin and perineum were common sites of infection. Fourteen patients required inotropic support and seventeen required transfusions. The median number of surgical procedures was 5 (range: 1–17). Group A Streptococcus was the most frequently identified pathogen. Five patients died. Being elderly, female sex and failure to use clindamycin as a first-line antibiotic were associated with significantly higher mortality.
Conclusions
In contrast to other recent series, group A streptococcal monomicrobial NSTI remains the most common presentation in our population. Survival is anticipated in young patients, regardless of premorbid status. Elderly patients have a poor prognosis. The negative predictive value of the LRINEC score is questioned. Use of clindamycin as a first-line antibiotic is supported.
Keywords: Necrotising fasciitis, Necrotising soft tissue infection, Group A Streptococcus
Necrotising soft tissue infection (NSTI) is a life threatening bacterial infection, characterised by rapid progress through tissue planes, resulting in necrosis.1 Described originally by Hippocrates in the fifth century BC as ‘erysipelas’, the term ‘necrotising fasciitis’ was first used in 1952,2 and has more recently been sensationalised in the popular media owing to its exceptional morbidity and mortality. While some cases arise in areas of tissue injury or infection, others arise de novo. Despite improvements in identification and recognition of the need for aggressive multidisciplinary management, mortality rates have remained largely static in the modern literature.3,4
In 2002 the Laboratory Risk Indicator for Necrotising Fasciitis (LRINEC) was introduced to improve diagnostic accuracy on the basis of features of the blood work on admission. However, subsequent studies have questioned the initial positive and negative predicted values of 0.98 and 0.96 respectively,3,5–7 suggesting that the scoring system, developed in a Chinese population, may not be as suitable in a Western patient cohort.8 Prognostic indicators reported in the literature include age,6,9 female sex,9 poor functional status (ASA [American Society of Anesthesiologists] grade 4),10 emergency surgery,6 site of infection,9 hospital transfer,6 sepsis,6 malignancy11 and absence of hyperbaric oxygen therapy.5,11–16 Importantly, prognosis is also based on the microbiological profile of the infection11 with substantial variations in the reported microbiological spectra observed mirroring variations in variables including geography, climate, population pressures and socioeconomic status.17
In the UK, management is largely guided by data extracted from non-UK populations that may not adequately reflect our population or the microbiological spectra encountered. It is our observation that in our UK metropolitan population, prognosis can be difficult to predict based on the presenting features including premorbid health, site of infection, LRINEC score and surgical intervention. It was hypothesised that the NSTI in a UK metropolitan population is not embraced adequately by the current literature. A retrospective chart review of our experience was conducted to establish whether any of the features of presentation and/or management were predictive of outcome so as to guide our future management of these infections.
Methods
All patients referred with a query of NTSI were seen urgently by the specialist registrar on call and, in addition, by the consultant on call if the clinical suspicion favoured emergency surgical intervention. Patients were prescribed clindamycin and ciprofloxacin with/without benzylpenicillin unless an allergy was declared. Surgery was performed by the registrar and/or consultant on call with a second procedure scheduled for the following day in each case subject to anaesthetic and/or intensivist approval. Each case was discussed with the intensive care unit (ICU) consultant on call with an admission threshold determined by the ICU consultant. Further surgical procedures were scheduled as necessary.
For extremity wounds, it was not our policy to use a tourniquet. Postoperative wound dressings consisted of povidone-iodine soaked gauze in the first instance. Imaging to aid diagnosis was used when the clinical picture was equivocal. While it did not form part of our formal protocol, a LRINEC score was established on arrival and prior to surgical debridement. In some patients, a serum lactate study was performed. Hyperbaric oxygen therapy was not a feature of management owing to lack of general availability.
Inclusion and exclusion criteria
All patients presenting directly to the Royal Free Hospital or University College Hospital in London between January 2007 and July 2013 and diagnosed with NSTI (namely necrotising adipositis, fasciitis and/or myositis and Fournier’s gangrene)7 confirmed subsequently by histological analysis were included. Patients referred for secondary reconstruction, having undergone emergency management elsewhere, were excluded, as were all cases of severe cellulitic infections not requiring surgical debridement and surgically debrided infections with no proven histological diagnosis.
Data extraction
In each case, patient age, premorbid history, sex, smoking status, site of infection, associated signs and symptoms, predisposing aetiology, antibiotic regimen (and subsequent changes), microbiological growth, intensive and anaesthetic management, surgical management, duration of admission and outcome were noted.
Statistical analysis
Contingency variables were analysed using Fisher’s exact test. Univariate analysis of independent predictors of mortality was performed. Based on these results, three clinically relevant variables (age, female sex and deviation from the use of clindamycin in the initial antibiotic management) were selected for Pearson’s correlation and logistic regression analysis. A p-value of <0.05 was considered statistically significant. All reported p-values are two-sided. Analyses were performed using OpenStat software (http://StatPages.info/miller/OpenStatMain.htm).
Results
Twenty-four patients (16 men, 8 women) were identified with a mean age of 57 years (standard deviation [SD]: 18 years). Further evaluation of the age distribution revealed two distinct cohorts, with means of 46 years (SD: 10 years) and 80 years (SD: 6 years) (Fig 1). This observation was consistent for both male and female patients. Four of the men and none of the women were smokers.
Figure 1.
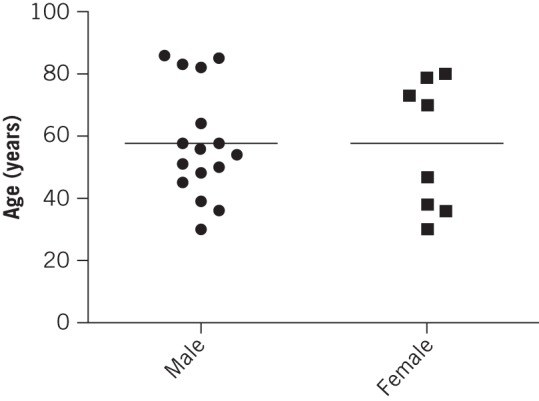
Patient distribution by age and sex
The most frequently presenting symptoms were pain and erythema. Sepsis was the most common sign (Fig 2). Between 0 and 4 (median: 2) significant co-morbidities were identified in the patient cohort. After hypertension (present in nine cases), the next most common co-morbidities were hypercholesterolaemia and type 2 diabetes mellitus, present in seven cases each. Blood borne viruses (human immunodeficiency virus and hepatitis C) were identified in five cases and four patients were dependent on alcohol and/or drugs. These co-morbidities were exclusive to the younger cohort. Two patients were taking corticosteroids and a further two were undergoing cancer chemotherapy on admission. No patients were taking non-steroidal anti-inflammatory drugs on admission.
Figure 2.
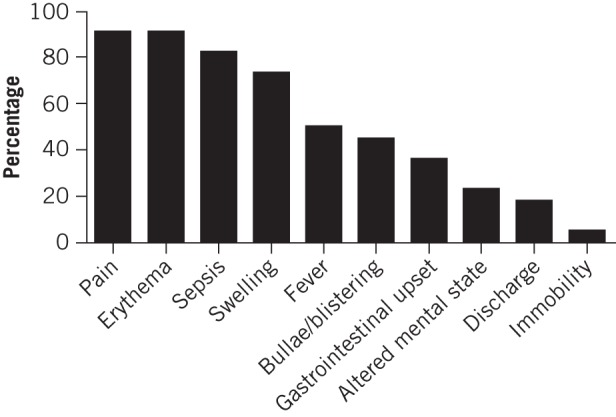
Frequency of presenting symptoms
Imaging was used to aid diagnosis in seven cases (computed tomography in six cases, and ultrasonography and plain film radiography in one case each). The site of infection was perineum/penis/groin in seven cases, left lower extremity in seven cases, right lower extremity in six cases, abdomen/flanks in three cases, right upper extremity in two cases, and left upper extremity, back and buttocks in one case each. A predisposing cause was identified in 12 of the 24 cases: 3 intravenous drug use injection sites, 3 penetrating wounds including 1 surgical wound, 3 abscesses/bursitis, 1 fungal foot infection, 1 pilonidal sinus and 1 anal fissure secondary to intercourse. (The latter two were both referred by our general surgical colleagues.)
The mean length of hospital stay was 61 days (SD: 43 days). There were five deaths overall, at days 5, 49, 64, 70 and 93 following admission. The LRINEC score on admission was available for 16 of the 24 patients, with a mean of 5.8 (SD: 3.3). For the sixteen scores available, four patients scored 0–2 and three of these were among five patients who died. By contrast, no patients among the 12 with scores greater than 2 died (odds ratio [OR]: 58, p=0.007). Serum lactate levels were available for 12 patients, revealing a mean of 4.2mEq/l (SD: 3.1mEq/l).
ICU management
Of the 24 patients, 19 were admitted to the ICU following surgical debridement. An additional patient was referred from the ICU. The median duration of ICU admission was 14 days (range: 1–70 days), representing a median of 27% (range: 1–77%) of the total inpatient stay. The 19 patients were initially intubated and 6 required an eventual tracheostomy. The patient referred from the ICU had a tracheotomy before referral. Seventeen patients required allogeneic transfusion and fourteen required inotropic support. Twenty-two patients (including three not admitted to the ICU) required nutritional supplementation via a nasogastric tube.
Surgical management
The first debridement was performed within 12 hours of referral in 18 of the 24 cases. In the remaining six cases, a mean delay of 49.5 hours (SD: 31 hours) was observed. The median delay between the first and second debridement was 24 hours (range: 13–336 hours). The median number of debridements was 2 and the median number of total surgical procedures performed was 5 (range: 1–17).
Microbiology
Group A Streptococcus (S pyogenes) was the most frequently identified pathogen, implicated in over a third of cases at initial debridement (Table 1). An additional two cases were attributed to other streptococcal species (S oralis and S anginosus) while two subsequent infections were also attributed to Streptococcus spp. Escherichia coli was identified in six cases primarily and in one further case in a subsequent tissue sample. Mixed anaerobic bacteria including Bacteroides spp, Peptostreptococcus spp and Clostridium spp were also identified in six cases. Methicillin resistant Staphylococcus aureus (MRSA) was only found in one case, during subsequent debridements, most likely as a nosocomial superinfection. Pseudomonas spp (all P aeruginosa) were implicated in only one case primarily but also in two subsequent nosocomial infections. There were 15 monomicrobial and 9 polymicrobial infections. Other monomicrobial infections included E coli in three cases, and Acinetobacter baumannii, P aeruginosa and Serratia marcescens in one case each.
Table 1.
The microbiological profile of each infective episode
| Organism | Frequency of positive cultures | |
|---|---|---|
| Initial presentation | Subsequent cultures | |
| Group A Streptococcus | 9 | 0 |
| Escherichia coli | 6 | 1 |
| Mixed anaerobes | 6 | 0 |
| Enterococcus spp | 4 | 0 |
| Staphylococcus aureus | 3 | 0 |
| Streptococcus spp (excluding Group A Streptococcus) | 2 | 2 |
| Staphylococcus spp (coagulase negative) | 2 | 1 |
| Klebsiella spp | 2 | 0 |
| Pseudomonas spp | 1 | 2 |
| Acinetobacter spp | 1 | 1 |
| Serratia spp | 1 | 0 |
| Aerococcus spp | 1 | 0 |
| Haemophilus influenzae | 1 | 0 |
| Methicillin resistant Staphylococcus aureus | 0 | 1 |
| Candida albicans | 0 | 1 |
Antimicrobial management
Clindamycin was prescribed in 19 cases on admission and ciprofloxacin was prescribed in 18 cases. Both were each prescribed in one further case subsequently. Benzylpenicillin was the next most common antibiotic prescribed, in 14 cases. The remainder were prescribed infrequently. The most common second-line antibiotic prescribed was piperacillin/tazobactam and antibiotics of the carbapenem class (meropenem and ertapenem), in four cases each. Additionally, piperacillin/tazobactam was prescribed on admission in two cases and ertapenem was prescribed in one case. All first-line antibiotics were prescribed empirically and all second-line antibiotics were prescribed on the advice of the clinical microbiology team, either to provide further cover for the organisms implicated in NSTI or to manage nosocomial superinfections. The antimicrobial prescribing practice is summarised in Figure 3.
Figure 3.
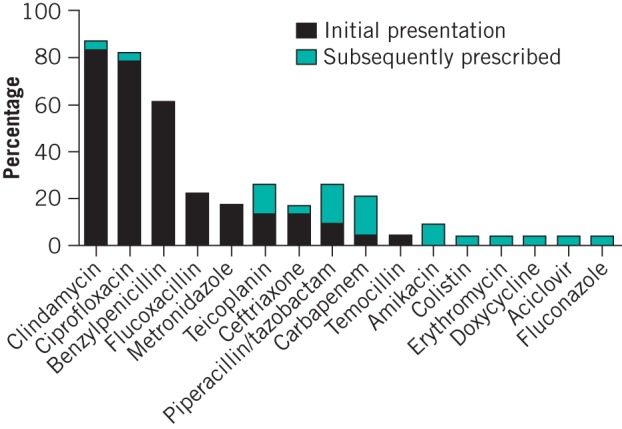
Frequency of antibiotic use
Mortality
Five patients died and a further three were discharged (not to home but to rehabilitation). One further patient died of unrelated causes within 12 months. Those who died in hospital did so between days 5 and 93 following admission. In the elderly cluster of 8 patients, there were 4 deaths, compared with 1 death in the remaining 16 patients. Univariate analyses (Fisher’s exact test) revealed this to be significant (OR: 15, p=0.028). Of the five deaths, four were women (including three elderly women). The remaining case was an elderly male patient (OR: 15, p=0.028).
Of the 19 patients prescribed clindamycin on admission, 17 survived and 2 died. Of the five cases not prescribed clindamycin on admission, three died and two survived (OR: 13, p=0.042). All three deaths were women, including one young female patient. None of the other variables evaluated revealed any association with patient mortality (Table 2). Logistic regression analysis of age, sex and clindamycin use revealed that no single factor was independently predictive of mortality in this series.
Table 2.
Univariate analysis of independent predictors of death
| Factor | Odds ratio | p-value |
| Age >60 years | 15 | 0.028 |
| Female sex | 15 | 0.028 |
| Deviation from guideline use of clindamycin on admission | 13 | 0.042 |
| Delay >24h to first debridement | 5.7 | 0.18 |
| Delay >12h to first debridement | 4.2 | 0.29 |
| Inotropic support | 1.3 | 1.0 |
| >2 co-morbidities | 0.48 | 0.63 |
| Group A Streptococcus | 0.34 | 0.61 |
| Smoker | 0.31 | 0.54 |
Discussion
The importance of adopting an evidence-based approach to NSTI lies not only with the severe spectrum of morbidity and mortality observed but with the fact that it remains rare (and so prior clinical exposure is limited) yet is evolving17,18 and becoming more common.8 As a result, the global experience of NSTI presentation and management must be appreciated in the context of local disease patterns so that emerging trends may be identified and management practice evolved to keep pace.
Our mortality rate of 21% is similar to rates published in the literature.4,19–21 One series (from a regional hospital in Australia) reported a mortality rate of only 8.6%.22 All 20 patients were managed in an ICU setting, with a mean time from presentation to surgery of 20 hours and a median of 4 operative procedures. Consequently, management and (it may be assumed) clinical severity was similar to our series. However, the Australian series was different in one crucial aspect. The authors did not report (as we did) an isolated population of elderly patients. Indeed, the eldest patient in the Australian study was 62 years. A more suitable comparison with this study would be the 16 youngest patients in our series, only one of whom died (6%).
The site of infection was the lower extremity in 13 of the 24 cases, with only 3 cases occurring in the upper limbs. This predilection for the lower limb has been reported elsewhere.8,19,23 Our symptom profile was similar to other reports,11,16,23 confirming that most cases of NSTI, irrespective of type or microbiology of infection, present similarly, with pain, erythema and swelling. Importantly, these symptoms are non-specific, emphasising the need for a high index of clinical suspicion. The relatively high premorbid rate of type 2 diabetes mellitus is similar to that in other published series.18,21,22
The predominance of group A Streptococcus as the offending organism, either alone or in combination with others, is similar to one report from 201322 but at odds with other studies.1,23 The fact that there were no cases of Vibrio vulnificus (type 3) NSTI in our series is probably due to the metropolitan nature of our catchment area. We did not find (as others have)3 that clostridial infections were independent predictors of mortality in our series. Again, this is probably due to the lack of clostridial infections in our cohort, which would be expected to be a feature of NSTI in rural populations.
Multiresistant nosocomial infections such as MRSA were not attributed to any case in our series. Evidence from the literature is divided on the extent to which these organisms are implicated, with some studies showing a low incidence of MRSA implicated NSTI19,24 while others report the emergence of community associated, MRSA implicated, monomicrobial NSTI.17,18,21,25,26 This may reflect pervasive antibiotic misuse and could yet have implications for the UK. The majority of NSTI cases in our series were monomicrobial.18,21 This contrasts with Elliott et al, who reported monomicrobial infections in only 28 of 182 cases.1 Overall, the microbiological profile of our cohort was very different to some of the others published,1,23 lending credence to our view that local data are crucial when instigating a policy for the management of NSTI and underlining the need for caution when interpreting the results of dissimilar series.
Interestingly, no fewer than ten different antibiotics were prescribed initially despite having an established antibiotic protocol. This observation could be explained by the fact that a number of cases were referred by other specialties and antibiotics were prescribed prior to referral. In some cases, it was felt sensible to continue the antibiotics prescribed, pending definitive cultures.
Four patients underwent surgery more than twenty-four hours following referral. In each case, the patient was admitted via another specialty and was referred subsequently to our service, and in each case, the clinical picture was equivocal when first evaluated, evolving over the course of the first day following referral. In all four cases, the first evaluation was performed by a registrar, with a consultant evaluation sought later as the clinical picture evolved. While we did not find that time from referral to first or, indeed, second surgical debridement influenced survival, of the 4 patients who underwent their first debridement after 24 hours, 2 died. Delayed debridement has been implicated in mortality in other reports5,16 but the numbers were too small to demonstrate a statistical correlation here. By operating on 83% of cases within 24 hours of admission, our practice compares favourably with others.22
The median number of surgical procedures performed in our series, at 5, was greater than that in other series.5,19 It may be argued that this suggests inexperienced surgeons are performing inadequate debridements, necessitating more trips to theatre. However, these data include theatre visits for skin grafting and other reconstructive procedures, and a consultant plastic surgeon was involved in at least one debridement in each case. Moreover, the median number of debridements was 2. The case requiring 17 theatre visits was mostly for change of negative pressure wound therapy dressings while awaiting a graftable wound bed. As a result, we feel it is more likely that these data are simply a sign of the reconstructive efforts required.
The high rate of ICU admission in our series (20/24 cases) suggests a clinically more severe cohort than in some other series.20,24 This may also reflect the availability of the ICU and/or the willingness of our ICU colleagues to engage in the management of these infections. One patient was already on the ICU when referred to us.
Importantly, on retrospective application, we did not find that the LRINEC score was useful in the diagnosis of NSTI. In fact, the LRINEC score was 0 for two patients, one of whom later died. It is difficult to provide an adequate explanation for our finding that a low LRINEC score was predictive of mortality. As these patients died on days 5, 64 and 70 following admission, the number of variables that may have influenced their outcome during their hospital stay, particularly in the latter two cases, is likely to be substantial. Certainly, there is no evidence to support the conclusion that these cases were treated more conservatively, owing to their relatively benign presentation.
The alternative explanation is that we have identified a subset of patients, presenting in a relatively benign manner, that subsequently deteriorate rapidly despite aggressive intervention. These data support the case for diagnosis to be made on clinical grounds and reinforce the requirement for having a low index of suspicion when confronted with potential cases of NSTI.27 We did not assess the procalcitonin ratio from day 1 to day 2 and acknowledge that this requires further evaluation.28
The number of cases included are a sign of both the rarity of this condition (an estimated 4 cases per 100,000 person-years)15 and the strict criteria used to define our cohort, the express purpose of which was to ensure our analyses were based only on true cases of NSTI. It was therefore not possible to evaluate outcomes in terms of premorbid health or medication owing to insufficient statistical sensitivity.
Missing data in our series include patient body mass index, implicated in NSTI,6 and an ICU scoring system such as the Acute Physiology and Chronic Health Evaluation (APACHE) II or III, used by some authors to stratify clinical severity. Moreover, just as the findings from North American and South-East Asian studies must be interpreted with caution when seeking to extrapolate data relevant to our patient cohort, our findings reflect NSTI in a UK metropolitan population and caution is advised when extrapolating these results the other way.
While substantial resources are required to manage these patients adequately, the evidence from our study suggests that good outcomes can be achieved through early, aggressive surgical and ICU management in a multidisciplinary setting. It seems clear that both a UK-wide database is needed, to evaluate the evolution of case presentations in this country, and a national standard for the management of NSTI, to mitigate variations in practice likely to arise as a result of lack of experience in the management of these rare cases in the community hospital setting and to further stratify risk. These outcomes must be audited against international data.
Conclusions
Our experience of NSTI in a UK metropolitan population exhibits crucial differences from the trends observed in the recent international literature. In our series, NSTI was principally monomicrobial in nature, with group A Streptococcus still the most common organism implicated. It follows that deviation from the accepted standard of clindamycin at first presentation may affect outcome adversely thereafter. We also observed an isolated population of elderly patients, who may present insidiously but exhibit a significantly poorer outcome. In our population, NSTI remains a clinical diagnosis where favourable outcomes may be anticipated, especially in younger patients, when early diagnosis is combined with aggressive surgical and ICU management in a multidisciplinary setting. Caution is advised when designing protocols based on non-UK studies.
Source of funding
GG is funded by a grant from the Academy of Medical Sciences.
References
- 1. Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg 2000; 179: 361–366. [DOI] [PubMed] [Google Scholar]
- 2. Wilson B. Necrotizing fasciitis. Am Surg 1952; 18: 416–431. [PubMed] [Google Scholar]
- 3. Anaya DA, McMahon K, Nathens AB et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005; 140: 151–157. [DOI] [PubMed] [Google Scholar]
- 4. Fontes RA, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg 2000; 8: 151–158. [DOI] [PubMed] [Google Scholar]
- 5. McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995; 221: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mills MK, Faraklas I, Davis C et al. Outcomes from treatment of necrotizing soft-tissue infections: results from the National Surgical Quality Improvement Program database. Am J Surg 2010; 200: 790–796. [DOI] [PubMed] [Google Scholar]
- 7. Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg 2009; 208: 279–288. [DOI] [PubMed] [Google Scholar]
- 8. Bernal NP, Latenser BA, Born JM, Liao J. Trends in 393 necrotizing acute soft tissue infection patients 2000–2008. Burns 2012; 38: 252–260. [DOI] [PubMed] [Google Scholar]
- 9. Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Ann Surg 1996; 224: 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faraklas I, Stoddard GJ, Neumayer LA, Cochran A. Development and validation of a necrotizing soft-tissue infection mortality risk calculator using NSQIP. J Am Coll Surg 2013; 217: 153–160.e3. [DOI] [PubMed] [Google Scholar]
- 11. Hsiao CT, Weng HH, Yuan YD et al. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26: 170–175. [DOI] [PubMed] [Google Scholar]
- 12. Boyer A, Vargas F, Coste F et al. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 2009; 35: 847–853. [DOI] [PubMed] [Google Scholar]
- 13. Dworkin MS, Westercamp MD, Park L, McIntyre A. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiol Infect 2009; 137: 1,609–1,614. [DOI] [PubMed] [Google Scholar]
- 14. Hong YC, Chou MH, Liu EH et al. The effect of prolonged ED stay on outcome in patients with necrotizing fasciitis. Am J Emerg Med 2009; 27: 385–390. [DOI] [PubMed] [Google Scholar]
- 15. Kaul R, McGeer A, Low DE et al. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Am J Med 1997; 103: 18–24. [DOI] [PubMed] [Google Scholar]
- 16. Wong CH, Chang HC, Pasupathy S et al. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85: 1,454–1,460. [PubMed] [Google Scholar]
- 17. Kao LS, Lew DF, Arab SN et al. Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: a multicenter study. Am J Surg 2011; 202: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller LG, Perdreau-Remington F, Rieg G et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352: 1,445–1,453. [DOI] [PubMed] [Google Scholar]
- 19. Angoules AG, Kontakis G, Drakoulakis E et al. Necrotising fasciitis of upper and lower limb: a systematic review. Injury 2007; 38 Suppl 5: S19–S26. [DOI] [PubMed] [Google Scholar]
- 20. Das DK, Baker MG, Venugopal K. Risk factors, microbiological findings and outcomes of necrotizing fasciitis in New Zealand: a retrospective chart review. BMC Infect Dis 2012; 12: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsitsilonis S, Druschel C, Wichlas F et al. Necrotizing fasciitis: is the bacterial spectrum changing? Langenbecks Arch Surg 2013; 398: 153–159. [DOI] [PubMed] [Google Scholar]
- 22. Bucca K, Spencer R, Orford N et al. Early diagnosis and treatment of necrotizing fasciitis can improve survival: an observational intensive care unit cohort study. ANZ J Surg 2013; 83: 365–370. [DOI] [PubMed] [Google Scholar]
- 23. Salvador VB, San Juan MD, Salisi JA, Consunji RJ. Clinical and microbiological spectrum of necrotizing fasciitis in surgical patients at a Philippine university medical centre. Asian J Surg 2010; 33: 51–58. [DOI] [PubMed] [Google Scholar]
- 24. Suwantarat N, Chow DC, Koss W et al. Histologically confirmed necrotizing fasciitis: risk factors, microbiology, and mortality in Hawaii. Int J Infect Dis 2012; 16: e886–e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frazee BW, Fee C, Lynn J et al. Community-acquired necrotizing soft tissue infections: a review of 122 cases presenting to a single emergency department over 12 years. J Emerg Med 2008; 34: 139–146. [DOI] [PubMed] [Google Scholar]
- 26. Lee TC, Carrick MM, Scott BG et al. Incidence and clinical characteristics of methicillin-resistant Staphylococcus aureus necrotizing fasciitis in a large urban hospital. Am J Surg 2007; 194: 809–812. [DOI] [PubMed] [Google Scholar]
- 27. Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: clinical suspicion should trump scoring systems. J Emerg Med 2013; 44: 928–931. [DOI] [PubMed] [Google Scholar]
- 28. Friederichs J, Hutter M, Hierholzer C et al. Procalcitonin ratio as a predictor of successful surgical treatment of severe necrotizing soft tissue infections. Am J Surg 2013; 206: 368–373. [DOI] [PubMed] [Google Scholar]


