Abstract
Introduction
The cost of fragility fractures to the UK economy is predicted to reach £2.2 billion by 2025. We studied our hip fracture population to establish whether national guidelines on fragility fracture prevention were being followed, and whether high risk patients were identified and treated by local care services.
Methods
Data on a consecutive series of trauma hip fracture admissions were collected prospectively over 14 months. National Institute for Health and Care Excellence (NICE) and National Osteoporosis Guideline Group (NOGG) recommendations and FRAX® risk calculations were applied to patients prior to their admission with a new hip fracture.
Results
Overall, 94 patients were assessed against national guidelines. The mean population age was 77 years. Almost a quarter (22%) of patients had suffered a previous fragility fracture. The mean FRAX® ten-year probability of hip fracture was 7%. According to guidelines, 45% of the study population required treatment, 35% fulfilled criteria for investigation and reassessment, and 20% needed no further management. In practice, 27% received treatment, 4% had undergone dual energy x-ray absorptiometry and were untreated, and 69% had not been investigated and were untreated. In patients meeting intervention thresholds, only 33% of those who required treatment were receiving treatment in practice.
Conclusions
In conjunction with NICE and NOGG recommendations, FRAX® was able to identify 80% of our fracture population as intermediate or high risk on the day of fracture. Correct management was evident in a third of cases with a pattern of inferior guideline compliance seen in a London population. There remains a lack of clarity over the duty of care in fragility fracture prevention.
Keywords: Osteoporotic fracture, Primary prevention, Secondary prevention, Hip fracture
Fragility fractures are responsible for 300,000 annual UK emergency department attendances and over 9 million annual fractures worldwide.1,2 The estimated direct medical cost of fragility fractures to the UK healthcare economy is expected to increase to £2.2 billion by 2025. The majority of these costs are associated with hip fracture care.3 The single greatest factor contributing to fragility fracture risk is osteoporosis, the management of which remains a priority if the burden of these potentially preventable injuries is to be reduced.
The National Institute for Health and Care Excellence (NICE) advises on the use of risk assessment tools in the care of patients who may be at risk of osteoporosis and secondary fragility fractures.4 A summary of the current guidelines is provided in Table 1. For those meeting specified criteria, absolute fracture risk should be estimated using a validated risk calculator.
Table 1.
Summary of National Institute for Health and Care Excellence guidelines4
| > Consider DXA in patients starting treatments that may affect bone density (eg goserelin for prostate cancer). |
| > Do not assess fracture risk routinely in people aged <50 years unless they have major risk factors. |
| > Consider assessment in women aged <65 years and men aged <75 years in the presence of risk factors (eg prior fragility fracture, current smoker, low BMI). |
| > Consider assessment in all women aged >65 years and all men aged >75 years. |
DXA = dual energy x-ray absorptiometry; BMI = body mass index
There are a number of risk assessment tools, three of which are validated for the assessment of osteoporosis: FRAX®, QFracture® and bone mineral density.5 For the purpose of this study we elected to use FRAX®, a computer-based algorithm from the World Health Organization Collaborating Centre for Metabolic Bone Diseases. The FRAX® algorithm calculates fracture probability from patient parameters and dichotomised variables, outputting the ten-year probability of a major osteoporotic fracture (humerus, wrist, spine, hip) and the ten-year probability of a hip fracture. In order to assist in the interpretation of outputted data, the National Osteoporosis Guideline Group (NOGG) has published management algorithms, underpinned by a health economic analysis, with defined assessment and intervention thresholds (Table 2).6
Table 2.
Summary of National Osteoporosis Guideline Group recommendations6
| > Postmenopausal women with a prior fragility fracture should undergo risk assessment although DXA may be appropriate for younger women. |
| > Men aged ≥50 years (with or without a fracture) but with a WHO risk factor or a BMI of <19kg/m2 should undergo FRAX® assessment. |
| > All postmenopausal women without a fracture but with a WHO risk factor or a BMI of <19kg/m2 should undergo FRAX® assessment. |
DXA = dual energy x-ray absorptiometry; WHO = World Health Organization; BMI = body mass index
Risk factors include body mass index, previous fragility fracture, parental history of hip fracture, current glucocorticoid treatment, smoking status, alcohol intake, and secondary causes of osteoporosis including type 1 diabetes and rheumatoid arthritis. Following FRAX® assessment, patients are classified as low, intermediate or high risk. Low risk patients are reassured and reassessed in five years or less depending on the clinical context. Intermediate risk patients require dual energy x-ray absorptiometry (DXA) assessment and recalculation of fracture risk. High risk patients should be considered for treatment without the need for DXA.
Current NICE and NOGG recommendations were applied to our fractured neck of femur population on the day of injury to establish whether current national guidelines were being followed, whether high risk patients were being identified, whether correct treatment was initiated and whether intervention thresholds were set at an appropriate level. Where deficiency was found, possible explanations were considered.
Methods
Data on a consecutive series of trauma hip fracture admissions were collected prospectively at a central London teaching hospital with 846 beds and 120,000 attendances per year in the accident and emergency department. Data proformas were completed on admission from the patient history and further details were extracted from the local electronic trauma database. These were cross-checked with clinical notes and the picture archiving and communication system for verification and acquisition of supplementary information.
For inclusion in the study, patients had to be admitted with a confirmed diagnosis of a neck of femur fracture between January 2011 and March 2012. A total of 120 consecutive patients were admitted during this period, 11 of whom were excluded owing to incomplete data. For FRAX® assessment, patients suffering high energy trauma and those aged younger than 50 years or over 90 years were excluded, as proposed by the original model. This excluded a further 15 patients, leaving 94.
Results
The mean age of the study population was 77.4 years (range: 51–89 years). The mean body mass index was 23.1kg/m2 (standard deviation [SD]: 3.82kg/m2). There were 64 female and 30 male patients. The majority (80%) of the population were resident in London with locally registered general practitioners, 19% were from outside of London and 1% were international, reflecting the transient population seen in a central London hospital. Over half (57.8%) of the fractures were intracapsular and 42.2% were extracapsular. A third (32.7%) of the patients were treated with a cemented hemiarthroplasty, 29.1% with a dynamic hip screw, 18.7% with a cephalomedullary nail, 10.2% with cannulated screws and 9.3% with a total hip replacement.
Across all age groups, the mean number of risk factors per patient was 0.91 (SD: 0.3). The most common risk factors were smoking, followed by a prior fragility fracture (Table 3). The mean ten-year probabilities of fragility fracture or hip fracture were 14% (SD: 6.7 percentage points) and 7% (SD: 3.1 percentage points) respectively (Table 4).
Table 3.
FRAX® clinical risk factors
| Age group | |||||
|---|---|---|---|---|---|
| 50–59 (n=7) | 60–69 (n=10) | 70–79 (n=27) | 80–89 (n=50) | Mean (SD) | |
| Previous fragility fracture | 0% | 30% | 26% | 32% | 22% (14.8pp) |
| Parental hip fracture | 0% | 10% | 0% | 12% | 6% (6.4pp) |
| Smoking | 43% | 30% | 19% | 14% | 26% (12.9pp) |
| Glucocorticoid treatment | 0% | 0% | 11% | 8% | 5% (5.6pp) |
| Rheumatoid arthritis | 0% | 10% | 0% | 2% | 3% (4.8pp) |
| Secondary OP risk factor | 14% | 20% | 7% | 20% | 15% (6.2pp) |
| Alcohol | 14% | 30% | 7% | 6% | 14% (11.1pp) |
| Mean risk factors per patient | 0.71 | 1.30 | 0.70 | 0.94 | 0.91 (0.3pp) |
OP = osteoporosis; SD = standard deviation; pp = percentage point
Table 4.
Mean ten-year FRAX® fracture probabilities
| Age | Major osteoporotic fracture | Hip fracture |
|---|---|---|
| 50–59 yrs | 4.51 (SD: 2.1) | 0.66 (SD: 0.2) |
| 60–69 yrs | 11.10 (SD: 4.1) | 4.10 (SD: 3.2) |
| 70–79 yrs | 16.39 (SD: 2.3) | 8.04 (SD: 5.1) |
| 80–89 yrs | 24.15 (SD: 7.4) | 15.41 (SD: 6.4) |
SD = standard deviation
According to FRAX® scores calculated on the day of fracture, 45% of the total study population should have been on treatment, 35% fulfilled criteria for DXA and reassessment, and 20% needed no further investigation or treatment. In practice, 27% were on treatment, 4% had undergone DXA but were not treated, and 69% were not on treatment and had not received DXA. A subanalysis of management according to FRAX® outcome is depicted in Figure 1.
Figure 1.
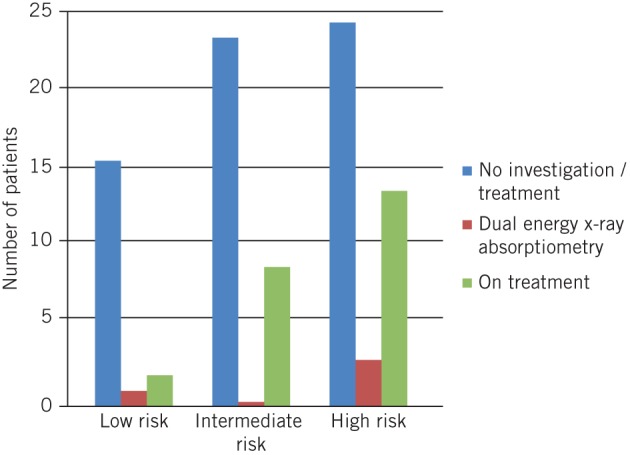
Management of study population according to FRAX® outcome
Application of NOGG recommendations revealed that 35% of patients resident in London who required either treatment or investigation on the day of fracture had been managed correctly compared with 54% of patients resident outside of London (Fig 2). An analysis of patients definitively meeting NOGG intervention thresholds revealed that 33% of the total study population who should have been receiving treatment before the day of fracture were indeed receiving treatment in practice. This equated to 27% of the relevant London population and 56% of patients from outside of London (Fig 3).
Figure 2.
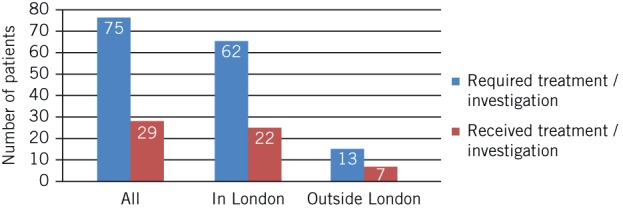
Comparison of recommended versus actual management according to FRAX® scores and National Osteoporosis Guideline Group recommendations
Figure 3.
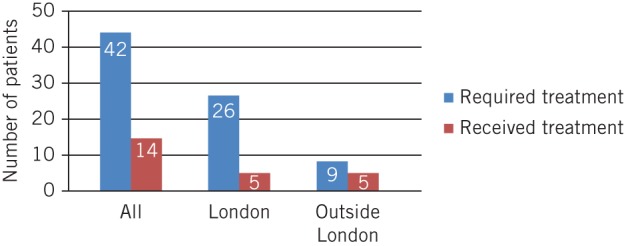
Comparison of recommended versus actual management according to FRAX® scores and National Osteoporosis Guideline Group (NOGG) recommendations for those patients definitively meeting NOGG intervention thresholds
In the study population, eight patients had undergone DXA. Seven of these required treatment according to NOGG recommendations. However, only four received treatment. Examining the population of postmenopausal women with a prior fragility fracture (n=20), 45% were on treatment, 5% had received DXA but were not treated, and 50% were not on treatment and had not received DXA.
Discussion
The results of this study suggest that patients at risk of fragility fractures remain underdiagnosed and undertreated, with 67–70% of patients receiving incorrect management according to current guidelines. One concern is the lack of adherence to guidelines witnessed in the breakdown of management according to FRAX® outcomes (Fig 1).
In our study population, 19 patients (20%) were classified as low risk on FRAX® scoring and required future reassessment only. In practice, one patient underwent unnecessary DXA without subsequent treatment initiation and two patients were initiated on bone protection without investigation. Thirty-three patients (35%) were classified as intermediate risk on the FRAX® score and warranted DXA and reassessment but no record was found of DXA in any of these patients. Twenty-four patients were not on therapy and had no record of DXA, and of the nine patients receiving therapy, none had undergone prior DXA. Forty-two patients (44%) were classified as high risk on the FRAX® score and, according to guidelines, should have been commenced on treatment without further investigation. Despite this, 25 (60%) of these patients were untreated. Seven high risk patients had undergone DXA and three of these were not subsequently commenced on treatment.
Application of NOGG recommendations demonstrated that 30% of the study population had undergone correct investigation or had been initiated on treatment correctly (35% London vs 54% outside of London). A similar pattern was seen when analysing those patients definitively meeting the intervention threshold, with 27% of London patients versus 56% of out of London patients on qualifying therapy.
It is recommended that all postmenopausal women with a prior fragility fracture should be considered for treatment without the need for further risk assessment. Under half (45%) of this subpopulation in our study were on appropriate treatment, 5% had undergone bone mineral density measurement but treatment was omitted and the remaining 50% were found to be on no treatment without any explanation or noted contraindication.
These results are representative of the trend seen in recent studies evaluating the care of patients at risk of fragility fractures and the efficacy of risk calculators including FRAX® in identifying an at-risk population.7 The publication of Falling Standards, Broken Promises in 2011, a national audit of falls and bone health in older people, showed unacceptable variation in the quality of services for care and prevention of falls and fractures.8 The audit found that patients were not routinely receiving essential aspects of care for falls prevention or bone health with subsequent exposure to a greater risk of further falls and fractures.
In March 2013 data in the online publication of UK specific figures from the latest Global Burden of Disease report showed musculoskeletal disorders accounting for 31.3% of the total burden of disability in the UK,9 making it the single biggest cause of disability. The report demonstrated that this growing burden, particularly from musculoskeletal disorders including fragility fractures, required an integrated and strategic response to achieve improvement.
As the subject of recent focus and debate, the reasons for poor guideline adherence are unclear. A potential explanation might be the lack of clarity in ownership of duty of care. The single greatest predictor of future fragility fracture is prior fracture (22% of our study population), an event that tends to present to secondary care. However, in the UK, where a number of health professionals (both in primary and secondary care) are involved in the fracture pathway, it remains unclear who holds responsibility for establishing and maintaining secondary prevention. At present, just 37% of trusts have access to a fracture prevention service and most patients are unevaluated until a subsequent fracture.8
The advent of risk calculators arose through the appreciation of the difficulty in identification of those patients at risk of fracture and those who would benefit from primary prevention. A number of these calculators, including FRAX®, are currently available to clinicians. Nevertheless, as recently demonstrated by Bolland et al,10 the algorithms underpinning these calculators are not constant and the inclusion or exclusion of key variables (eg the competing risk of mortality) can produce contradictory risk estimates in the same patient. Conflicting outputs may discourage the use of tools that can prove highly effective when correctly implemented.
In addition, as demonstrated in a study evaluating the efficacy of FRAX® assessment in a Swiss population,7 further challenges lie in determining location specific FRAX® thresholds in order to define appropriate intervention thresholds. This process is most suited to prospective population-based studies. Such studies afford the opportunity to calibrate and make secondary adjustments to threshold values. Examples of this occurring can be found in France, Canada and Holland.11–13 A fifth (20%) of our study population was identified as low risk, 75% of whom were over the age of 70 years. While not definitive evidence of a lack of sensitivity, we may find that with time and further research UK thresholds will become refined, and the positive predictive value will increase.
The benefits of therapy once a diagnosis is made are well proven. Alendronate (a nitrogen containing bisphosphonate),14 raloxifene (a selective oestrogen receptor modulator),15 strontium (a dual action bone agent)16,17 and denosumab (a human monoclonal antibody)18 have all demonstrated in randomised placebo controlled trials a significant reduction in the risk of fragility fracture. With the exception of denosumab, which has proven to have greater efficacy in postmenopausal women at intermediate to high risk of fracture as assessed by FRAX®,19 the effect of agents appear to be independent of FRAX® score at the point of fracture. The wide variety in proven treatment options and preparations should increase the ease of prescription. However, our data and previous studies suggest this is not the case.
Our sample size in this prospective consecutive series is small albeit representative of the pattern of trauma in a central London teaching hospital. It was not possible to distinguish between cases where risk assessments had been performed but further investigation and treatment had been consciously omitted and those where simply no risk assessment was performed. In order to aid in the interpretation of data, it was assumed that patients without prior DXA who had not been prescribed treatment fell into the latter group. Additional context may have been acquired through an appreciation of the number of patients who had appropriate evaluation and treatment in the primary care setting, therefore receiving successful fracture prevention. Unfortunately, these data were not available to us.
Furthermore, compliance was not assessed. Twenty-five patients in this study sustained a neck of femur fracture despite a recorded prescription of a bisphosphonate. While it was not possible to demonstrate a history of non-compliance, it has been established previously that the desired goal of keeping patients with osteoporosis on long-term treatment is not being achieved adequately in actual practice, and the potential social and economic implications of this behaviour are substantial.20 Responsibility lies with both the doctor and patient if we are to succeed in tackling this important public health burden.
Conclusions
In conjunction with NICE and NOGG recommendations, FRAX® scores were able to identify 80% of our fracture population as intermediate or high risk on the day of fracture. Correct management was evident in up to 33% of cases with a pattern of inferior guideline compliance in London. Investigations were performed unnecessarily and national recommendations were not followed in the interpretation of results, with documented examples of superfluous treatment prescription and omission of qualifying therapy.
Explanations are unlikely to be found in lack of awareness of this growing burden or confusion over efficacy of available treatment. There is disparity between the output of individual risk calculators, which might create confusion and discourage usage, and further research is required to calibrate intervention thresholds and improve the predictive value of current algorithms. Of more concern is a national trend in lack of clarity over the duty of care of these high risk patients. Improved communication between primary and secondary care, nationally agreed care pathways, and expansion of current fracture prevention services in primary and secondary care are all required to ease this growing burden.
References
- 1.Kanis JA, Oden A, Johnell O et al The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001; 12: 417–427. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1,726–1,733. [DOI] [PubMed] [Google Scholar]
- 3.Burge RT, Worley D, Johansen A et al The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ 2001; 4: 51–62. [Google Scholar]
- 4.Osteoporosis Overview. National Institute for Health and Care Excellence. http://pathways.nice.org.uk/pathways/osteoporosis/ (cited April 2014).
- 5.National Clinical Guideline Centre. Osteoporosis: Fragility Fracture Risk. London: NCGC; 2012. [Google Scholar]
- 6.National Osteoporosis Guideline Group. Guideline for the Diagnosis and Management of Osteoporosis. London: NOGG; 2014. [Google Scholar]
- 7.Aubry-Rozier B, Stoll D, Krieg MA et al What was your fracture risk evaluated by FRAX® the day before your osteoporotic fracture? Clin Rheumatol 2013; 32: 219–223. [DOI] [PubMed] [Google Scholar]
- 8.Treml J, Husk J, Lowe D, Vasilakis N. Falling Standards, Broken Promises. London: RCP; 2011. [Google Scholar]
- 9.Murray CJ, Richards MA, Newton JN et al UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013; 381: 997–1,020. [DOI] [PubMed] [Google Scholar]
- 10.Bolland MJ, Jackson R, Gamble GD, Grey A. Discrepancies in predicted fracture risk in elderly people. BMJ 2013; 346: e8669. [DOI] [PubMed] [Google Scholar]
- 11.Sornay-Rendu E, Munoz F, Delmas PD, Chapurlat RD. The FRAX tool in French women: how well does it describe the real incidence of fracture in the OFELY cohort? J Bone Miner Res 2010; 25: 2,101–2,107. [DOI] [PubMed] [Google Scholar]
- 12.Fraser LA, Langsetmo L, Berger C et al Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int 2011; 22: 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalmohamed A, Welsing PM, Lems WF et al Calibration of FRAX ® 3.1 to the Dutch population with data on the epidemiology of hip fractures. Osteoporos Int 2012; 23: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black DM, Cummings SR, Karpf DB et al Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348: 1,535–1,541. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger B, Black DM, Mitlak BH et al Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. JAMA 1999; 282: 637–645. [DOI] [PubMed] [Google Scholar]
- 16.Meunier PJ, Roux C, Seeman E et al The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004; 350: 459–468. [DOI] [PubMed] [Google Scholar]
- 17.Reginster JY, Seeman E, De Vernejoul MC et al Strontium ranelate reduces the risk of nonvertebral fracture in postmenopausal women with osteoporosis Treatment of Peripheral Osteoporosis (TROPOS) study. J Clinl Endocrinol Metab 2005; 90: 2,816–2,822. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, San Martin J, McClung MR et al Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey EV, Johansson H, Oden A et al Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27: 1,480–1,486. [DOI] [PubMed] [Google Scholar]
- 20.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 2006; 38: 922–928. [DOI] [PubMed] [Google Scholar]


