Highlight
Maize lateral root primordia 1 encodes an auxin-inducible transcriptional activator confined to root primordia that is repressed by direct promoter binding of the Aux/IAA protein ROOTLESS WITH UNDETECTABLE MERISTEM 1.
Key words: Aux/IAA, crown root, differentiation zone, LATERAL ROOT PRIMORDIA 1 (LRP1), maize, RUM1, transcriptional activator.
Abstract
Only little is known about target genes of auxin signalling downstream of the Aux/IAA-ARF module. In the present study, it has been demonstrated that maize lateral root primordia 1 (lrp1) encodes a transcriptional activator that is directly regulated by the Aux/IAA protein ROOTLESS WITH UNDETECTABLE MERISTEM 1 (RUM1). Expression of lrp1 is confined to early root primordia and meristems and is auxin-inducible. Based on its primary protein structure, LRP1 is predicted to be a transcription factor. This notion is supported by exclusive LRP1 localization in the nucleus and its ability to activate downstream gene activity. Based on the observation that lrp1 transcription is completely repressed in the semi-dominant gain of function mutant rum1, it was demonstrated that the lrp1 promoter is a direct target of RUM1 proteins. Subsequently, promoter activation assays indicated that RUM1 represses the expression of a GFP reporter fused to the native promoter of lrp1. Constitutive repression of lrp1 in rum1 mutants is a consequence of the stability of mutated rum1 proteins which cannot be degraded by the proteasome and thus constitutively bind to the lrp1 promoter and repress transcription. Taken together, the repression of the transcriptional activator lrp1 by direct binding of RUM1 to its promoter, together with specific expression of lrp1 in root meristems, suggests a function in maize root development via the RUM1-dependent auxin signalling pathway.
Introduction
Plant roots are instrumental for water and nutrient uptake and for the anchorage of plants in the soil (Hochholdinger et al., 2004a). The monocotyledonous model plant Zea mays L. exhibits a complex root stock architecture which comprises embryonically formed primary and seminal roots and postembryonic lateral and shoot-borne roots (Hochholdinger and Tuberosa, 2009). The postembryonic root system makes up the major backbone of the plant (Hochholdinger et al., 2004b). Lateral roots are, per definition, roots that emerge from other roots. Lateral roots are initiated in pericycle cells of all root-types of maize and emerge a few days after the formation of the main roots.
In Arabidopsis, the initiation of lateral roots starts with the dedifferentiation of pericycle founder cells located at the xylem pole and leads to the formation of a small primordium which finally breaks the outer tissues to emerge from the parental root (Dolan et al., 1993). By contrast, lateral roots in maize are initiated from pericycle and endodermal cells at the phloem poles (Hochholdinger and Zimmermann, 2008).
The plant hormone auxin plays a pivotal role in the co-ordination of almost all developmental processes including root patterning. Auxin maxima in phloem pole pericycle cells are required for the initiation of lateral root primordia in maize (Jansen et al., 2012). The maize rum1 mutant is defective in the initiation of lateral root primordia in primary roots and displays an 83% reduction in polar auxin transport in mutant primary roots compared with the wild-type (Woll et al., 2005). The rum1 gene encodes Aux/IAA10 (von Behrens et al., 2011). Aux/IAA (Auxin/Indole-3-Acetic Acid) proteins are involved in auxin signal transduction by interacting with ARF (Auxin Response Factor) proteins. These Aux/IAA–ARF complexes regulate the transcription of early auxin-responsive genes such as Aux/IAAs, SAURs, and GH3s by ARF binding to their auxin-responsive elements (AuxREs) in their promoters (Reed, 2001; Woodward and Bartel, 2005). An increase in cellular auxin levels leads to a rapid degradation of Aux/IAA proteins by the 26S proteasome and, as a consequence, ARF mediated transcription of downstream auxin-responsive target genes (Abel, 2007).
The plant specific family of SHORT INTERNODES-RELATED SEQUENCE (SRS) proteins in Arabidopsis consists of ten members (Eklund et al., 2010a). The proteins encoded by this gene family display a zinc-finger motif (Fridborg et al., 1999) and two putative nuclear localization signals (NLSs) (Eklund et al., 2010a). Moreover, they contain a C-terminal IGGH domain which was shown to be required for homodimerization (Eklund et al., 2010a). LRP1 (LATERAL ROOT PRIMORDIUM 1), a member of the SRS family, is involved in early lateral root formation in Arabidopsis (Smith and Fedoroff, 1995). Arabidopsis lrp1 transcripts were only detected during the early stages of lateral and adventitious root primordia formation but not at the later stages of primordia development before the emergence of these structures (Smith and Fedoroff, 1995). SWP1, which is involved in the regulation of flower timing, represses Arabidopsis lrp1 by histone deacetylation (Krichevsky et al., 2009). Yeast two-hybrid assays demonstrated the ability of Arabidopsis LRP1 to form homodimers and heterodimers with members of the SRS family (Kuusk et al., 2006).
In the present study it has been demonstrated that lrp1 activity in maize is regulated by binding of the Aux/IAA protein RUM1 to the lrp1 promoter. Subsequent analyses demonstrated that LRP1 functions as a transcriptional activator of downstream gene expression which is in line with its localization in the nucleus. In summary, the auxin-inducible LRP1 protein is involved in maize auxin signal transduction downstream of rum1 which regulates the initiation of lateral and seminal roots.
Material and methods
Plant growth and auxin treatment
Seeds of the maize inbred line B73 were germinated in paper rolls as previously described (Ludwig et al., 2013). Five-day-old maize seedlings were treated with 5 μM of the auxin analogue 1-NAA, maize primary roots were subsequently harvested 0, 1, 2, or 3h after 1-NAA treatment (Ludwig et al., 2013), then immediately frozen in liquid nitrogen and stored at -80 °C for subsequent analyses.
Semi-quantitative RT-PCR and qRT-PCR experiments
Semi-quantitative RT-PCR experiments were performed with RNA extracted from pools of primary root samples. For root-type-specific expression studies in the wild-type, lrt1 (Hochholdinger and Feix, 1998) and rum1 (Woll et al., 2005) mutant background, separate pools of primary roots with a length of >4cm were harvested, frozen in liquid nitrogen, and processed immediately for total RNA isolation using Trizol (Invitrogen). At this stage, lateral root formation was initiated in all wild-type primary roots as illustrated by Feulgen-staining experiments (data not shown). Subsequently, samples were incubated overnight with RNAse-free DNAseI (Invitrogen) and reverse transcription was performed using SuperScriptII (Invitrogen) reverse transcriptase according to the manufacturer’s protocol. PCR was performed using the oligonucleotide primers ZmLrp1-semi-fw and ZmLrp1-semi-rv (ZmLrp1, GRMZM2G077752; see Supplementary Table S1 at JXB online) and LA-Taq Polymerase (TaKaRa) following the manufacturer’s guidelines. The housekeeping gene actin1 (Genebank AC: AY104722) was used as standard with the oligonucleotide primers Actin-fw and Actin-rv (see Supplementary Table S1 at JXB online). All PCR experiments were repeated in four biological replicates to confirm the reproducibility of the results.
For qRT-PCR experiments, total RNA was extracted from pools of different root tissues and subsequently treated with RNase-free DNaseI as previously described (von Behrens et al., 2011). cDNA was synthesized from total RNA via the qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA), and q-RT-PCR experiments were performed as previously described (Zhang et al., 2014). Four biological replicates in three technical replicates for each pool of different root tissues were analysed in qRT-PCR experiments. Lrp1 transcripts were assayed relative to myosin (von Behrens et al., 2011). The oligonucleotide primers ZmLrp1-qPCR-fw and ZmLrp1-qPCR-rv, and oligonucleocleotide primers Myosin-fw and Myosin-rv of the reference gene myosin1 (486090G09.x1) were used to analyse these gene expression patterns (see Supplementary Table S1 at JXB online).
Transcript abundance of lrp1 after auxin induction was assayed relative to myosin for each time point. Differential gene expression was determined by Student′s t test (*, P ≤0.05; **, P ≤0.01; ***, P ≤0.001; n=4).
Subcellular localization
In order to construct the maize lrp1–GFP plasmid, the open reading frame of lrp1 was PCR amplified from the GAL4DB-LRP1 plasmid (Lab AC: 761) using ZmLrp1-KpnI-fw and ZmLrp1-BspHI-rv, and then introduced into the plant transformation vector CF203 at the restriction sites KpnI and BspHI (Karin Schumacher, University of Heidelberg) yielding a construct containing a constitutive cauliflower mosaic virus (CaMV) 35S promoter at the 5′ end of the coding sequence of lrp1 and a 3′ in-frame GFP sequence followed by an rbcs E9 terminator (Lab AC: 755). The subcellular localization experiment was performed by transiently transforming the plasmid 35S::ZmLrp1–GFP into Arabidopsis Col-0 protoplasts grown in suspension culture for 3 d in the dark. Protoplasts were generated according to Negrutiu et al. (1987). Transformation was performed with the PEG method (Merkle et al., 1996) and incubated overnight in the dark at 26 °C in MSCol medium (Liu et al., 2003). The transformed protoplasts were directly examined with a HCX PL APO 63x/1.2W CORR water-immersion objective (Leica Microsystems, Wetzlar, Germany) of a TCS SP2 AOBS confocal microscope (Leica Microsystems). GFP was excited with an argon laser at 488nm and the emitted fluorescence was detected with an argon–krypton laser at 509nm. Image processing was performed with Leica Confocal Software (Leica Microsystems). Epifluorescence images were taken from the identical protoplast that was analysed for green fluorescence localization.
Transient luciferase expression assays
The control effector vector containing the GAL4 DNA binding domain (Lab AC: 1002), the reporter vector with firefly luciferase (LUC, Lab AC: 1004), and the reference reporter vector with Renilla luciferase (Lab AC: 999) were constructed as previously described (Majer et al., 2012). For generating the effector plasmid GAL4DB-LRP1, the full-length coding sequence of lrp1 was amplified by nested PCR with oligonucleotide primers ZmLrp1-luc-fw, ZmLrp1-luc-rv and ZmLrp1-luc-SmaI-fw, ZmLrp1-luc-KpnI-rv from cDNA of 5-day-old maize primary roots (see Supplementary Table S1 at JXB online). These sequences were subsequently introduced into the control effector at the SmaI and KpnI sites (Lab AC: 761). The reporter, effector, and reference plasmids were co-transformed into Arabidopsis Col-0 protoplasts (Li et al., 2010b).
LUC assays were performed with the dual-luciferase reporter assay system (Promega) via a TriStar multimode microplate reader LB 941 (Berthold, Bad Wildbad, Germany). Measurement of LUC activity was repeated three times for each transformant and LUC values were normalized with the internal control Renilla LUC values. Each transformation was independently repeated three times.
In situ hybridization analyses
Root samples were fixed in 4% formaldehyde in phosphate-buffered saline overnight, dehydrated in an increasing ethanol series, and embedded in paraffin wax (Paraplast plus; Sigma-Aldrich). Tissue sections (7 μm) were prepared using a Leica rotary microtome 2035 and transferred on Superfrost Plus slides (Microm). Templates for all in situ hybridization probes were cloned into the pGEM-T Easy vector (Promega) and amplified using a combination of a gene-specific and a M13 forward or M13 reverse primer, respectively. All probes were transcribed using an in vitro transcription kit containing digoxigenin-labelled UTP (Roche). In situ hybridization experiments were performed according to Jackson (1991).
EMSA (Electrophoretic Mobility Shift Assay)
A 75bp probe containing an AuxRE motif in the central position was amplified from the 2kb promoter sequence of lrp1 with the oligonucleotide primers ZmLrp1-EMSA-fw and ZmLrp1-EMSA-rev from pZmLrp1-pGEM (Lab AC: 387) and subcloned into pGEM-T easy (Lab AC: 496). EMSA experiments were performed as described in the Promega technical bulletin TB110 (www.promega.com/tbs/tb110/tb110.pdf). The 75bp probe used for the DNA-protein binding reaction was labelled with [γ-32P]-ATP (Hartmann Analytic, Braunschweig, Germany) with a T4 polynucleotide kinase (Fermentas, St Leon-Roth, Germany). 30 μg of the RUM1 raw protein extract containing recombinant GST-RUM1 fusion proteins were incubated with [γ-32P]-ATP-labelled DNA fragments and 1 μg of poly-(dI-dC) in 10× buffer (100mM TRIS pH 7.5; 500mM NaCl; 10mM EDTA; 10mM DTT) in a total volume of 30 μl. The reaction product was analysed on a 4% non-denaturing polyacrylamide gel. The specificity of GST-RUM1 binding was controlled by using 50× excess of the specific competitor (unlabelled target sequence) and λ-DNA (Fermentas) as the unspecific competitor, respectively.
Promoter activation assays
The promoter fragments containing AuxRE of lrp1 were amplified by PCR with oligonucleotide primers pZmLrp1-XbaI-fw and pZmLrp1-XhoI-rv (see Supplementary Table S1 at JXB online) from pZmLrp1-pGEM (Lab AC: 387) using Pfu DNA Polymerase (Fermentas) and cloned into the GFP reporter vector (pZmLrp1-pGTKan, Lab AC: 747). The full-length open reading frame of rum1 was cloned into the binary vector pUC-SPYCE which contains a C-terminal c-Myc-tag as previously described (von Behrens et al., 2011). Five micrograms of reporter construct pZmLrp1-pGTKan was co-transformed with 20 μg of effector vector RUM1-SPYCE or blank-SPYCE (von Behrens et al., 2011). The pBT8-35SLUCm3 vector was used for normalization of the transformation efficiency (Li et al., 2010a). The transfected protoplasts were incubated as described above. GFP fluorescence was measured after or without treatment with 10 μM MG-132 for 2h. These experiments were replicated independently three times.
Results
Phylogeny and synteny of the maize LRP1-LIKE family
The Arabidopsis SHORT INTERNODES-RELATED SEQUENCE (SRS) gene family comprises ten members. Among those, the LRP1 gene encodes a protein that is expressed during early lateral primordia formation. To gain a comprehensive overview of this plant-specific gene family of transcription factors in maize, the Arabidopsis LRP1 protein sequence (Genbank AC: AT5G12330) was used as a query for homology searches in maizegdb.org. As a result, nine SHORT INTERNODES-RELATED SEQUENCE (SRS) proteins designated LRP1 and LRP1-Like1 (LRL1) to LRP1-Like8 (LRL8) were identified (see Supplementary Table S2 at JXB online). All nine maize genes were assigned to the two maize subgenomes which emerged as a consequence of a whole genome duplication 5–12 million years ago. Hence, all members of the maize gene family were already present in the maize genome before the last whole genome duplication (Schnable et al., 2011; www.skraelingmountain.com/datasets/maize_indexed_by_subgenome.csv). Maize lrl1, lrl3, lrl4, lrl6, and lrl8 were assigned to subgenome 1, while lrp1, lrl2, lrl5, and lrl7 were assigned to subgenome 2. Among the nine genes, three pairs of paralogues were identified (see Supplementary Table S2 at JXB online).
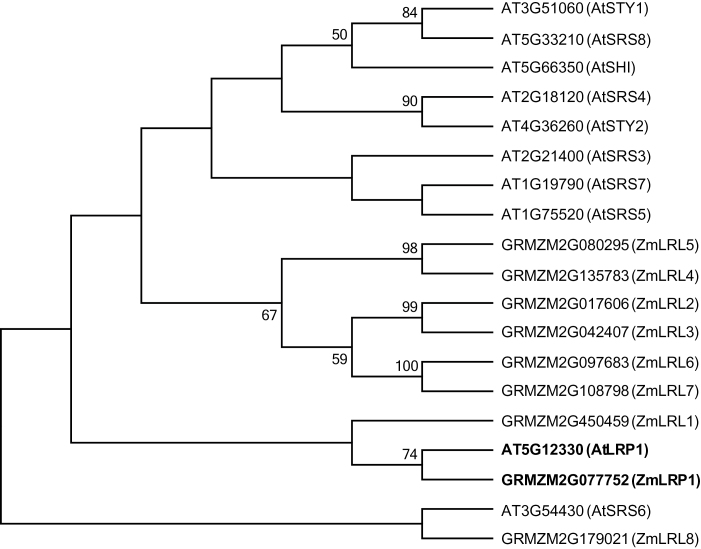
To study the relationship between the Arabidopsis and maize protein families phylogenetic reconstructions were performed (Fig. 1). The resulting phylogenetic tree revealed that maize GRMZM2G077752, which was designated LRP1, is the closest homologue of AtLRP1. Maize LRL1, which is not a paralogue of maize LRP1, is also closely related to these two proteins. For all other proteins, no one-to-one correlation of homologous pairs was established. The bootstrap values of the two members of a small outgroup AtSRS6 and maize LRL8 were too small to establish a significant correlation between these sequences. All other maize and Arabidopsis proteins in Fig. 1 group in an Arabidopsis- and a maize specific clade. These phylogenetic relations support the notion of functional diversification of most maize and Arabidopsis SRS proteins with the exception of the protein pair maize LRP1/Arabidopsis AtLRP1, which might have conserved their molecular function to some extent during evolution.
Fig. 1.
The plant-specific SRS protein family. Phylogenetic relationships of the maize and Arabidopsis SRS families were generated by MEGA5. Maize LRP1 and Arabidopsis AtLRP1 are highlighted in bold.
Based on this result, the maize LRP1 protein was subjected to a detailed functional characterization.
Maize lrp1 is expressed in lateral and crown root primordia and is auxin-inducible
Root-type and development-specific expression patterns of maize lrp1 were analysed in the inbred line B73 by qRT-PCR. Transcripts of lrp1 were detected in all root types (Fig. 2A). Expression in crown roots was significantly higher than in all other root types under analysis (see Supplementary Table S3 at JXB online). By contrast, expression in young primary roots of 1–2cm length was significantly lower than in all other tissues. Finally, expression in seminal roots was significantly higher than in all analysed stages of primary root development but significantly lower than expression in crown roots. In summary, primary, seminal, and crown roots displayed discrete expression levels that distinguished between these root types. Lateral roots displayed a similar expression level as seminal roots that was significantly higher than 1–2cm primary roots but lower than crown roots.
Fig. 2.
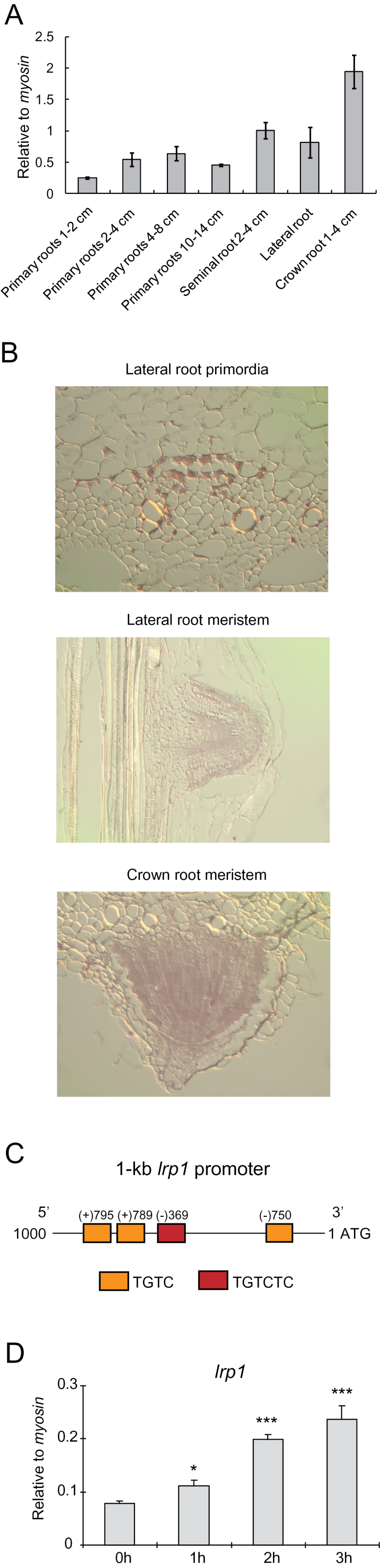
Expression patterns of lrp1 in root tissues. (A) qRT-PCR analyses demonstrated the expression of lrp1 in seven specific different root tissues. (B) In situ hybridization experiments revealed the expression of lrp1 in crown root meristems and lateral root primordia and meristems of maize roots. (C) AuxRE analysis of 1-kb lrp1 promoters of maize. TGTCTC is highlighted by a red box, and TGTC is denoted by a yellow box. (D) Expression of lrp1 in primary roots of wild-type seedlings after auxin treatment with 5 μM 1-NAA for 0, 1, 2, or 3h, assayed by qRT-PCR relative to myosin (*, P ≤0.05; **, P ≤0.01; ***, P ≤0.001; n=4). (This figure is available in colour at JXB online.)
To study expression of lrp1 in more detail, in situ hybridization experiments were performed in postembryonic lateral roots and crown roots. These root types were selected because the formation of these roots can be observed after germination, while primary and seminal roots are formed early during embryo development deep inside the maize seed. In both root types, expression of lrp1 was confined to early root primordia and meristems (Fig. 2B).
Promoter analysis of 1kb upstream of the ATG start codon of lrp1 revealed both types of auxin response elements (AuxRE) 5′-TGTCTC-3′ (once) and 5′-TGTC-3′ (three times) (Fig. 2C). Auxin-inducibility of lrp1 was tested by qRT-PCR in 5-day-old maize B73 primary roots at 5 μM 1-NAA (Fig. 2D). The experiment demonstrated that lrp1 is auxin inducible with a >3-fold increase of expression within 3h.
LRP1 is localized in the nucleus and acts as a transcriptional activator
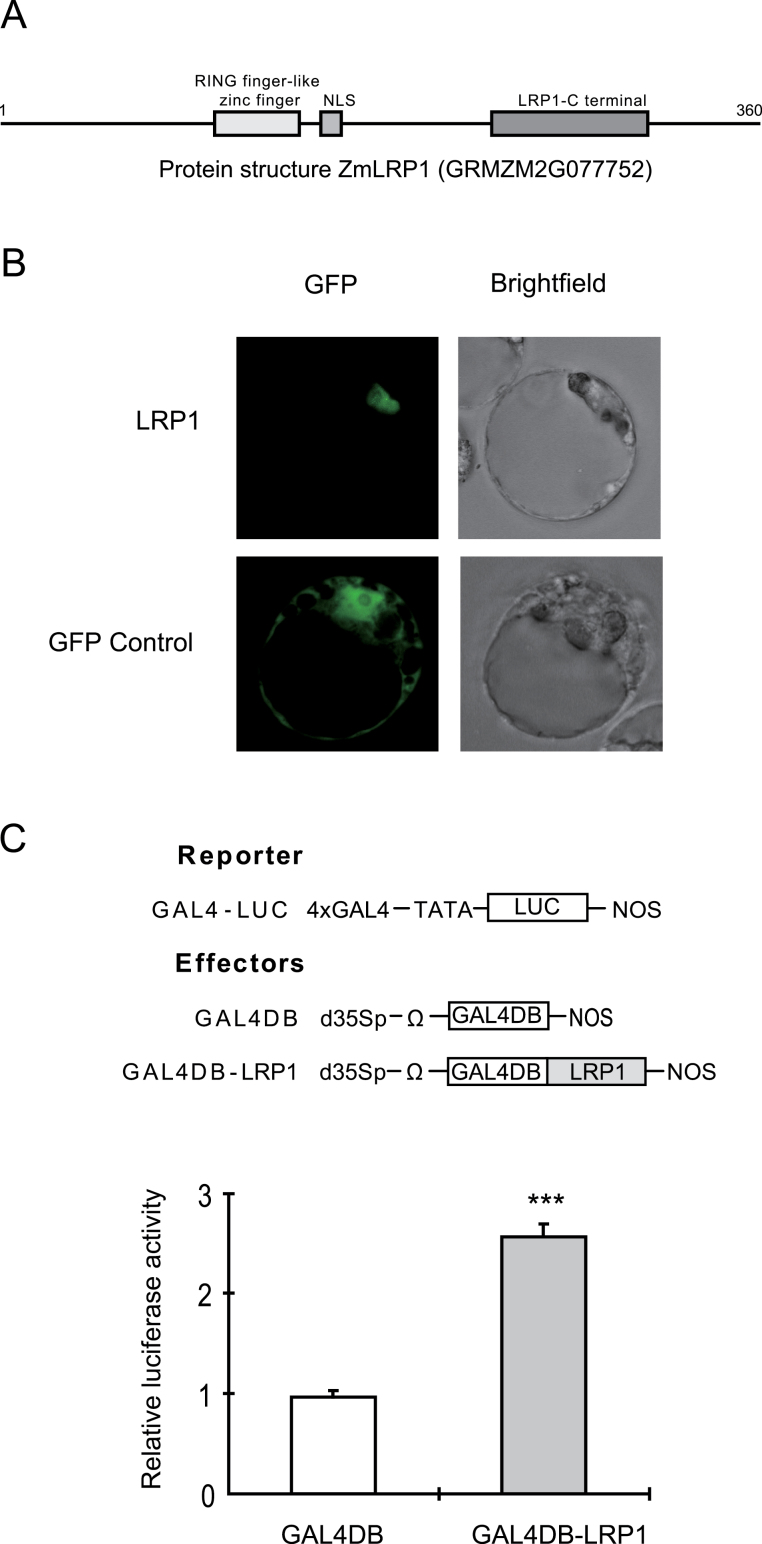
The conserved RING finger-like zinc finger, which is a putative DNA binding domain, the nuclear localization signal (NLS), and the LRP1-C terminal domain, which is predicted to be a protein-protein interaction domain (Fig. 3A), suggest that maize LRP1 functions as a transcription factor. To determine the functionality of the predicted nuclear localization signal (NLS) subcellular localization experiments expressing LRP1–GFP fusion proteins in Arabidopsis Col-0 protoplasts were performed. LRP1–GFP proteins were exclusively localized in the nucleus (Fig. 3B). By contrast, the GFP control protein displayed a constitutive localization in the nucleus and the cytoplasm (Fig. 3B).
Fig. 3.
LRP1 acts as a transcriptional activator. (A) The protein structure of maize LRP1. The RING-like zinc finger domain, nuclear localization signal, and LRP1 C-terminal domain are shaded in grey. (B) Subcellular localization of LRP1. The GFP control proteins are localized in both the cytoplasm and the nucleus while the wild-type LRP1–GFP fusion protein localizes only to the nucleus. (C) LRP1 transient co-transfection assay. After co-transformation of Arabidopsis Col-0 protoplasts with the reporter construct GAL4-LUC, the effector construct GAL4DB and GAL4DB-LRP1, and the reference construct, the relative luciferase activities are assayed. All luciferase activities are expressed relative to values obtained with the GAL4DB control (GAL4DB set arbitrarily at 1). (This figure is available in colour at JXB online.)
To investigate the capacity of LRP1 to control transcription of downstream genes, a luciferase reporter assay was performed by transiently co-transfecting Arabidopsis protoplasts. The effector plasmids consisted of the yeast GAL4 DNA binding domain (GAL4DB) as a control or the GAL4DB fused in-frame with the coding sequence of lrp1 (GAL4DB-LRP1) driven by a dual 35S promoter (Fig. 3C). The reporter plasmid included the LUC gene driven by the minimal TATA box of the cauliflower mosaic virus 35S promoter with four GAL4-binding sites immediately upstream (Fig. 3C). Co-expression of the GAL4-LUC reporter with GAL4DB-LRP1 effector plasmids resulted in a 2.6-times increase of LUC activity compared with co-expression with the GAL4DB control effector (Fig. 3C). These results suggest that LRP1 activates downstream gene expression and functions as a transcriptional activator.
RUM1 interacts with the promoter of the lrp1 gene
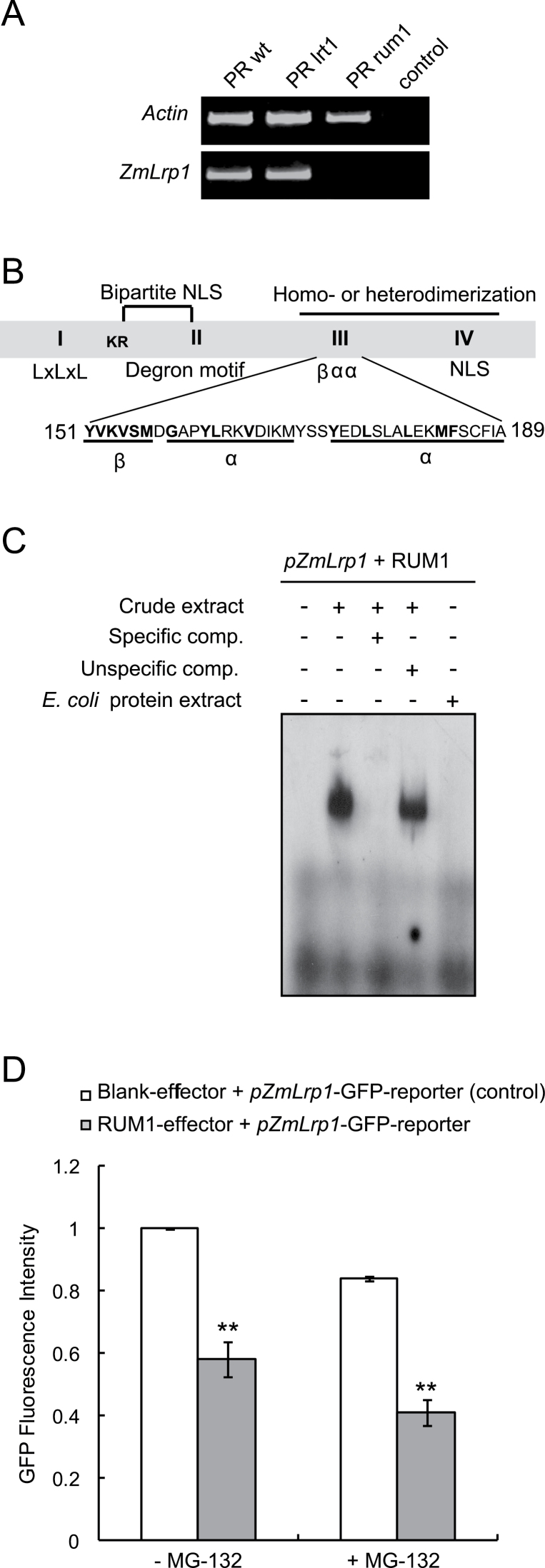
In situ hybridization experiments (Fig. 2B) demonstrated that lrp1 was expressed in lateral root primordia and meristems. In maize, the mutants lrt1 (Hochholdinger and Feix, 1998) and rum1 (Woll et al., 2005) are defective in lateral root formation in primary roots. Therefore, expression of lrp1 in primary roots of these mutants was measured by semi-quantitative RT-PCR (Fig. 4A). Transcripts of lrp1 were detected in wild-type and mutant lrt1 primary roots. However, lrp1 was not expressed in primary roots of the mutant rum1 (Fig. 4A) suggesting that this gene is regulated by the Aux/IAA protein RUM1 (AC: GRMZM2G037368) which is a central regulator of auxin signalling. The role of lrp1 in auxin signalling was supported by the observation that lrp1 is auxin-inducible (Fig. 2D). Aux/IAA proteins regulate the activity of downstream auxin-responsive genes by protein–protein interaction with ARF proteins. However, it has also been suggested that Aux/IAA proteins might directly bind to the promoter motif AuxRE of auxin-responsive genes (Paciorek and Friml, 2006). RUM1 contains a βαα motif in domain III which was predicted to act in DNA-binding (Abel et al., 1994) (Fig. 4B). Therefore, to test whether lrp1 is a direct target of RUM1, an EMSA (Electrophoretic Mobility Shift Assay) experiment with recombinant GST-tagged RUM1 was performed (Fig. 4C). It was demonstrated that RUM1 is able to bind to a 75bp promoter sequence of lrp1 containing an AuxRE (Auxin Responsive Element) motif in a central position (Fig. 4C, lane 2). Specificity of binding was demonstrated by a 50× excess of unlabelled promoter sequence containing the AuxRE motif which prevents sufficient amounts of labelled promoter sequences to bind to RUM1 and generate a detectable shift (lane 3). Moreover, unspecific competition by a 50× excess of λ-DNA which was not bound by RUM1 supported the specificity of the binding (lane 4). To confirm that RUM1 rather than another protein of the bacterial protein lysate binds to the subjected DNA oligonucleotides, crude protein lysate from BL21 cells expressing only GST was applied as a negative control (lane 5).
Fig. 4.
Molecular interaction of RUM1 with lrp1. (A) Semi-quantitative RT-PCR with cDNA based on RNA isolated from wild-type, lrt1 and rum1 primary roots (PR) indicates an absence of lrp1 transcripts in the primary root of the mutant rum1. The water control was negative. (B) Structure of RUM1 protein with conserved domains I, II, III, and IV. Domain I contains the sequence LxLxL which plays a putative role as transcriptional repressor (Tiwari et al., 2004). Degron motif GWPPV in Domain II is responsible for interaction with the SCFTIR complex. Domains III and IV are involved in homo or heterodimerization (Kim et al., 1997; Ulmasov et al., 1997). Domain III contains a βαα motif which is predicted to act in DNA-binding (Abel et al., 1994). The conserved hydrophobic residues characteristic of the predicted amphipathic βαα fold are in bold. (C) Direct binding of RUM1 to [γ-32P]-ATP labelled 75bp lrp1 promoter fragments containing a central AuxRE motif. A 50× excess of unlabelled 75bp probe was used as a specific competitor while a 50× excess of λ-DNA was used as an unspecific competitor. (D) lrp1 promoter activation by RUM1. RUM1 effector co-expressed with lrp1 promoter-driven GFP reporter gene (RUM1+pZmLrp1–GFP-reporter) in Arabidopsis protoplasts under standard conditions or MG-132 treatment after co-transformation. GFP fluorescence intensity was quantified by flow cytometry. The normalization of transformation efficiencies was performed according to Li et al. (2010a). The co-transformation of the blank-effector and pZmLrp1–GFP-reporter was used as a negative control. Statistical significance between the two experiments were determined by Student′s t test (*, P ≤0.05; **, P ≤0.01).
A promoter activation assay was performed in Arabidopsis protoplasts to survey the regulation of lrp1 gene activity by RUM1. In this experiment, relative GFP fluorescence of a reporter plasmid was monitored. A native lrp1 promoter fragment was fused upstream of the GFP fragment (pZmLrp–GFP reporter) and cotransfected with RUM-effector or Blank-effector constructs (see the Materials and methods). It was demonstrated that the presence of RUM1 repressed the expression of the GFP reporter fused to the native lrp1 promoter by 42% relative to the control which was arbitrarily set at 1 (Fig. 4D) after transformation efficiency normalization (see the Materials and methods). When the same experiment was performed adding the proteasome inhibitor MG-132, the expression of GFP under the control of the native lrp1 promoter was further repressed by the presence of RUM1 proteins by 52% relative to the +MG-132 control. This significantly stronger repression in the presence of MG132 is in line with the notion that proteasomal degradation of the Aux/IAA protein RUM1 is inhibited by MG-132, which further represses GFP activity via stabilizing RUM1 binding to the lrp1 promoter (Fig. 4D).
Discussion
The Arabidopsis SHORT INTERNODES-RELATED SEQUENCE (SRS) family of plant-specific transcription factors comprises ten members (Smith and Fedoroff, 1995). Among those, the LRP1 gene is expressed during early lateral primordia formation (Smith and Fedoroff, 1995). Homology searches in the maize genome (maizegdb.org) identified a total of nine homologous maize genes designated lrp1 and lrp1-like1 (lrl1) to lrl8 (see Supplementary Table S2 at JXB online).
About 5–12 mya maize underwent a whole genome duplication which led to the emergence of two subgenomes designated maize 1 and maize 2 (Schnable et al., 2011). Over time, a process called partial fractionation resulted in the loss of one or both copies of the duplicated genes. In the maize lrp1-like gene family, three pairs of paralogous genes (66% of the gene family members) have been retained, while for three additional genes (33% of the gene family members) one paralogue was lost by partial fractionation (see Supplementary Table S2 at JXB online). Similarly, in the larger Aux/IAA gene family of maize, seven pairs of paralogues have been retained (52% of ancient genes) in addition to 13 genes (48% of ancient genes) assigned to a subgenome without a paralogue (Ludwig et al., 2013). Moreover, the maize Aux/IAA family also displays seven genes which emerged by single copy duplications after the last whole genome duplication (Ludwig et al., 2013) while no such gene was observed for the lrp1-like gene family. In general, partial fractionation resulted in more gene loss in subgenome maize 2 compared with subgenome 1 (Schnable and Freeling, 2011). This tendency was also observed for the lrp1-like gene family where two of three (66%) genes assigned to a subgenome without having a paralogue belonged to subgenome 1. Similarly nine of 14 such Aux/IAA genes of maize (64%) were assigned to subgenome 1.
Phylogenetic reconstructions revealed that, among the ten Arabidopsis and nine maize family members, only for maize LRP1 and Arabidopsis AtLRP1 can significant homology based on a bootstrap value >70 be assigned on a one-to-one basis. All other maize and Arabidopsis proteins, except AtSRS6 and ZmLRL8 which represent an outgroup, belong to maize- and Arabidopsis-specific clades. Hence, for only one of nine (11%) maize lrp1-like genes it was possible to determine the Arabidopsis homologue (Fig. 1). A similar frequency was observed in a previous phylogenetic study of the LATERAL ORGANS BOUNDARIES DOMAIN (LBD) family of maize (Majer and Hochholdinger, 2011). For only five out of 43 (12%) lbd genes, were pairs of homologous unambiguous maize/Arabidopsis genes identified (Majer and Hochholdinger, 2011). The difficulty of defining homologous genes between monocot species such as maize and the dicot model Arabidopsis is a consequence of the limited colinearity between these species (Brendel et al., 2002).
In the present study, it was demonstrated by in situ hybridization experiments that the maize lrp1 gene is expressed in emerging lateral root primordia and meristems (Fig. 2B). This is consistent with the expression pattern of AtLRP1 which is involved in Arabidopsis lateral root formation (Smith and Fedoroff, 1995). In addition, it was revealed that lrp1 is also expressed in shoot-borne crown-root primordia of maize (Fig. 2A). The maize root stock is primarily determined by postembryonically formed shoot-borne crown roots while Arabidopsis does not form such roots (Hochholdinger and Zimmermann, 2008). Hence, the homologous maize and Arabidopsis lrp1 genes display conserved expression patterns in lateral roots which are present in both species but also a diversity of expression as a consequence of the structural variation of root stock architecture.
Auxin signal transduction plays a critical role in maize root development as illustrated by root-deficient mutants that are impaired in auxin signal transduction. For instance, the LOB domain protein RTCS controls shoot-borne root initiation (Taramino et al., 2007) while the Aux/IAA protein RUM1 controls lateral root formation in maize (von Behrens et al., 2011). Both genes are auxin-inducible and belong to the classical auxin signalling pathway which also includes the AUXIN RESPONSE FACTORS (ARFs) (Okushima et al., 2007). Aux/IAA genes control the activity the auxin signalling mainly by regulating the expression of downstream transcription factors although only few such factors such as LOB domain proteins have been identified (Benjamins and Scheres, 2008; Lau et al., 2011). In the present study it was demonstrated that lrp1 is a transcription factor involved in auxin signal transduction.
Based on its protein structure containing a DNA binding domain, a nuclear localization signal and a protein–protein interaction domain, maize LRP1 was predicted to be a transcription factor (Fig. 3A). First, it was demonstrated that LRP1–GFP is localized to the nucleus (Fig. 3B). Nuclear localization has also been demonstrated for AtSTY1 another member of this gene family, by transient expression in onion epidermal cells and in protoplasts of the moss Physcomitrella patens (Eklund et al., 2010a). Moreover, it was demonstrated that maize LRP1 acts as a transcriptional activator which was confirmed by transient luciferase assays (Fig. 3C). This result was consistent with results for the gene family members AtSTY1 and PpSHI of Physcomitrella patens which both act as transcriptional activators regulating downstream transcription (Eklund et al., 2010a, b).
A role of LRP1 in auxin signal transduction based on the observation that lrp1 transcription is repressed in the semi-dominant mutant rum1 has been demonstrated in the present study (Fig. 4A). It has been shown that RUM1 binding to the promoter of lrp1 represses transcription of this gene (Fig. 4C, D). The classical model of Aux/IAA function suggests an indirect control of downstream transcription via the interaction with ARF proteins which bind to downstream auxin-responsive elements AuxRE (Quint and Gray, 2006). Direct DNA-binding of Aux/IAA proteins has been suggested previously (Paciorek and Friml, 2006) although no experimental data for such a function of Aux/IAA proteins were thus far available. In Arabidopsis, it was demonstrated that SWP1 represses AtLRP1 by histone acetylation. The swp1-1 mutant seedlings showed a consistent increase in primary root length (Krichevsky et al., 2009). AtLRP1 belongs to the family of SHI proteins (Kuusk et al., 2006) which is involved in cell proliferation and expansion in different developmental contexts. Similarly, maize LRP1 could act as regulator of cell division during the early stages of lateral root formation through a RUM1-dependent pathway.
The results obtained in the present study suggest at least two distinct functions for RUM1. It was previously demonstrated that RUM1 acts as a transcriptional repressor interacting with ZmARF25 or ZmARF34, thereby regulating the transcription of auxin-responsive genes in pericycle cells of primary roots (von Behrens et al., 2011). A second function of RUM1 suggested here might be conferred by a direct binding to promoters of downstream genes. The binding of RUM1 to the promoter of lrp1 has been demonstrated in this study which might play a role in lateral root formation as indicated by the expression pattern of lrp1 in lateral root primordia. Hence, RUM1 might exert an ARF mediated or direct function as transcriptional repressor regulating the activity of downstream auxin-responsive genes.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Sequences of oligonucleotide primers used in this study (restriction sites underlined).
Supplementary Table S2. Characteristics of the lrp1-like gene family in maize (pairs of paralogues are highlighted in different shades of grey).
Supplementary Table S3. Statistical analysis of differential lrp1 gene expression in various root types according to Fig. 2A.
Acknowledgements
We would like to thank Karin Schumacher (University of Heidelberg, Germany) for providing the CF203–GFP vector and Changzheng Xu (INRES, University of Bonn, Germany) for modifying the LUC vectors. Furthermore, we would like to thank Caterina Brancato (ZMBP, Transformation Unit, University of Tuebingen, Germany) for the preparation of protoplasts and excellent technical support. This project was supported by a DFG (Deutsche Forschungsgemeinschaft) grant to FH and a CSC (China Scholarship Council) fellowship to YZ.
References
- Abel S. 2007. Auxin is surfacing. ACS Chemical Biology 2, 380–384. [DOI] [PubMed] [Google Scholar]
- Abel S, Oeller PW, Theologis A. 1994. Early auxin-induced genes encode short-lived nuclear proteins. Proceedings of the National Academy of Sciences, USA 91, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Scheres B. 2008. Auxin: the looping star in plant development. Annual Reviews in Plant Biology 59, 443–465. [DOI] [PubMed] [Google Scholar]
- Brendel V, Kurtz S, Walbot V. 2002. Comparative genomics of Arabidopsis and maize: prospects and limitations. Genome Biology 3, Reviews1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. 1993. Cellular organization of the Arabidopsis thaliana root. Development 119, 71–84. [DOI] [PubMed] [Google Scholar]
- Eklund DM, Staldal V, Valsecchi I, et al. 2010. a . The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. The Plant Cell 22, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Thelander M, Landberg K, et al. 2010. b Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens . Development 137, 1275–1284. [DOI] [PubMed] [Google Scholar]
- Fridborg I, Kuusk S, Moritz T, Sundberg E. 1999. The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein. The Plant Cell 11, 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Feix G. 1998. Early post-embryonic root formation is specifically affected in the maize mutant Irt1 . The Plant Journal 16, 247–255. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. 2004. a . From weeds to crops: genetic analysis of root development in cereals. Trends in Plant Science 9, 42–48. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Tuberosa R. 2009. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology 12, 172–177. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. 2004. b Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany 93, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Zimmermann R. 2008. Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology 11, 70–74. [DOI] [PubMed] [Google Scholar]
- Jackson DP. 1991. In situ hybridisation in plants. In: Bowles DJ, Gurr SJ, McPhereson M, eds. Molecular plant pathology: a practical approach, Oxford: Oxford University Press, 163–166. [Google Scholar]
- Jansen L, Roberts I, De Rycke R, Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philosophical Transactions of the Royal Society London B Biological Sciences 367, 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. 1997. Protein–protein interactions among the Aux/IAA proteins. Proceedings of the National Academy of Sciences, USA 94, 11786–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A, Zaltsman A, Kozlovsky SV, Tian GW, Citovsky V. 2009. Regulation of root elongation by histone acetylation in Arabidopsis. Journal of Molecular Biology 385, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusk S, Sohlberg JJ, Magnus Eklund D, Sundberg E. 2006. Functionally redundant SHI family genes regulate Arabidopsis gynoecium development in a dose-dependent manner. The Plant Journal 47, 99–111. [DOI] [PubMed] [Google Scholar]
- Lau S, De Smet I, Kolb M, Meinhardt H, Jurgens G. 2011. Auxin triggers a genetic switch. Nature Cell Biology 13, 611–615. [DOI] [PubMed] [Google Scholar]
- Li M, Berendzen KW, Schoffl F. 2010. a . Promoter specificity and interactions between early and late Arabidopsis heat shock factors. Plant Molecular Biology 73, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Doll J, Weckermann K, Oecking C, Berendzen KW, Schoffl F. 2010. b Detection of in vivo interactions between Arabidopsis class A-HSFs, using a novel BiFC fragment, and identification of novel class B-HSF interacting proteins. European Journal of Cell Biology 89, 126–132. [DOI] [PubMed] [Google Scholar]
- Liu LH, Ludewig U, Frommer WB, von Wiren N. 2003. AtDUR3 encodes a new type of high-affinity urea/H+ symporter in Arabidopsis . The Plant Cell 15, 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig Y, Zhang YX, Hochholdinger F. 2013. The maize (Zea mays L.) AUXIN/INDOLE-3-ACETIC ACID gene family: phylogeny, synteny, and unique root-type and tissue-specific expression patterns during development. PLoS ONE 8, e78859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer C, Hochholdinger F. 2011. Defining the boundaries: structure and function of LOB domain proteins. Trends in Plant Science 16, 47–52. [DOI] [PubMed] [Google Scholar]
- Majer C, Xu C, Berendzen KW, Hochholdinger F. 2012. Molecular interactions of ROOTLESS CONCERNING CROWN AND SEMINAL ROOTS, a LOB domain protein regulating shoot-borne root initiation in maize (Zea mays L.). Philosophical Transactions of the Royal Society London B Biological Sciences 367, 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle T, Leclerc D, Marshallsay C, Nagy F. 1996. A plant in vitro system for the nuclear import of proteins. The Plant Journal 10, 1177–1186. [DOI] [PubMed] [Google Scholar]
- Negrutiu I, Shillito R, Potrykus I, Biasini G, Sala F. 1987. Hybrid genes in the analysis of transformation conditions. 1. Setting up a simple method for direct gene transfer in plant protoplast. Plant Molecular Biology 8, 363–373. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. 2007. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis . The Plant Cell 19, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Friml J. 2006. Auxin signaling. Journal of Cell Science 119, 1199–1202. [DOI] [PubMed] [Google Scholar]
- Quint M, Gray WM. 2006. Auxin signaling. Current Opinion in Plant Biology 9, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW. 2001. Roles and activities of Aux/IAA proteins in Arabidopsis . Trends in Plant Science 6, 420–425. [DOI] [PubMed] [Google Scholar]
- Schnable JC, Freeling M. 2011. Genes identified by visible mutant phenotypes show increased bias toward one of two subgenomes of maize. PLoS ONE 6, e17855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable JC, Springer NM, Freeling M. 2011. Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proceedings of the National Academy of Sciences, USA 108, 4069–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Fedoroff NV. 1995. LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis . The Plant Cell 7, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramino G, Sauer M, Stauffer J, et al. 2007. The rtcs gene in maize (Zea mays L.) encodes a lob domain protein that is required for postembryonic shoot-borne and embryonic seminal root initiation. The Plant Journal 50, 649–659. [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. 2004. Aux/IAA proteins contain a potent transcriptional repression domain. The Plant Cell 16, 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Behrens I, Komatsu M, Zhang YX, Berendzen KW, Niu XM, Sakai H, Taramino G, Hochholdinger F. 2011. Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. The Plant Journal 66, 341–353. [DOI] [PubMed] [Google Scholar]
- Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F. 2005. Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1 . Plant Physiology 139, 1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. A receptor for auxin. The Plant Cell 17, 2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paschold A, Marcon C, et al. 2014. The Aux/IAA gene rum1 involved in seminal and lateral root formation controls vascular patterning in maize (Zea mays L.) primary roots. Journal of Experimental Botany 65, 4919–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.