Highlight
High expression of the FRD3 gene in the metal hyperaccumulator Arabidopsis halleri stems from complex zinc-regulated transcriptional and post-transcriptional mechanisms present in the non-hyperaccumulating Arabidopsis thaliana.
Key words: Alternative promoter, Arabidopsis halleri, gene regulation, transcript stability, translation, zinc homeostasis.
Abstract
In Arabidopsis thaliana, FRD3 (FERRIC CHELATE REDUCTASE DEFECTIVE 3) plays a central role in metal homeostasis. FRD3 is among a set of metal homeostasis genes that are constitutively highly expressed in roots and shoots of Arabidopsis halleri, a zinc hyperaccumulating and hypertolerant species. Here, we examined the regulation of FRD3 by zinc in both species to shed light on the evolutionary processes underlying the evolution of hyperaccumulation in A. halleri. We combined gene expression studies with the use of β-glucuronidase and green fluorescent protein reporter constructs to compare the expression profile and transcriptional and post-transcriptional regulation of FRD3 in both species. The AtFRD3 and AhFRD3 genes displayed a conserved expression profile. In A. thaliana, alternative transcription initiation sites from two promoters determined transcript variants that were differentially regulated by zinc supply in roots and shoots to favour the most highly translated variant under zinc-excess conditions. In A. halleri, a single transcript variant with higher transcript stability and enhanced translation has been maintained. The FRD3 gene thus undergoes complex transcriptional and post-transcriptional regulation in Arabidopsis relatives. Our study reveals that a diverse set of mechanisms underlie increased gene dosage in the A. halleri lineage and illustrates how an environmental challenge can alter gene regulation.
Introduction
Zinc is an essential micronutrient with numerous important functions in plants but becomes toxic when accumulated in excess (Broadley et al., 2007; Palmer and Guerinot, 2009; Nouet et al., 2011). Plants possess a complex metal homeostasis network that controls the metal supply to tissues throughout development, enabling them to cope with substantial temporal and spatial fluctuations in metal availability in their environment. So-called metal hyperaccumulation, found in approximately 500 plant species, represents a rare and extreme adaptation of the metal homeostasis network. Metal hyperaccumulator plants are able to maintain populations on soils contaminated with highly toxic levels of metals and accumulate extremely high metal concentrations (e.g. >0.3% zinc in leaf dry biomass) in their above-ground tissues (Verbruggen et al., 2009; Krämer, 2010; Hanikenne and Nouet, 2011).
The pseudometallophyte Arabidopsis halleri is a zinc and cadmium hypertolerant and hyperaccumulating species. A. halleri diverged from Arabidopsis thaliana and Arabidopsis lyrata, two non-accumulator and non-tolerant species between 3 and 5.8 million years ago and 0.4 and 2 million years ago, respectively (Yogeeswaran et al., 2005; Clauss and Koch, 2006; Roux et al., 2011). These three species represent an ideal experimental model to examine the mechanisms of evolution of a naturally selected extreme trait.
Metal transport, chelation, and detoxification play important roles in hyperaccumulation and hypertolerance in A. halleri. About 30 genes involved in these processes are constitutively highly expressed in A. halleri when compared with A. thaliana and/or A. lyrata (Krämer et al., 2007; Verbruggen et al., 2009; Krämer, 2010; Hanikenne and Nouet, 2011). For instance, HMA4 (HEAVY METAL ATPASE 4) is critical for high rates of root-to-shoot translocation of zinc by mediating xylem loading in roots and possibly the intercellular distribution in leaves. High expression of HMA4 is required for hyperaccumulation and hypertolerance in A. halleri (Talke et al., 2006; Courbot et al., 2007; Hanikenne et al., 2008). Increased gene product dosage of HMA4 was strongly selected for during the evolutionary history of A. halleri and evolved through tandem triplication and cis-activation of expression of all three gene copies (Hanikenne et al., 2008, 2013). The zinc transporter MTP1 (METAL TOLERANCE PROTEIN 1) is involved in zinc vacuolar storage (Krämer, 2005). MTP1 probably accommodates the high HMA4-dependent metal flux into A. halleri shoots and thus would play an important role in zinc tolerance. The MTP1 gene is constitutively highly expressed in both roots and shoots of A. halleri and is present in four to five copies in the A. halleri genome (Dräger et al., 2004; Talke et al., 2006; Willems et al., 2007; Shahzad et al., 2010).
Gene copy number variation compared with A. thaliana is very likely to contribute to the high expression of many other candidate genes in A. halleri, including several ZIP (ZRT-IRT-LIKE PROTEIN) genes (Talke et al., 2006). At least partially dependent on the activity of HMA4 (Hanikenne et al., 2008), high transcript levels of several ZIP genes presumably result in enhanced rates of root metal uptake or mobilization from root storage sites and contribute to metal partitioning between root and shoot tissues (Talke et al., 2006; Krämer et al., 2007; Lin et al., 2009). High expression of the NAS2 (NICOTIANAMINE SYNTHASE 2) gene provides increased nicotianamine levels for intercellular symplastic mobility of zinc towards the xylem in roots (Deinlein et al., 2012).
The FRD3 (FERRIC CHELATE REDUCTASE DEFECTIVE 3) gene is constitutively highly expressed in roots (15-fold) and shoots (6-fold) of A. halleri compared with A. thaliana (Talke et al., 2006). In A. thaliana, FRD3 encodes a plasma membrane transporter involved in citrate efflux into the xylem (Rogers and Guerinot, 2002; Green and Rogers, 2004; Durrett et al., 2007). Citrate is required for maintaining the apoplastic mobility of iron as a citrate complex in the xylem vessels and in leaf veins (Roschzttardtz et al., 2011). Iron(III)–citrate complexes are responsible for the translocation of an important fraction of iron to shoots (Rellán-Alvarez et al., 2010; Alvarez-Fernández et al., 2014). In A. thaliana, FRD3 is expressed in the root pericycle and vascular cylinder (Rogers and Guerinot, 2002; Green and Rogers, 2004). Mutants of frd3 are reduced in size and chlorotic, and have reduced citrate and iron contents in xylem sap. They accumulate iron in the root vascular cylinder and the leaf apoplast, accumulate manganese, copper, and zinc in both roots and shoots, and exhibit a constitutive root iron deficiency response (Delhaize, 1996; Rogers and Guerinot, 2002; Green and Rogers, 2004). FRD3 is also essential during embryo germination and pollen development (Roschzttardtz et al., 2011).
In an analysis of the natural diversity of zinc tolerance across A. thaliana accessions, Bay-0 was identified as a zinc-tolerant accession, whereas Shahdara (Sha) was zinc sensitive (Richard et al., 2011). A quantitative trait locus underlying the differential zinc tolerance between Bay-0 and Sha accessions mapped to FRD3 (Pineau et al., 2012). Differential zinc tolerance among A. thaliana accessions was linked to different regulation of FRD3 expression in response to excess zinc and altered function of the protein. A excess of zinc had a reduced impact on iron homeostasis in the zinc-tolerant Bay-0 accession, suggesting a function of FRD3 in the connection between zinc and iron homeostasis in A. thaliana (Pineau et al., 2012).
Similar to the role of FRD3 in A. thaliana, highly elevated expression of FRD3 in A. halleri may contribute to zinc tolerance and the ability of A. halleri to maintain iron homeostasis in zinc hyperaccumulating tissues (Shanmugam et al., 2011). FRD3 is a single-copy gene in A. halleri (Talke et al., 2006). This is in contrast to several other genes with key functions in hyperaccumulation and hypertolerance, of which enhanced expression is, at least in part, caused by gene copy number expansion (see above, and Talke et al., 2006; Hanikenne et al., 2008; Shahzad et al., 2010). To reveal the type of alteration underlying the high expression of FRD3 in A. halleri, we compared the determinants of gene expression of A. thaliana and A. halleri FRD3. Here, we have shown that promoter activities of FRD3 genes localize to identical spatial domains in both species, but the genes undergo differential and complex transcriptional and post-transcriptional regulation. In A. thaliana, two FRD3 transcript variants were transcribed from alternative transcription initiation sites. The abundances of the two transcripts were differentially regulated by the zinc supply, favouring a more efficiently translated variant upon zinc excess. Only this latter transcript variant has been retained in A. halleri, allowing enhanced translation. Our data highlight that mechanisms other than copy number expansion and cis-regulatory changes have contributed to altered metal homeostasis during the evolution of metal hyperaccumulation in A. halleri. Moreover, our results contribute a novel aspect to our understanding of the function of FRD3 in zinc homeostasis of both Arabidopsis species.
Materials and methods
Plant material, cultivation, and transformation
The experiments were conducted with A. halleri ssp. halleri (accession Langelsheim) (Talke et al., 2006; Hanikenne et al., 2008), A. thaliana [accession Columbia-0 (Col-0)] or zinc-tolerant (Bay-0, NIL-Bay) and zinc-sensitive (Sha, NIL-Sha) A. thaliana genotypes (Pineau et al., 2012). Stable transformation of A. halleri was performed using a tissue culture-based procedure (Hanikenne et al., 2008). Nicotiana tabacum transient transformations were performed by Agrobacterium infiltration (Docquier et al., 2004). All metal treatments were conducted under 11h light (100 µmol m–2 s–1, 22 °C)/13h dark (20 °C) in a climate-controlled growth chamber (Binder) for A. thaliana and under 16h light (100 µmol m–2 s–1)/8h dark at 19–21 °C in a growth chamber for A. halleri.
Plant experimental treatments
Plants were cultivated hydroponically in modified Hoagland’s medium or on solidified Hoagland’s medium supplemented with 0.8% (w/v) agar (Select Agar; Sigma-Aldrich) in square plastic Petri plates (Greiner Bio-One) (Talke et al., 2006; Hanikenne et al., 2008). Control medium included 1 µM Zn for A. thaliana and 5 µM Zn for A. halleri, and 10 µM FeIII-HBED [N,N’-di(2-hydroxybenzyl)ethylenediamine-N,N’-diacetic acid monohydrochloride] as the source of iron. Excess zinc (ZnSO4.7 H20) was added to the medium as indicated. For zinc deficiency (0 µM Zn) experiments, zinc was omitted from the medium.
A. thaliana seeds were germinated on 0.5× MS medium (Duchefa Biochemie) supplemented with 1% (w/v) sucrose and 0.8% (w/v) agar (Select Agar; Sigma-Aldrich). Four days after germination, the seedlings were transferred to Hoagland agar medium to start the treatments. Root and shoot tissues were harvested separately after 17 d treatments.
A. halleri individuals were cloned vegetatively by direct rooting in hydroponic medium for 4.5 weeks before initiating experimental treatments. Root and shoot tissues were harvested separately after 3 weeks of treatment.
FRD3 promoter cloning
The promoter sequence of the A. halleri FRD3 gene was identified in an A. halleri BAC library (Hanikenne et al., 2008) screened with an AhFRD3 probe (Talke et al., 2006) as described previously (Benderoth et al., 2006). A total of two positive BAC clones was obtained. BAC DNAs were digested with XbaI (a restriction site for XbaI is present 317bp downstream of the AhFRD3 translation initiation codon) and then ligated using T4 DNA ligase. An inverse PCR using primers 5′-CGGCGGTTGGAGTCTCCATTGCCA-3′ and 5′-CGCCGCTAGCTGAGCCGCTCCTAAAC-3′ on the ligation products allowed the amplification of a 2050bp fragment, including the 5′ extremity of the AhFRD3 coding sequence and 1733bp upstream of the ATG, which was cloned and sequenced. The AhFRD3 promoter sequences from the two BACs represented two alleles and were 97% similar, with the exception of a 155bp insertion in one allele.
Cloning
5′ Rapid amplification of cDNA ends (RACE) was conducted using a SMART RACE cDNA amplification kit (Clontech). All FRD3 sequences were PCR amplified (see Supplementary Table S1 available at JXB online for primer sequences) using a proofreading polymerase (Pfu polymerase, Promega; and Bio-X-ACT Long, Bioline) and verified by sequencing.
The AhFRD3 promoter sequence was amplified from genomic DNA of the LAN3.1 genotype of A. halleri ssp. halleri (Hanikenne et al., 2008). The AtFRD3Full (4506bp) and AtFRD3Trunc (2220bp) promoter fragments were amplified from genomic DNA of A. thaliana (Col-0). All promoter fragments, which included the promoter, 5′-untranslated region (5′UTR) and the first 27bp of the respective FRD3 coding sequence were directionally cloned into pENTR/D TOPO (Invitrogen) and then transferred by Gateway recombination into the pMDC163 binary vector in translational fusion with the β-glucuronidase (GUS) gene (Curtis and Grossniklaus, 2003).
The 5′UTRL (89bp) and 5′UTRS (102bp) of AtFRD3, and the 5′UTR (103bp) of AhFRD3 (see Fig. 1) were amplified from A. thaliana and A. halleri cDNA libraries, respectively. 5′UTR fragments were fused to the green fluorescent protein (GFP) coding sequence using a KpnI restriction site included in the primer sequences (Supplementary Table S1), and the 5′UTR–GFP cassettes were subcloned at the AscI and PacI sites of the pMDC32 binary vector downstream from the 35S promoter (Curtis and Grossniklaus, 2003). GFP alone and the luciferase gene (LUC) alone were inserted at the AscI and PacI sites of pMDC32 to generate controls.
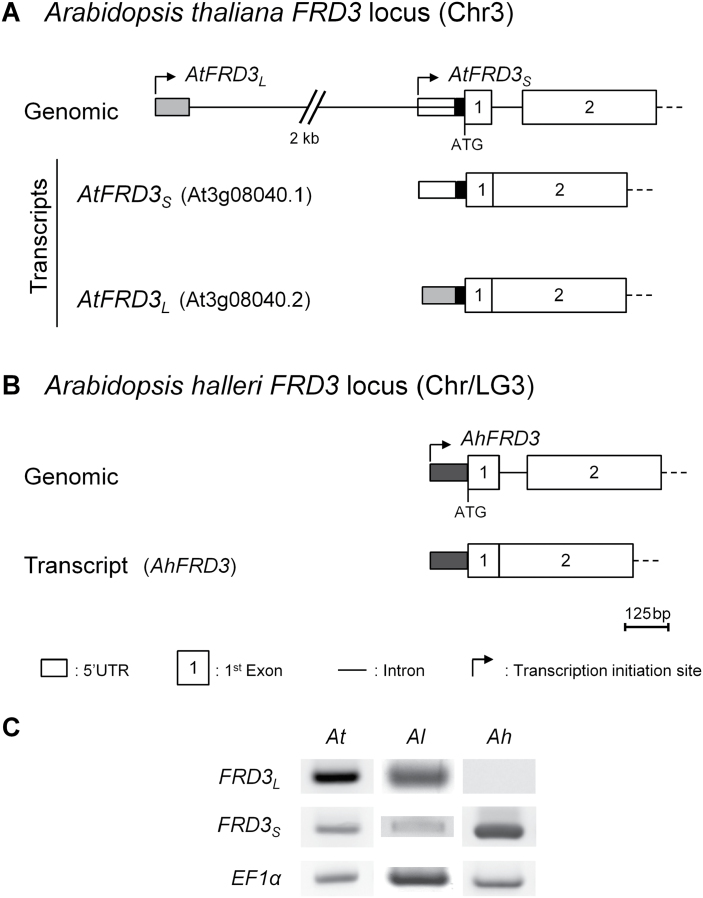
Fig. 1.
Transcription initiation sites of FRD3 genes. (A, B) Organization of the FRD3 genomic loci and transcripts in A. thaliana (A) and A. halleri (B). For narrow rectangles denoting the 5′UTR, distinct white, grey shading, or black represent alternative segments of the FRD3 sequence. (C) FRD3 transcript variants (FRD3 L and FRD3 S) were detected with specific primers by qualitative RT-PCR in shoots of A. thaliana (At), A. lyrata (Al) and A. halleri (Ah). EF1α was used as a control.
Analysis of GUS reporter lines
Histochemical GUS staining (Jefferson et al., 1987) was carried out on T3 homozygous A. thaliana seedlings grown on 0.5× MS medium, and on A. halleri transformants regenerated in tissue culture under selection. Transverse sections of tissues were prepared and imaged as described previously (Hanikenne et al., 2008). Ten independent lines were analysed for each construct for whole mounts, and at least three representative lines for cross-sections. Fluorimetric quantitative GUS activity assays were performed as described elsewhere (Jefferson et al., 1987; Hanikenne et al., 2008).
Gene expression analyses
Total RNAs were prepared using an RNeasy Plant Mini kit with on-column DNase treatment (Qiagen), and cDNAs were synthesized using a RevertAid H Minus First Strand cDNA Synthesis kit with Oligo dT (Thermo Scientific). Steady-state transcript levels were determined following a robust reverse transcription (RT)-PCR procedure (see Supplementary Table S2 available at JXB online for primer sequences). Briefly, after PCR amplification with GoTaq polymerase (Promega), PCR products were separated in a 1.5 % LE agarose gel (SeaKem; Lonza) in sodium borate buffer (Brody et al., 2004). As an inter-run calibrator, a common DNA fragment was loaded on each gel, enabling gel-to-gel comparisons. After gel staining with GelRed (Biotium), the gels were imaged with identical settings using a G:BOX imaging system (Syngene), and absolute band intensities were determined in Photoshop (Adobe Systems Software). Expression values were normalized to EF1α and the inter-run calibrator. Relative AtFRD3 S steady-levels were calculated as a percentage of total AtFRD3 transcripts using the following formula: AtFRD3 S/(AtFRD3 S+AtFRD3 L)×100.
Analysis of metal contents
Root and leaf tissues were harvested separately. Shoot tissues were rinsed in Milli-Q water, whereas root tissues were desorbed and washed as described previously (Talke et al., 2006). Dried tissue samples were acid- digested in DigiPrep tubes with 3ml of ≥65% (w/w) HNO3 (Sigma-Aldrich) on a DigiPrep Graphite Block Digestion System (SCP Science) as follows: 15min at 45 °C, 15min at 65 °C, and 90min at 105 °C. After cooling, sample volumes were adjusted to 10ml with Milli-Q water and 200 µl of ≥65% HNO3. Element concentrations were determined by inductively coupled plasma-atomic emission spectroscopy (Vista AX, Varian).
Cordycepin assay
Cordycepin assays (Gutiérrez et al., 2002) were conducted with 12-d-old A. thaliana seedlings, A. halleri root fragments of 3- to 4-week-old plants, and fragments of tobacco leaf transiently transformed with the genes of interest. Samples were first incubated in Hoagland control medium for 30min at room temperature under slight shaking in the light. At time 0 (T0), samples were vacuum infiltrated with Hoagland control medium containing 100 µM cordycepin (Sigma-Aldrich) for 30 s and then incubated at room temperature in the light. Subsamples (10 A. thaliana seedlings or A. halleri root fragments or five tobacco leaf explants) were harvested at regular time intervals.
Transcript levels were determined by real-time RT-PCR in 384-well plates with an ABI Prism 7900HT system (Applied Biosystems) using MESA GREEN qPCR MasterMix (Eurogentec) as described previously (Talke et al., 2006), including three technical replicates for each sample/primer pair (see Supplementary Table S3 available at JXB online for primer sequences). Transcript levels were normalized to T0 and fitted by non-linear regression according to the following exponential decay formula: Y=(Y 0–plateau)×exp(–K×X)+plateau with fixed parameters (plateau >0, Y 0=1, K>0). Plateau is the Y value at infinite times, K is the rate constant expressed in inverse minutes, and Y 0 is the Y value when X (time) is 0. Half-life was then computed as ln2/K. To test statistical differences between estimated half-life, we followed this non-linear regression by an extra-sum-of-squares F comparison test.
GFP quantification and imaging
At 3–4 d after transient co-transformation with GFP and LUC constructs, tobacco leaf fragments (1cm2) were cut off either for protein extraction or for confocal imaging. Total proteins were extracted from leaf fragments using the CCLR buffer of a Luciferase Assay System kit (Promega). GFP was quantified in protein extracts by fluorimetry (excitation at 485nm and emission at 535nm) using a Victor 3 plate spectrophotometer (Perkin Elmer). GFP fluorescence levels were normalized to LUC activity (Petit et al., 2001) determined using the Luciferase Assay System kit and a tube luminometer (Lumat LB 9501, Berthold).
GFP was imaged by confocal microscopy ensuring that the maximal fluorescence signal was not saturating the photomultiplier tubes, as described previously (Rausin et al., 2010). For GFP quantification, Z-optical sections of 45 and 65 µm were taken in randomly selected zones of leaf fragments. The resulting images were then stacked and three regions of interest were selected in each visible nucleus. GFP signal intensity was measured as the mean grey intensity in each region of interest, and the mean grey intensity for each construction was then computed.
Secondary structure prediction
The structure and minimum free energies of the mature AtFRD3 L and AtFRD3 S full-length transcripts were calculated using Centroidfold with default settings (Sato et al., 2009).
Statistical analysis
All data evaluation and statistics were done using GraphPad Prism 5 (GraphPad Software).
Accession numbers
The sequence of the AhFRD3 promoter is available through EBI (http://www.ebi.ac.uk), accession no. Hx2000040684. The AtFRD3 TAIR accession number is At3g08040 (http://www.arabidopsis.org).
Results
Alternative transcription initiation sites
In A. thaliana, two FRD3 transcript variants, At3g08040.1 and At3g08040.2 (The Arabidopsis Information Resource, http://www.arabidopsis.org), possess distinct transcription initiation sites (Fig. 1A). We verified the transcription initiation sites and expression of the two transcript variants in A. thaliana by 5′RACE. A short transcript (AtFRD3 S or At3g08040.1) was initiated 102bp upstream of the AUG translation initiation codon (Fig. 1A). The long transcript (AtFRD3 L or At3g08040.2) was initiated 2726bp upstream of the AUG. The corresponding pre-mRNA contained a large intron (2637 nt) in the 5′UTR. The mature transcript possessed a 5′UTR of 89 nt (Fig. 1A). The AtFRD3 L and AtFRD3 S transcripts shared 27 nt upstream of the AUG. Out of 17 independently sequenced 5′RACE clones, 13 corresponded to AtFRD3 L and four to AtFRD3 S.
In A. halleri, 5′RACE detected a single transcript (AhFRD3) corresponding to the short variant of A. thaliana, out of 12 independent clones sequenced, with a 103bp 5′UTR not spanning an intron (Fig. 1B). AtFRD3 S and AhFRD3 shared an evolutionary conserved transcription initiation site and their 5′UTRs were 95% identical.
Variant-specific primers were designed based on A. thaliana sequences, which allowed the detection of two FRD3 transcript variants in both A. thaliana and A. lyrata (Fig. 1C). Again, a single transcript variant was detected in A. halleri (Fig. 1C). This suggests that the presence of two FRD3 transcript variants is the ancestral state in the genus Arabidopsis, whereas a single transcript in A. halleri corresponds to the derived state (Supplementary Fig. S1, available at JXB online). In silico analyses of available genomic sequences for FRD3 orthologues suggested the presence of two FRD3 transcript variants in other Brassicaceae, with either the annotation of two variants (Boechera stricta) or sequence conservation at splicing and transcript initiation sites (Capsella rubella, Brassica rapa, and Eutrema halophilum) (http://www.phytozome.net).
Conserved expression profiles
The most detailed analysis of the AtFRD3 expression profile was reported by Roschzttardtz et al. (2011) using a GUS reporter construct that included 1751bp upstream of the ATG as promoter. This reporter construct included the proximal site of transcription initiation only (Fig. 1A, Supplementary Fig. S2 available at JXB online). To determine the expression profile of an AtFRD3 promoter sequence that included both transcription initiation sites, and to examine whether the expression profiles of FRD3 are conserved in A. thaliana and A. halleri, we generated fusions to the GUS reporter. Three constructs were made using full-length (4506bp, pAtFRD3Full) and truncated (2220bp, pAtFRD3Trunc) AtFRD3 promoters, including both transcript initiation sites and the proximal site alone, respectively, and the AhFRD3 promoter (1885bp) (Supplementary Fig. S2). The pAtFRD3 promoter fragments shared 88% sequence identity with pAhFRD3 in the first 269bp upstream from the ATG. Upstream from this proximal fragment, sequence divergence precluded an alignment.
Spatial patterns of reporter activity for all three constructs were highly similar in both A. thaliana and A. halleri (Fig. 2). The FRD3 promoters were active in the root pericycle and vascular cylinder in both species, as described for the A. thaliana gene (Green and Rogers, 2004; Roschzttardtz et al., 2011), and in leaves, mostly in vascular tissues, in hydathodes and in mesophyll cells (Fig. 2). Only a slight GUS coloration could be detected for pAtFRD3Trunc in A. halleri leaves (Fig. 2). Moreover, two GUS transcripts were initiated from the full AtFRD3 promoter in reporter lines of both species (Supplementary Fig. S3 available at JXB online).
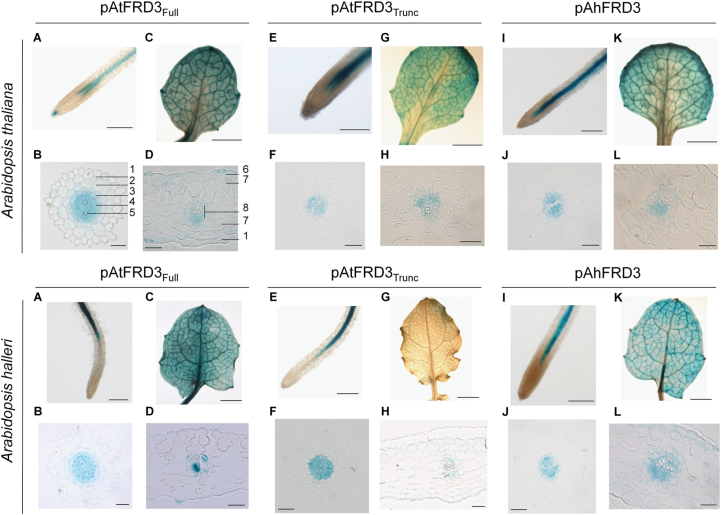
Fig. 2.
Localization of FRD3 promoter activity in A. thaliana and A. halleri. Histochemical detection of GUS activity (blue) directed by a full (pAtFRD3Full) (A–D) and a truncated (pAtFRD3Trunc) (E–H) A. thaliana FRD3 promoter, or the A. halleri FRD3 (pAhFRD3) promoter (I–L) in whole mounts (A, C, E, G, I, K) and transverse sections (B, D, F, H, J, L) of roots (A, B, E, F, I, J) and leaves (C, D, G, H, K, L) of 3-week-old A. thaliana (top) and A. halleri (bottom) plants. Note that only weak GUS staining was observed for pAtFRD3Trunc in A. halleri shoots. Bars, 1.5mm (A, E, I), 5mm (C, G, K) and 25 µm (B, D, F, H, J, L). 1, epidermis; 2, cortex; 3, endodermis; 4, pericycle; 5, xylem; 6, stomata; 7, mesophyll, 8, vascular bundle.
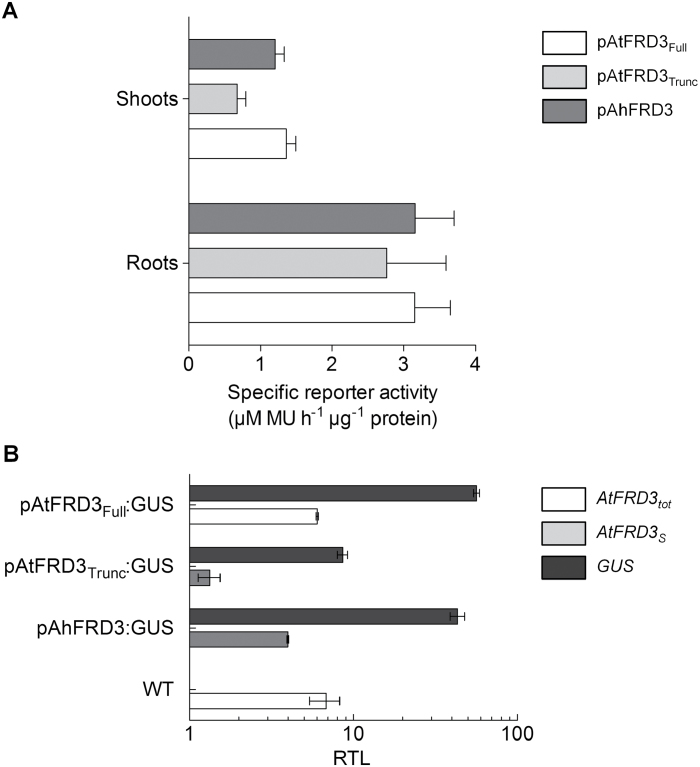
Quantitative analysis of GUS activity in protein extracts from A. thaliana seedlings suggested that the three promoters were more active in roots than in shoots. The pAhFRD3 promoter mediated high GUS transcript expression and high GUS activity in A. thaliana (Fig. 3), which reflected the high expression of FRD3 in A. halleri (Talke et al., 2006). This suggested a conserved regulation in cis for high expression of AhFRD3 in A. thaliana and A. halleri. Surprisingly, the two pAtFRD3 promoter fragments mediated equally high activity of the reporter protein (Fig. 3A). We quantified both GUS and endogenous AtFRD3 transcript levels in reporter lines and observed that GUS activity did not reflect the expression of the endogenous AtFRD3 gene in these plants (Fig. 3B). Indeed, GUS transcript levels were significantly higher than: (i) the total AtFRD3 transcript levels (AtFRD3 tot) in pAtFRD3Full lines expressing both long and short GUS transcript variants (Supplementary Fig. S3), and (ii) AtFRD3 S transcript levels in pAtFRD3Trunc lines, expressing only the short GUS transcript variant (Supplementary Fig. S3) (Fig. 3B).
Fig. 3.
FRD3 promoter activity in A. thaliana. (A) Specific GUS activity was quantified in total protein extracts from roots and shoots of A. thaliana seedlings expressing the GUS reporter gene under the control of a full-length (pAtFRD3Full) and a truncated (pAtFRD3Trunc) A. thaliana FRD3 promoter, or the A. halleri FRD3 (pAhFRD3) promoter. Homozygous seedlings from four independent lines for each construct (T3 generation) were grown on solidified control Hoagland medium. Roots and shoots were harvested separately and pooled per plate (15 seedlings), with two replicate Petri plates per line. Values are mean± SEM with n=4 independent lines from one experiment representative of two independent experiments. MU, 4-methylumbelliferone. (B) Expression analysis of the endogenous AtFRD3 and GUS genes in A. thaliana GUS reporter lines. Steady-state levels of GUS, total AtFRD3 (AtFRD3 tot), and short AtFRD3 (AtFRD3 S) transcripts were determined by real-time RT-PCR in 12-d-old A. thaliana seedlings expressing the GUS reporter gene under the control of a full-length (pAtFRD3Full) and a truncated (pAtFRD3Trunc) A. thaliana FRD3 promoter, or the A. halleri FRD3 (pAhFRD3) promoter. Values are means of three technical replicates of one representative line for each construct grown on solid Hoagland control medium. RTL, relative transcript level.
Differential gene regulation by zinc
So far, little evidence is available supporting a metal-dependent regulation of the FRD3 gene expression in A. thaliana or A. halleri (Rogers and Guerinot, 2002; Talke et al., 2006). We further examined the transcriptional regulation of FRD3 by zinc in root and shoot tissues of A. thaliana. Seedlings were exposed to zinc deficiency or to moderate zinc excess. Under these growth conditions, zinc concentrations in plant tissues reflected metal supply in the medium (Supplementary Fig. S4 available at JXB online) and the zinc-responsive gene AtZIP4 was strongly upregulated in both roots and shoots upon zinc deficiency (Supplementary Fig. S5 available at JXB online), as expected (Talke et al., 2006). The zinc treatments did not alter iron concentrations in the seedlings (Supplementary Fig. S4) or AtIRT1 expression (data not shown).
Upon growth under control conditions, AtFRD3 tot transcript levels were lower in shoots than in roots (Fig. 4A). Transcript levels of AtFRD3 tot in roots did not respond to the zinc supply in the medium, whereas they were significantly increased in shoots upon zinc deficiency (Fig. 4A).
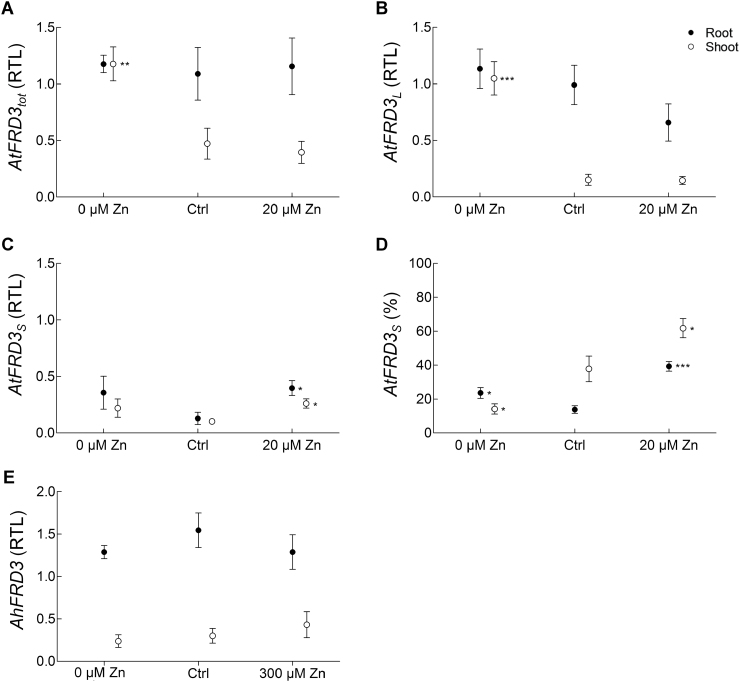
Fig. 4.
Dependence of transcript abundance of FRD3 variants on zinc supply in A. thaliana and A. halleri. Steady-state transcript levels for total AtFRD3 (AtFRD3 tot) (A), AtFRD3 L (B), AtFRD3 S (C), and AtFRD3 S (D) expressed as a percentage of total AtFRD3 transcript levels and for AhFRD3 (E). Steady-state transcript levels were determined in the roots and shoots of A. thaliana and A. halleri cultivated under control conditions (Ctrl), upon zinc deficiency (0 µM Zn) and zinc excess (20 µM Zn for A. thaliana and 300 µM Zn for A. halleri). Values were normalized to EF1α and an inter-run calibrator. The inter-run calibrator differed for each species, and thus transcript levels could only be compared within species. Values are means±SEM of four (A–D) or two (E) independent experiments. Independent experiments included pools of at least 25 A. thaliana seedlings grown on Hoagland agar medium plates (A–D) or six A. halleri plants grown hydroponically in Hoagland medium (E) for each condition. *P<0.05, **P<0.01, and ***P<0.001 according to one-way analysis of variance, followed by Dunnett’s test for multiple comparisons of means. RTL, relative transcript level.
We next examined changes in the levels of the two AtFRD3 transcript variants in response to zinc status. Under control conditions, AtFRD3 S was the minor form, representing ~14% of AtFRD3 transcripts in roots and ~40% in shoots, respectively (Fig. 4D). In both roots and shoots, the levels of AtFRD3 S increased upon zinc excess (Fig. 4C), whereas AtFRD3 L levels were unchanged (Fig. 4B). The differential regulation of the two transcripts by zinc excess significantly modified the AtFRD3 S/AtFRD3 L ratio, with AtFRD3 S representing ~40% of the total transcripts in roots and becoming the dominant form in shoots (~62%) (Fig. 4D). In contrast, AtFRD3 L was strongly induced by zinc deficiency in shoots (Fig. 4B), and this induction accounted for the increased levels of AtFRD3 tot transcripts (Fig. 4A).
For comparison, in hydroponically grown vegetative A. halleri plants, no significant zinc-dependent changes in AhFRD3 transcript levels were observed (Fig. 4E), whereas AhZIP4 transcript levels responded to zinc deficiency or excess (Supplementary Fig. S5), as expected (Talke et al., 2006). Zinc excess resulted in increased root iron contents, but reduced shoot iron levels (Supplementary Fig. S4).
Transcript stability
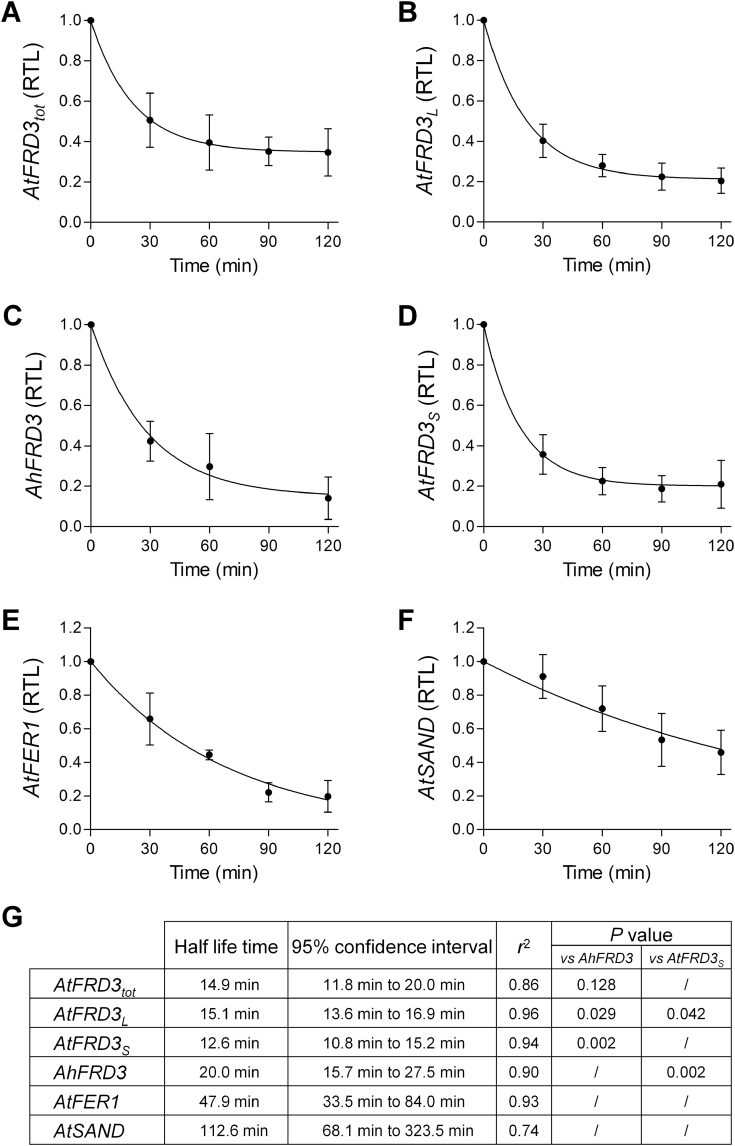
In A. thaliana and A. halleri, AtFRD3 tot and AhFRD3 transcripts accumulated at different steady-state levels, respectively. In A. thaliana, steady-state levels of the AtFRD3 L and AtFRD3 S transcript variants were also distinct. These differences could result from differences in either the rates of transcription or RNA stability. To discriminate between these alternative hypotheses, we determined the half-life time of FRD3 transcripts using cordycepin, an inhibitor of transcription, in A. thaliana seedlings and in roots of vegetative A. halleri plants. The half-life of AhFRD3 transcripts (20.0min) was slightly, but not significantly, longer than that of overall AtFRD3 tot transcripts (14.9min; Fig. 5). Moreover, the half-life of both AtFRD3 transcript variants was similar, with 12.6min for AtFRD3 S and 15.1min for AtFRD3 L (Fig. 5). The FRD3 transcripts could be considered as relatively unstable, compared with the half-life of control genes (47.9 and 112.6min for AtFER1 and AtSAND, respectively; Fig. 5) (Ravet et al., 2012). These data suggested that differences in steady-state transcript levels of AtFRD3 tot and AhFRD3 on the one hand, and of AtFRD3 L and AtFRD3 S on the other hand, were mostly stemming from differential transcription initiation rates. In contrast, AtFRD3 S and AhFRD3 displayed more substantial differences in transcript stabilities (~1.6-fold), which may contribute to overall differences in FRD3 transcript levels in the two species (Fig. 5G).
Fig. 5.
FRD3 transcript stability. (A–F) The half-life times of total AtFRD3 (AtFRD3 tot) (A), AhFRD3 (B), AtFRD3 long (AtFRD3 L) (C), and short (AtFRD3 S) (D) transcripts were determined in the presence of the transcriptional inhibitor cordycepin, with AtFER1 (E) and AtSAND (F) included as controls (Ravet et al., 2012). Seedlings (A. thaliana) and root segments (A. halleri) were collected at several time points after the onset of cordycepin treatment. Transcript levels were determined by real-time RT-PCR. Values were normalized to time 0 and fitted by non-linear regression. (G) Transcript half-life (in min), confidence interval at 95%, regression correlation for the fit of the curve (r 2) and P values of an extra-sum-of-squares F comparison test for statistical differences between estimated half-life. Values are means±SEM of nine (A, C, D, E, F) or four (B) independent experiments. Each independent experiment included at least three technical replicates. RTL, relative transcript level.
Differential translation efficiency
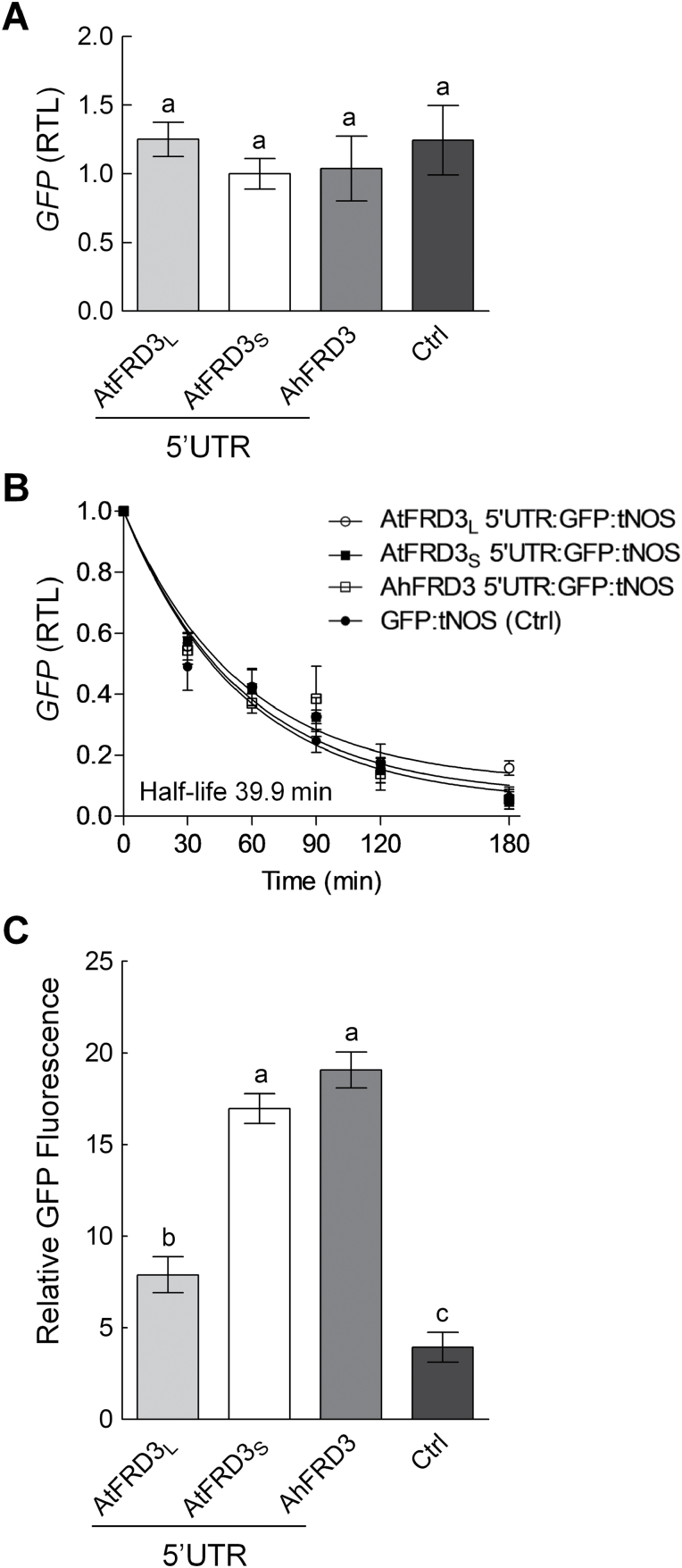
The two AtFRD3 transcript variants differed in their 5′UTR but were strictly identical in their coding sequences. This raised the question of the functional significance of the complex regulation of AtFRD3. We next tested the hypothesis that the alternative 5′UTRs alone had an impact on post-transcriptional processes using a reporter system (Dvir et al., 2013; Kim et al., 2014). To this end, the AtFRD3 L and AtFRD3 S 5′UTRs were cloned upstream of the GFP coding sequence, placed under the control of the cauliflower mosaic virus 35S promoter and transiently expressed in tobacco leaves. A similar construct was generated with the AhFRD3 5′UTR to allow comparison.
Relative abundances of GFP transcripts and their stabilities (half-life of 39.9min) were identical for all three constructs and for a 35S:GFP control (Fig. 6A, B), indicating that the 5′UTRs of FRD3 do not regulate transcriptional processes and transcript stability. In contrast, GFP protein levels were ~2.5-fold higher in tissues transformed with the AtFRD3 S and AhFRD3 5′UTRs by comparison with the AtFRD3 L 5′UTR, respectively (Fig. 6C). Qualitative analyses in vivo using confocal microscopy yielded comparable results (Supplementary Fig. S6 available at JXB online). These data suggested that FRD3 5′UTRs specify differential translation efficiencies.
Fig. 6.
Contributions of the 5′UTR of FRD3 transcript variants to steady-state transcript levels, transcript stability, and levels of the encoded protein. The AtFRD3 L, AtFRD3 S, and AhFRD3 5′UTR fused to the GFP coding sequence were transiently expressed in tobacco leaves by Agrobacterium infiltration under the control of a 35S promoter. A 35S:GFP control (Ctrl) lacking a 5′UTR was included in the experimental design. Leaf fragments were harvested 3–4 d post-infiltration. (A) Steady-state GFP transcript levels were normalized to HygB transcript levels generated in vivo from the introduced T-DNA and are given relative to AtFRD3 S samples. (B) GFP transcript stability (cordycepin assay). Infiltrated leaf fragments were collected at several time points after the onset of cordycepin treatment. GFP transcript levels were determined by real-time RT-PCR. Values were normalized to time 0 and fitted by non-linear regression. (C) Quantification of GFP protein levels in total protein extracts by fluorimetric assay normalized to LUC activity. A 35S:LUC construct was co-infiltrated with all GFP constructs. Different letters above bars indicate significantly different values (P<0.05) according to a t-test. Values are means±SEM of two (A, C) or three (B) independent experiments, respectively. Independent experiments included at least three leaf fragments in three technical replicates. RTL, relative transcript level.
As secondary structures are known to influence translation efficiency (Bugaut and Balasubramanian, 2012; Pichon et al., 2012), we used the Centroidfold algorithm (Sato et al., 2009) to predict the secondary structures of the full-length AtFRD3 transcript variants (Supplementary Fig. S7 available at JXB online). Globally, the two structures were very similar, but a disorganized loop at the 5′ extremity of AtFRD3 L (Supplementary Fig. S7A) was absent in AtFRD3 S (Supplementary Fig. S7B).
AtFRD3 regulation in zinc-tolerant and zinc-sensitive accessions
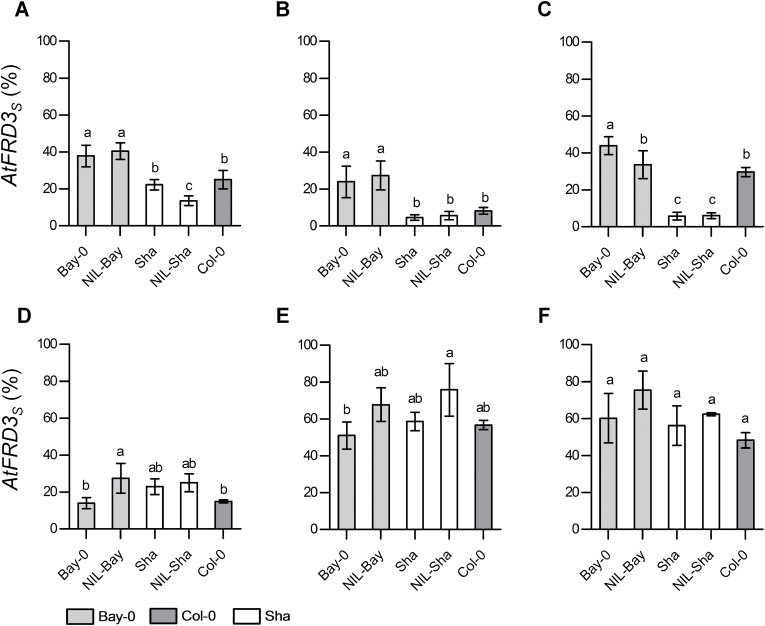
We next examined whether the transcriptional regulation of AtFRD3 tot and the AtFRD3 L and AtFRD3 S transcript variants by zinc differed in zinc-sensitive and zinc-tolerant A. thaliana genotypes. To do so, we made use of the Bay-0 and Sha accessions, as well as two near-isogenic lines, NIL-Bay and NIL-Sha, expressing the FRD3 Bay gene in a Sha genetic background and vice versa (Pineau et al., 2012). In roots, AtFRD3 tot transcript levels were unchanged by zinc in all genotypes including Col-0 (Supplementary Fig. S8 available at JXB online). In contrast, steady-state levels of AtFRD3 S were constitutively higher in roots of zinc-tolerant genotypes (Bay-0 and NIL-Bay) than in Col-0 in control conditions and were only increased slightly in response to zinc treatments (Fig. 7). In zinc-sensitive genotypes (Sha and NIL-Sha), steady-state levels of AtFRD3 S were similar to Col-0 in roots under control conditions. However, the upregulation of the short transcript levels observed in Col-0 in response to zinc excess was lost in Sha and NIL-Sha (Fig. 7). In shoots, levels of AtFRD3 tot were strongly increased under Zn deficiency in all genotypes (Supplementary Fig. S8). The AtFRD3 S/AtFRD3 L ratio as well as the upregulation of AtFRD3 L transcript levels under zinc deficiency were conserved across all five genotypes (Fig. 7).
Fig. 7.
Differential transcriptional regulation of FRD3 in zinc-tolerant and zinc-sensitive A. thaliana genotypes. Steady-state transcript levels were determined for the AtFRD3 short (AtFRD3 S) transcript in roots (A–C) and shoots (D–F) of 21-d-old A. thaliana seedlings grown under control conditions (Ctrl, 1 µM Zn) (B, E), under zinc deficiency (0 µM Zn) (A, D), and under zinc excess (20 µM Zn) (C, F) for 17 d. Bay-0 and NIL-Bay are zinc-tolerant genotypes, whereas Sha and NIL-Sha are zinc-sensitive genotypes (Pineau et al., 2012). Col-0 was included as reference genotype. AtFRD3 S levels are expressed as a percentage of total AtFRD3 transcripts (see Materials and methods). Total steady-state AtFRD3 transcript levels are given in Supplementary Fig. S8. Values were normalized to EF1α and an inter-run calibrator. Values are means±SEM of two independent experiments. Each independent experiment included at least 25 pooled seedlings per treatment and genotype. Different letters above histogram bars indicate significantly different values (P<0.05) within treatments according to a one-way analysis of variance followed by Dunnett’s test for multiple comparison of means.
Discussion
Here, we examined the regulation of FRD3 expression in two closely related Brassicaceae, A. thaliana and A. halleri, with contrasting metal homeostasis (Verbruggen et al., 2009; Krämer, 2010; Hanikenne and Nouet, 2011).
FRD3 is expressed in both roots and shoots
We showed that the spatial pattern of AtFRD3 promoter activity was conserved in roots and shoots of both A. thaliana and A. halleri. In roots, the AtFRD3 promoter was highly active in the pericycle and vascular cylinder in roots (Fig. 2), as described previously (Rogers and Guerinot, 2002; Green and Rogers, 2004; Roschzttardtz et al., 2011). In A. thaliana roots, iron chelation by citrate, which depends on FRD3 function, is of critical importance for root-to-shoot mobility of iron in the xylem (Rogers and Guerinot, 2002; Green and Rogers, 2004; Durrett et al., 2007), and FRD3 contributes to zinc tolerance (Pineau et al., 2012).
FRD3-mediated citrate release into the apoplast also contributes to lateral iron transport from xylem to leaf parenchyma and from the phloem to surrounding tissues in old leaves (Roschzttardtz et al., 2011; Schuler et al., 2012). The AtFRD3 promoter was active in leaf veins and mesophyll cells (Fig. 2). Whereas several studies concluded that AtFRD3 is not expressed in leaves (Rogers and Guerinot, 2002; Green and Rogers, 2004; Roschzttardtz et al., 2011), others reported AtFRD3 expression in leaves using either ATH1 microarrays (Supplementary Fig. S9) or quantitative RT-PCR (Talke et al., 2006). We detected AtFRD3 expression in leaves of both seedlings (Fig. 4A–C) and adult plants (data not shown), but steady-state transcript levels were much lower in leaves than in roots of adult plants. This suggests that AtFRD3 expression in leaves changes dynamically with developmental stages or environmental conditions, which may account for apparent discrepancies in the literature.
Two transcript variants and two promoters for AtFRD3
The AtFRD3 gene gives rise to two transcript variants from alternative transcription initiation sites. A full-length AtFRD3 promoter fragment of approximately 4500bp initiated expression of the two transcripts predominantly in vascular tissues of both A. thaliana and A. halleri (Fig. 2, Supplementary Fig. S3). By contrast, a truncated AtFRD3 promoter including the proximal transcription initiation site contained the elements required for cell-type-specific expression only in A. thaliana and not in leaves of A. halleri. The lack of expression of pAtFRD3Trunc in A. halleri leaves could result either from the absence of the required cis-elements or from the need for a specific trans-factor present only in A. thaliana shoots. Moreover, the data suggest that AtFRD3 transcription is initiated at two core promoters of similar function, one distal and one proximal to the ATG. The presence of TATA boxes ~30bp upstream of both transcription initiation sites supports this hypothesis. Both AtFRD3 promoter fragments specified constitutive and high expression of the GUS gene in both species, at levels similar to AhFRD3 promoter-driven GUS expression (Figs 2 and 3). In A. thaliana reporter lines, the GUS expression levels generated by the full-length AtFRD3 promoter were substantially higher than endogenous FRD3 transcript levels (Fig. 3B), suggesting that this promoter fragment does not contain all cis-elements required to control AtFRD3 expression. In A. thaliana, AtFRD3 transcription may be downregulated by an as-yet-unidentified repressive cis-regulatory element that was not included in the constructs tested in our study. Further work will be required to identify this putative repressor element. The full-length AtFRD3 promoter fragment (4506bp) included the entire intergenic region between AtFRD3 and the 5′ upstream gene (At3g08490), indicating that the repressor element is located either at a greater distance upstream from AtFRD3, within transcribed regions of AtFRD3 (coding sequence, introns, 3′UTR), or in 3′ sequences further downstream from the gene (Ito et al., 2003; Baek et al., 2011; Cao et al., 2014). It is possible that different cis regions of the AtFRD3 gene interact in regulating steady-state AtFRD3 transcript levels and that absence of these interactions in our promoter–reporter constructs contribute to higher-than-expected GUS transcript levels in A. thaliana.
Hidden regulation of AtFRD3 by zinc
Whereas the total transcript levels of FRD3 in A. thaliana roots (Fig. 4A) appeared unresponsive to zinc, we showed here that the steady-state levels of the two transcript variants of AtFRD3 responded differentially to zinc supply (Fig. 4B, C). In control conditions, AtFRD3 L was the predominant form, whereas AtFRD3 S levels were upregulated upon zinc excess in roots (Fig. 4B–D). In shoots, total AtFRD3 transcript levels increased under zinc deficiency (2.5-fold), which was attributable to AtFRD3 L (Fig. 4A, B). In addition, the regulation of the AtFRD3 transcript variants differed between zinc-tolerant and zinc-sensitive A. thaliana accessions, with specific responsiveness to zinc excess (FRD3 S) and a conserved response to zinc deficiency (FRD3 L) (Fig. 7, Supplementary Fig. S8). Thus, in both accessions the responses to zinc excess and zinc deficiency were distinct, suggesting distinct regulatory mechanisms for each variant transcript.
As exposure to excess zinc can result in secondary iron deficiency (Mendoza-Cozatl et al., 2008; Fukao et al., 2011; Shanmugam et al., 2011, 2013; Pineau et al., 2012), it may be postulated that the zinc-dependent regulation of FRD3 reported here was an iron-deficiency response. However, the zinc concentration used was relatively low compared with previous studies (Shanmugam et al., 2011; Pineau et al., 2012), avoiding major toxicity effects on seedlings. This treatment did not cause major alterations in iron concentrations in plants (Supplementary Fig. S5A), suggesting a possible direct regulation of AtFRD3 in response to zinc. Similarly, the zinc-deficiency treatment did not alter iron concentrations in shoot tissues (Supplementary Fig. S5A). The specific regulation of AtFRD3 L observed upon zinc deficiency in shoots probably represents a direct response to this condition. The functional significance of the upregulation of AtFRD3 L by zinc deficiency in shoots remains unclear.
Alternative transcription initiation sites, transcript stability, and impact on translation
The AtFRD3 gene displays complex regulation: two short-lived transcripts, which differ in their 5′UTR only, are initiated from alternative transcription initiation sites and have different translation efficiencies. Large-scale studies in A. thaliana have revealed that, across all transcripts encoded in the genome, mRNA stability varies enormously, with half-life values ranging from 12min to 24h (with a mean of 6h and a median of 2h) (Gutiérrez et al., 2002; Narsai et al., 2007). The AtFRD3 transcript variants group among the transcripts of low stability. In A. thaliana, this would allow rapid shifts from one variant to the other in response to changes in zinc status. A low stability of endogenous AtFRD3 transcripts may partly contribute to the observed differences between GUS transcript levels (~30min half-life; data not shown) and endogenous FRD3 transcript levels in A. thaliana reporter lines (Fig. 3B).
The production of alternative transcripts from a single gene is a widespread phenomenon in eukaryotes and is responsible for proteome diversity. Alternative transcripts can be generated through two types of event: alternative splicing and alternative transcription initiation sites. Both types of event are highly frequent in mammals (Davuluri et al., 2008; Pan et al., 2008; Ma et al., 2009; Shabalina et al., 2010) and plants (Tanaka et al., 2009; Filichkin et al., 2010).
We could not directly evaluate protein accumulation in planta in response to metal treatments as in our hands it was neither possible to raise antibodies against FRD3 nor to detect expression of FRD3 proteins tagged with GFP or a FLAG tag in tissues of either transiently or stably transformed plants (data not shown). Instead, we assessed the functional impact of alternative 5′UTRs on transcript levels, transcript stability, and translation using GFP reporter constructs (Dvir et al., 2013; Kim et al., 2014; Remy et al., 2014). The 5′UTR of AtFRD3 S transcripts gave rise to ~2.5-fold higher protein levels inferred from relative GFP fluorescence compared with AtFRD3 L suggesting a more efficient translation (Fig. 6). Differential translation of the two transcript variants probably amplified the effect of the differential transcriptional changes incurred by the two variants upon zinc treatment (Fig. 4). This complex regulation affords increased protein accumulation, and probably increased citrate efflux into the apoplast, upon zinc excess, even when total AtFRD3 transcript levels remain unchanged. Increased expression of AtFRD3, as observed in a nicotianamine synthase quadruple (nas4x-2) mutant, indeed resulted in increased citrate levels in the xylem sap (Schuler et al., 2012). Similarly, 5′UTR variants of AtZIF2 resulting from alternative splicing promote distinct translation efficiencies and contribute differentially to zinc tolerance (Remy et al., 2014).
There are several mechanisms by which the 5′UTR can influence translation, via the presence of sequence elements controlling ribosome binding and translation initiation, such as the presence of micro-open reading frames (micro-ORFs) (Alatorre-Cobos et al., 2012) or secondary structures (Bugaut and Balasubramanian, 2012; Pichon et al., 2012). In A. thaliana, efficient translation initiation requires a short 5′UTR (between 40 and 175 nt), the absence of micro-ORFs, a specific sequence context around the AUG, and a high A content (Kawaguchi and Bailey-Serres, 2005). We detected no micro-ORF in AtFRD3 transcript variants, and both share a similarly low G+C content with 37.5% for AtFRD3 L and 34.2% for AtFRD3 S. We did not detect the presence of a TAGGGTTT cis element, which confers higher translatability to mRNAs in A. thaliana (Liu et al., 2012). After splicing, both AtFRD3 L and AtFRD3 S 5′UTRs share a similar size. Prediction of the secondary structure of the two transcript variants using Centroidfold (Sato et al., 2009) suggested the presence of a disorganized loop structure in the 5′UTR of AtFRD3 L, which is absent in AtFRD3 S (Supplementary Fig. S7). This difference in secondary structure may account for the distinct translation determined by the two variant 5′UTRs.
A. halleri maintains an ancestral highly expressed and translation-efficient FRD3 transcript
In contrast to A. thaliana, the expression of A. halleri FRD3 was not regulated in response to alterations in zinc supply (Fig. 4E), as shown previously (Talke et al., 2006). The spatial pattern of expression of AhFRD3 was conserved compared with AtFRD3 in roots and shoots of A. halleri and A. thaliana (Fig. 2). Expression of AhFRD3 in shoots corresponded to expectations based on transcript profiling in A. halleri (Talke et al., 2006). AhFRD3 was also expressed in anthers and in the embryo of A. thaliana (data not shown), as reported recently for AtFRD3 (Roschzttardtz et al., 2011).
Whereas the transcription of two FRD3 transcript variants appears to be a shared feature in Brassicaceae, A. halleri constituted an exception with a single short FRD3 transcript (Fig. 1, Supplementary Fig. S1). The transcription initiation sites of AhFRD3 and AtFRD3 S were evolutionarily conserved (Fig. 1), with both transcripts displaying highly similar 5′UTRs (94.2% identity and a low G+C content of ~34%) that determined equally efficient translation (Fig. 6, Supplementary Fig. S6). AhFRD3 transcripts, however, displayed slightly higher transcript stabilities (~1.6-fold) in comparison with AtFRD3 S transcripts (Fig. 5). In addition, as suggested above based on our finding that the AtFRD3 promoter fragment examined in this study directs high constitutive expression, AtFRD3 transcription may be subject to repression through an as-yet-unidentified cis-regulatory element in A. thaliana. Parsimoniously, we suggest that any repressor element present at the AtFRD3 locus has been lost at the AhFRD3 locus, resulting in constitutively high expression of AhFRD3 in A. halleri.
Based on our observations and the considerations detailed above, we propose the following hypothetic scenario, with two loss-of-function events, for the evolution of high expression of FRD3 in A. halleri. The ancestral short transcript variant (FRD3 S), with a short intronless, translation-efficient 5′UTR and pre-existing regulatory responsiveness to zinc excess, was favoured and retained during the evolutionary history of A. halleri. Together with a derepression through mutations in cis and enhanced transcript stability, this allowed constitutively high expression of FRD3 in A. halleri (Talke et al., 2006). Further work will be required to identify the cis-regulatory elements responsible for AhFRD3 high promoter activity.
In A. halleri and in zinc-tolerant A. thaliana genotypes, constitutively higher expression of the FRD3 S transcripts appeared to be associated with increased zinc tolerance (Figs 4E and 7) and possibly with increased metal accumulation. Indeed, constitutive expression of FRD3 S may be responsible for the higher accumulation of zinc observed in shoots of Bay-0 (Pineau et al., 2012). In A. halleri, increased FRD3-mediated release of citrate into the xylem might also contribute to zinc mobility towards the shoot and participate in hyperaccumulation. Indeed, a recent study showed that zinc is predominantly bound to organic acids, including citrate, in the xylem sap of A. halleri (Cornu et al., 2015). Further work, including targeted silencing of FRD3 in A. halleri, will be required to test the function of FRD3 in zinc tolerance.
So far, higher expression levels of metal homeostasis genes have been linked to gene copy number expansion and/or (cis-)regulatory changes in A. halleri compared with A. thaliana (Talke et al., 2006; Hanikenne et al., 2008; Shahzad et al., 2010). In contrast, FRD3 is a single-copy gene, and our data suggest an original path for the evolution of high expression of FRD3 in A. halleri building on complex regulation mechanisms already present in the non-hyperaccumulating ancestor of A. halleri. This suggests that a variety of types of molecular alterations underlie this extreme naturally selected trait.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Distribution of the FRD3 L and FRD3 S transcript variants in Arabidopsis relatives.
Supplementary Fig. S2. FRD3 promoter GUS reporter constructs.
Supplementary Fig. S3. Detection of GUS transcripts in reporter lines.
Supplementary Fig. S4. Metal ion concentrations in A. thaliana and A. halleri plant tissues.
Supplementary Fig. S5. Transcript levels of ZIP4 in response to zinc deficiency and excess in A. thaliana and A. halleri.
Supplementary Fig. S6. Effect of FRD3 variant 5′UTRs onto the translation of a downstream fused GFP-encoding transcript.
Supplementary Fig. S7. Secondary structure prediction of the AtFRD3 transcript variants.
Supplementary Fig. S8. Differential transcriptional regulation of AtFRD3 in zinc-tolerant and zinc-sensitive A. thaliana genotypes.
Supplementary Fig. S9. FRD3 expression profile in A. thaliana.
Supplementary Table S1. List of primers used to generate genetic constructs.
Supplementary Table S2. List of RT-PCR primers.
Supplementary Table S3. List of real-time RT-PCR primers.
Acknowledgements
We thank Dr J. Kroymann for screening the A. halleri BAC library, Dr O. Richard for the kind gift of seeds of A. thaliana NILs and accessions, Dr N. Verbruggen for helpful discussions, and C. Hamilton, S. Crépin, M. Schloesser, M. Scheepers, and M.C. Requier for technical support. Funding was provided by the F.R.S.–FNRS (FRFC-2.4583.08, PDR-T.0206.13) (MH), the University of Liège (SFRD-12/03) (MH), the Belgian Program on Interuniversity Poles of Attraction (IAP no. P6/19), the German Federal Ministry of Education and Research Biofuture grant 0311877 (UK), and the European Union RTN ‘Metalhome’, contract HPRN-CT-2002-00243 (UK). MH is Research Associate of the FNRS. JBC is a doctoral fellow (F.R.I.A.).
Glossary
Abbreviations:
- Col-0
Columbia-0
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- LUC
luciferase
- micro-ORF
micro-open reading frame
- RACE
rapid amplification of cDNA ends
- RT-PCR
reverse transcription PCR
- UTR
untranslated region.
References
- Alatorre-Cobos F, Cruz-Ramírez A, Hayden CA, Pérez-Torres C-A, Chauvin A-L, Ibarra-Laclette E, Alva-Cortés E, Jorgensen RA, Herrera-Estrella L. 2012. Translational regulation of Arabidopsis XIPOTL1 is modulated by phosphocholine levels via the phylogenetically conserved upstream open reading frame 30. Journal of Experimental Botany 63, 5203–5221. [DOI] [PubMed] [Google Scholar]
- Alvarez-Fernández A, Díaz-Benito P, Abadía A, López-Millán AF, Abadía J. 2014. Metal species involved in long distance metal transport in plants. Frontiers in Plant Science 5, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Jiang J, Chung J-S, Wang B, Chen J, Xin Z, Shi H. 2011. Regulated AtHKT1 gene expression by a distal enhancer element and DNA methylation in the promoter plays an important role in salt tolerance. Plant and Cell Physiology 52, 149–161. [DOI] [PubMed] [Google Scholar]
- Benderoth M, Textor S, Windsor AJ, Mitchell-Olds T, Gershenzon J, Kroymann J. 2006. Positive selection driving diversification in plant secondary metabolism. Proceedings of the National Academy of Sciences, USA 103, 9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. 2007. Zinc in plants. New Phytologist 173, 677–702. [DOI] [PubMed] [Google Scholar]
- Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE. 2004. Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques 37, 598, 600, 602. [DOI] [PubMed] [Google Scholar]
- Bugaut A, Balasubramanian S. 2012. 5′-UTR RNA G-quadruplexes: translation regulation and targeting. Nucleic Acids Research 40, 4727–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Kumimoto RW, Gnesutta N, Calogero AM, Mantovani R, Holt BF. 2014. A Distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis . The Plant Cell 26, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss MJ, Koch MA. 2006. Poorly known relatives of Arabidopsis thaliana . Trends in Plant Science 11, 449–459. [DOI] [PubMed] [Google Scholar]
- Cornu J, Deinlein U, Horeth S, Braun M, Schmidt H, Weber M, Persson DP, Husted S, Schjoerring JK, Clemens S. 2015. Contrasting effects of nicotianamine synthase knockdown on zinc and nickel tolerance and accumulation in the zinc/cadmium hyperaccumulator Arabidopsis halleri . New Phytologist 206, 738–750. [DOI] [PubMed] [Google Scholar]
- Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, Verbruggen N. 2007. A major QTL for Cd tolerance in Arabidopsis halleri co-localizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiology 144, 1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri R, Suzuki Y, Sugano S, Plass C, Huang T. 2008. The functional consequences of alternative promoter use in mammalian genomes. Trends in Genetics 24, 167–177. [DOI] [PubMed] [Google Scholar]
- Deinlein U, Weber M, Schmidt H, et al. 2012. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. The Plant Cell 24, 708–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E. 1996. A metal-accumulator mutant of Arabidopsis thaliana . Plant Physiology 111, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docquier S, Tillemans V, Deltour R, Motte P. 2004. Nuclear bodies and compartmentalization of pre-mRNA splicing factors in higher plants. Chromosoma 112, 255–266. [DOI] [PubMed] [Google Scholar]
- Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U. 2004. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. The Plant Journal 39, 425–439. [DOI] [PubMed] [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE. 2007. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir S, Velten L, Sharon E, Zeevi D, Carey LB, Weinberger A, Segal E. 2013. Deciphering the rules by which 5′-UTR sequences affect protein expression in yeast. Proceedings of the National Academy of Sciences, USA 110, E2792–E2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin SA, Priest HD, Givan SA, Shen R, Bryant DW, Fox SE, Wong W-K, Mockler TC. 2010. Genome-wide mapping of alternative splicing in Arabidopsis thaliana . Genome Research 20, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao Y, Ferjani A, Tomioka R, Nagasaki N, Kurata R, Nishimori Y, Fujiwara M, Maeshima M. 2011. iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis . Plant Physiology 155, 1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LS, Rogers EE. 2004. FRD3 controls iron localization in Arabidopsis . Plant Physiology 136, 2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez RA, Ewing RM, Cherry JM, Green PJ. 2002. Identification of unstable transcripts in Arabidopsis by cDNA microarray analysis: rapid decay is associated with a group of touch- and specific clock-controlled genes. Proceedings of the National Academy of Sciences, USA 99, 11513–11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Kroymann J, Trampczynska A, Bernal M, Motte P, Clemens S, Krämer U. 2013. Hard selective sweep and ectopic gene conversion in a gene cluster affording environmental adaptation. PLoS Genetics 9, e1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Nouet C. 2011. Metal hyperaccumulation and hypertolerance: a model for plant evolutionary genomics. Current Opinion in Plant Biology 14, 252–259. [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U. 2008. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4 . Nature 453, 391–395. [DOI] [PubMed] [Google Scholar]
- Ito T, Sakai H, Meyerowitz EM. 2003. Whorl-specific expression of the SUPERMAN gene of Arabidopsis is mediated by cis elements in the transcribed region. Current Biology 13, 1524–1530. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Bailey-Serres J. 2005. mRNA sequence features that contribute to translational regulation in Arabidopsis . Nucleic Acids Research 33, 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lee G, Jeon E, Sohn Ej, Lee Y, Kang H, Lee Dw, Kim DH, Hwang I. 2014. The immediate upstream region of the 5′-UTR from the AUG start codon has a pronounced effect on the translational efficiency in Arabidopsis thaliana . Nucleic Acids Research 42, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U. 2005. MTP1 mops up excess zinc in Arabidopsis cells. Trends in Plant Science 10, 313–315. [DOI] [PubMed] [Google Scholar]
- Krämer U. 2010. Metal hyperaccumulation in plants. Annual Review of Plant Biology 61, 517–534. [DOI] [PubMed] [Google Scholar]
- Krämer U, Talke IN, Hanikenne M. 2007. Transition metal transport. FEBS Letters 581, 2263–2272. [DOI] [PubMed] [Google Scholar]
- Lin YF, Liang HM, Yang SY, Boch A, Clemens S, Chen CC, Wu JF, Huang JL, Yeh KC. 2009. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytologist 182, 392–404. [DOI] [PubMed] [Google Scholar]
- Liu M-J, Wu S-H, Chen H-M, Wu S-H. 2012. Widespread translational control contributes to the regulation of Arabidopsis photomorphogenesis. Molecular Systems Biology 8, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li-Ling J, Huang Q, Chen X, Hou L, Ma F. 2009. Systematic analysis of alternative promoters correlated with alternative splicing in human genes. Genomics 93, 420–425. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. 2008. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. The Plant Journal 54, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Millar AH, O’Toole N, Small I, Whelan J. 2007. Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana . The Plant Cell 19, 3418–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet C, Motte P, Hanikenne M. 2011. Chloroplastic and mitochondrial metal homeostasis. Trends in Plant Science 16, 395–404. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Guerinot ML. 2009. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nature Chemical Biology 5, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. 2008. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nature Genetics 40, 1413–1415. [DOI] [PubMed] [Google Scholar]
- Petit JM, van Wuytswinkel O, Briat JF, Lobréaux S. 2001. Characterization of an iron-dependent regulatory sequence involved in the transcriptional control of AtFer1 and ZmFer1 plant ferritin genes by iron. Journal of Biological Chemistry 276, 5584–5590. [DOI] [PubMed] [Google Scholar]
- Pichon X, Wilson LA, Stoneley M, Bastide A, King HA, Somers J, Willis AE. 2012. RNA binding protein/RNA element interactions and the control of translation. Current Protein and Peptide Science 13, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau C, Loubet S, Lefoulon C, Chalies C, Fizames C, Lacombe B, Ferrand M, Loudet O, Berthomieu P, Richard O. 2012. Natural variation at the FRD3 MATE transporter locus reveals cross-talk between Fe homeostasis and Zn tolerance in Arabidopsis thaliana . PLoS Genetics 8, e1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausin G, Tillemans V, Stankovic N, Hanikenne M, Motte P. 2010. Dynamic nucleocytoplasmic shuttling of an Arabidopsis SR splicing factor: role of the RNA-binding domains. Plant Physiology 153, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravet K, Reyt G, Arnaud N, Krouk G, Djouani E-B, Boucherez J, Briat J-F, Gaymard F. 2012. Iron and ROS control of the DownSTream mRNA decay pathway is essential for plant fitness. EMBO Journal 31, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Alvarez R, Giner-Martínez-Sierra J, Orduna J, Orera I, Rodríguez-Castrillón JA, García-Alonso JI, Abadía J, Alvarez-Fernández A. 2010. Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: new insights into plant iron long-distance transport. Plant and Cell Physiology 51, 91–102. [DOI] [PubMed] [Google Scholar]
- Remy E, Cabrito TR, Batista RA, Hussein MAM, Teixeira MC, Athanasiadis A, Sá-Correia I, Duque P. 2014. Intron retention in the 5′UTR of the novel ZIF2 transporter enhances translation to promote zinc tolerance in Arabidopsis . PLoS Genetics 10, e1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard O, Pineau C, Loubet S, Chalies C, Vile D, Marques L, Berthomieu P. 2011. Diversity analysis of the response to Zn within the Arabidopsis thaliana species revealed a low contribution of Zn translocation to Zn tolerance and a new role for Zn in lateral root development. Plant, Cell, & Environment 34 1065–1078. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Guerinot ML. 2002. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis . The Plant Cell 14, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschzttardtz H, Seguela-Arnaud M, Briat JF, Vert G, Curie C. 2011. The FRD3 citrate effluxer promotes iron nutrition between symplastically disconnected tissues throughout Arabidopsis development. The Plant Cell 23, 2725–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Castric V, Pauwels M, Wright SI, Saumitou-Laprade P, Vekemans X. 2011. Does speciation between Arabidopsis halleri and Arabidopsis lyrata coincide with major changes in a molecular target of adaptation? PLoS ONE 6, e26872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Hamada M, Asai K, Mituyama T. 2009. CentroidFold: a web server for RNA secondary structure prediction. Nucleic Acids Research 37, W277–W280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M, Rellán-Álvarez R, Fink-Straube C, Abadía J, Bauer P. 2012. Nicotianamine functions in the phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis . The Plant Cell 24, 2380–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina S, Spiridonov A, Spiridonov N, Koonin E. 2010. Connections between alternative transcription and alternative splicing in mammals. Genome Biology and Evolution 2, 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad Z, Gosti F, Frérot H, Lacombe E, Roosens N, Saumitou-Laprade P, Berthomieu P. 2010. The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri . PLoS Genetics 6, e1000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Lo JC, Wu CL, Wang SL, Lai CC, Connolly EL, Huang JL, Yeh KC. 2011. Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana—the role in zinc tolerance. New Phytologist 190, 125–137. [DOI] [PubMed] [Google Scholar]
- Shanmugam V, Lo J-C, Yeh K-C. 2013. Control of Zn uptake in Arabidopsis halleri: a balance between Zn and Fe. Frontiers in Plant Science 4, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke IN, Hanikenne M, Krämer U. 2006. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri . Plant Physiology 142, 148–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Koyanagi KO, Itoh T. 2009. Highly diversified molecular evolution of downstream transcription start sites in rice and Arabidopsis . Plant Physiology 149, 1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. 2009. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist 181, 759–776. [DOI] [PubMed] [Google Scholar]
- Willems G, Dräger DB, Courbot M, Gode C, Verbruggen N, Saumitou-Laprade P. 2007. The genetic basis of zinc tolerance in the metallophyte Arabidopsis halleri ssp. halleri (Brassicaceae): an analysis of quantitative trait loci. Genetics 176, 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogeeswaran K, Frary A, York TL, Amenta A, Lesser AH, Nasrallah JB, Tanksley SD, Nasrallah ME. 2005. Comparative genome analyses of Arabidopsis spp.: inferring chromosomal rearrangement events in the evolutionary history of A. thaliana . Genome Research 15, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.