Highlight
Mycorrhiza effects on the direct pathway for plant phosphorus uptake were modulated by mycorrhizal phosphorus input to the roots, rather than by mycorrhizal colonization itself.
Key words: Arbuscular mycorrhizal (AM) fungi, colonized root patch, direct pathway uptake (DPU), Medicago truncatula, mtpt4-mutant, Pi transporters, resource allocation, soil P level.
Abstract
Two pathways exist for plant Pi uptake from soil: via root epidermal cells (direct pathway) or via associations with arbuscular mycorrhizal (AM) fungi, and the two pathways interact in a complex manner. This study investigated distal and local effects of AM colonization on direct root Pi uptake and root growth, at different soil P levels. Medicago truncatula was grown at three soil P levels in split-pots with or without AM fungal inoculation and where one root half grew into soil labelled with 33P. Plant genotypes included the A17 wild type and the mtpt4 mutant. The mtpt4 mutant, colonized by AM fungi, but with no functional mycorrhizal pathway for Pi uptake, was included to better understand effects of AM colonization per se. Colonization by AM fungi decreased expression of direct Pi transporter genes locally, but not distally in the wild type. In mtpt4 mutant plants, direct Pi transporter genes and the Pi starvation-induced gene Mt4 were more highly expressed than in wild-type roots. In wild-type plants, less Pi was taken up via the direct pathway by non-colonized roots when the other root half was colonized by AM fungi, compared with non-mycorrhizal plants. Colonization by AM fungi strongly influenced root growth locally and distally, and direct root Pi uptake activity locally, but had only a weak influence on distal direct pathway activity. The responses to AM colonization in the mtpt4 mutant suggested that in the wild type, the increased P concentration of colonized roots was a major factor driving the effects of AM colonization on direct root Pi uptake.
Introduction
Phosphate rock as a fertilizer is important for crop production worldwide. It is a limited and non-renewable natural resource, being depleted at an increasing rate, while demand for food production also increases (Cordell et al., 2009; Gilbert, 2009). Soil inorganic phosphate (Pi) is essential for the proper growth and functioning of plants, and plant growth and yield are adversely affected when Pi supply is limited (Epstein and Bloom, 2005; Marschner and Marschner, 2012). Therefore, maximizing the efficiency of plant acquisition of soil P with limited or no fertilizer application is important for the future of agriculture (López-Arredondo et al., 2014).
Root epidermal cells and roots hairs take up Pi from the soil via the direct pathway for Pi uptake (DPU), and this creates a nutrient depletion zone around the root due to the low mobility of P in soil (Smith et al., 2011). Furthermore, plants need to overcome a large electrochemical potential gradient in order to move Pi into cells (Schachtman et al., 1998). As a consequence, plants have evolved strategies that can increase the acquisition of root-derived P (Lambers et al., 2010). One strategy for foraging for nutrients beyond the depletion zone of roots is the formation of arbuscular mycorrhizas, an association between the plant and specialized soil fungi (Smith and Read, 2008). Arbuscular mycorrhizas increase plant uptake of soil nutrients such as P, Zn, Ca, and Cu when they are in limited supply, thus improving plant nutrition (Marschner and Dell, 1994). The formation of mycorrhizas creates an arbuscular mycorrhizal (AM) pathway for Pi uptake (MPU), which interacts with the DPU (Smith et al., 2003, 2004). Pi uptake via the MPU is often reduced with increasing soil P concentration, but this effect is influenced by the AM fungal species (Facelli et al., 2014).
While the formation of mycorrhizas can greatly enhance plant P acquisition, and thence plant growth, there are some instances where mycorrhizas do not appear to provide a benefit in terms of growth or P nutrition (Grace et al., 2009). The AM pathway can still be active, but ‘hidden’, when an AM growth or P response is absent, and can actually dominate Pi uptake (Smith et al., 2003; Nagy et al., 2009). Previously, mycorrhizas have been shown to contribute up to 100% of a plant’s P (Smith et al., 2004). Thus, the activity of the AM pathway when no growth or P response is observed cannot be discounted (Poulsen et al., 2005; Bucher, 2007), and further investigation into this area is needed, especially into the interplay between the DPU and MPU, at both molecular and physiological levels (Smith et al., 2011).
Root Pi transporter genes involved in the direct P uptake pathway can be down-regulated in AM plants, independent of the external P status or extent of AM colonization (Paszkowski et al., 2002; Glassop et al., 2005; Harrison, 2005; Christophersen et al., 2009). The genes that encode both the direct and AM Pi transporters have been described in many plant species, including Medicago truncatula (H. Liu et al., 1998; Javot et al., 2007; Liu et al., 2008). The direct pathway Pi transporter genes MtPT1, MtPT2, and MtPT3 are closely related low-affinity Pi transporters, belonging to the PHT1 family (Bucher et al., 2001; Liu et al., 2008). The phosphate transporter gene MtPT1 is an important direct Pi transporter gene that appears to be expressed exclusively in the root epidermal cells, cortex, and root hairs (Chiou et al., 2001; Liu et al., 2008). Transcript and protein levels are closely correlated for MtPT1, and increase greatly in response to Pi starvation (Chiou et al., 2001). Furthermore, MtPT1 expression levels (transcripts and protein) decrease when the plant becomes colonized by AM fungi (Chiou et al., 2001; Liu et al., 2008). The phosphate transporter gene MtPT2 acts in a similar way to MtPT1 with regards to Pi fertilization and AM colonization (H. Liu et al., 1998). However, promoter activity for MtPT2 is strongly located in the vascular cylinder (Xiao et al., 2006), indicating a role in plant P translocation. The direct phosphate transporter gene MtPT3 is down-regulated by Pi fertilization, and in roots colonized by three species of AM fungi (Grunwald et al., 2009), and is suggested to be expressed in the vascular tissue of roots (Liu et al., 2008). Two other direct Pi transporter genes have been identified, also belonging to the PHT1 family: MtPT5, a high-affinity Pi transporter, expressed in all parts of the root except the vascular tissue, and affected only by some AM fungi; and MtPT6, which is unaffected by Pi, but has reduced expression in AM plants (Grunwald et al., 2009). Thus, numerous Pi transporter genes are working to transport soil Pi directly via the roots in M. truncatula.
Of particular importance to the present study is the low-affinity Pi transporter MtPT4. This protein has been identified as a mycorrhiza-induced Pi transporter in M. truncatula (Harrison et al., 2002; Javot et al., 2007). The gene encoding MtPT4 is exclusively expressed in AM roots, specifically in cells containing arbuscules (Harrison et al., 2002; Grunwald et al., 2009). Loss of the MtPT4 function is detrimental to the AM symbiosis because it causes mature arbuscules to degenerate, leading to premature arbuscule death (Javot et al., 2007). Arbuscule development can be restored in the mtpt4 mutant, under low soil N conditions (Javot et al., 2011).
Expression of direct Pi transporter genes in non-mycorrhizal plants is regulated via Pi starvation signalling pathways, which also regulate various other signalling components. While most of the signalling components of Pi starvation responses have been described in the non-mycorrhizal model plant species Arabidopsis thaliana, many are also present in AM plants (Smith et al., 2011). Phosphate starvation-induced (PSI) genes are important components of the Pi starvation regulatory pathways, along with Pi transporters, phytohormones, transcription factors, and microRNAs (Rouached et al., 2010). A PSI gene in M. truncatula, Mt4 (Burleigh and Harrison, 1997), is down-regulated in roots by Pi fertilization and AM colonization (Burleigh and Harrison, 1999). Plant responses to Pi-depleted soil can occur locally via changes in root system architecture (e.g. root hair formation), as well as systemically (long-distance) through phosphate homeostasis (Martín et al., 2000; Franco-Zorrilla et al., 2004; Péret et al., 2011). Additionally, plant responses to Pi starvation can be physiological or molecular in nature, such as changes to the allocation of resources (photosynthate) and regulation of PSI genes (Raghothama, 1999). The inhibition of AM colonization by high soil P levels works systemically, as it is linked to shoot P concentrations rather than local P concentrations in roots (Breuillin et al., 2010; Balzergue et al., 2011).
It has previously been suggested that mycorrhizas may have a limited long-distance impact upon the direct Pi pathway (Grønlund et al., 2013). This suggestion was based on experiments with Pisum sativum (pea) plants that were grown at just one soil P level (7.5mg P kg–1 soil) and were incompletely investigated for expression of direct Pi transporter genes (PsPT1 only). Therefore, it was not possible to conclude if the mycorrhiza-related effect on the direct Pi uptake pathway resulted from an increased P status in AM plants or from more direct interactions between the AM fungus and the DPU (Smith et al., 2011).
Those limitations in Grønlund et al. (2013) were addressed in the present study with M. truncatula in split-root systems. In addition to the A17 wild type, the mtpt4 mutant M. truncatula genotype (Harrison et al., 2002; Javot et al., 2007) served to separate the effects of plant Pi signalling and of AM colonization on the regulation of plant P uptake by the direct and AM pathways. Furthermore, three soil P levels served to investigate that regulation in terms of shoot P status.
The hypotheses tested were (i) that localized root colonization by AM fungi will reduce DPU activity locally but not in a distal, non-colonized patch of root; (ii) that mycorrhiza-induced effects on DPU activity can be separated into P-derived and direct AM effects by comparing the wild-type and mtpt4 genotypes; and (iii) that colonized and non-colonized patches of roots will experience differences in root growth and P nutrition.
Materials and methods
Soil and plants
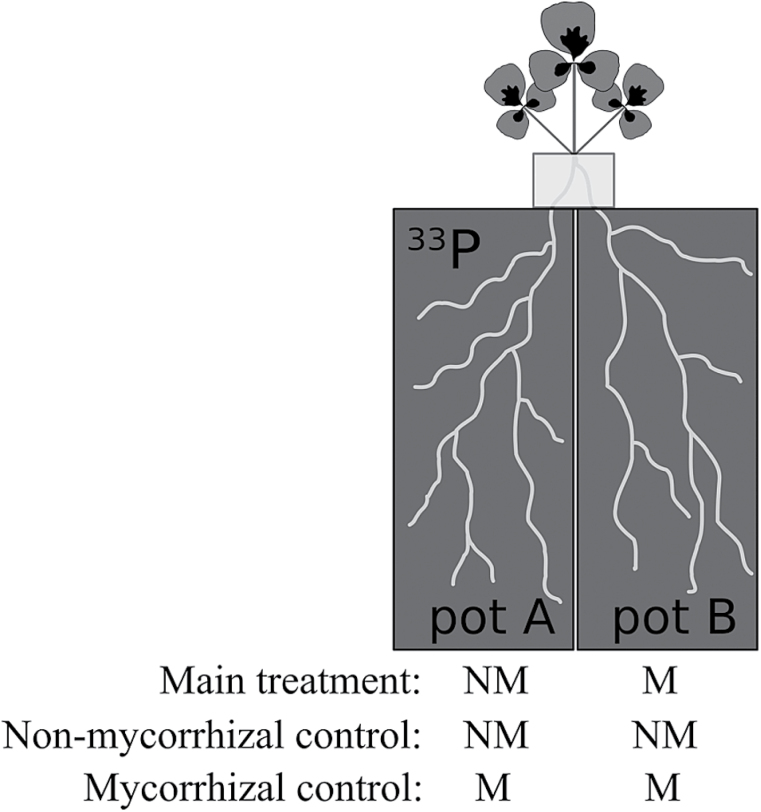
The M. truncatula cultivar Jemalong A17 (referred to as the wild type hereafter) and mutant mtpt4-1 were used in this study, kindly provided by Maria Harrison. Seeds of both genotypes were scarified, surface-sterilized, imbibed, and pre-germinated (following Grunwald et al., 2009). Pre-germinated seeds were sown (day 0) in small pots containing 160g of a semi-sterile (15 kGy, 10 MeV electron beam) 1:1 soil/sand mix, which was supplemented with basal nutrients (as per Merrild et al., 2013) including 10mg P kg–1 (as KH2PO4), and mixed thoroughly. Seedlings grew for 16 d before being moved to split-pots (following Grønlund et al., 2013). Shoot and root P contents were 0.04mg and 0.04mg P plant–1 in wild-type plants, respectively, and 0.06mg and 0.05mg P plant–1 in mtpt4 plants, on the day of transfer. The root system of each plant was divided into two halves of a similar size and planted in a split-root system consisting of two adjoining pots with one root half in each (see Fig. 1). Pots contained a semi-sterile 1:1 soil/sand mixture, as above. Three soil P concentrations were established by mixing KH2PO4 into the growth medium at 0, 20, and 50mg P kg–1. These P additions resulted in levels of 0.5M NaHCO3-extractable P (Olsen et al., 1954) of 5.0±0.2, 10.1±0.3, and 19.9±0.9mg P kg–1 soil and are referred to as P0, P20, and P50 hereafter. While the M. truncatula wild type was grown in all soil P treatments, the mutant M. truncatula mtpt4 was grown at P20 only.
Fig. 1.
Set up of split-pots and AM fungal inoculation treatments (not to scale). The left-hand pot (pot A) had unlabelled soil layers at the bottom (150g) and top (100g) of the pot, and 600g of soil labelled with 33P in between the two unlabelled layers; see the Marerials and methods for further details. NM, non-mycorrhizal; M, mycorrhizal.
The split-root treatments were (i) M,M with AM fungal inoculum in both root halves; (ii) NM,M with one root half non-inoculated and the other inoculated with AM fungi; and (iii) NM,NM with both root halves non-inoculated (see Fig. 1). Soil in M pots was mixed with 75g of Rhizophagus irregularis (previously named Glomus intraradices, Schüßler and Walker, 2010) BEG87 inoculum, consisting of a mixture of dry soil, spores, and root fragments of a Trifolium subterraneum pot culture. Pot A contained 600g of soil mixed with 0.5 kBq g–1 of carrier-free H3 33PO4. The specific activity in 0.5M NaHCO3 extracts of the 33P-labelled P0, P20, and P50 soils was 29.7±2.7, 12.4±1.5, and 7.1±0.5 kBq mg P–1, respectively. Isotope labelling was used to quantify the Pi uptake from pot A.
A layer of unlabelled, non-inoculated soil was weighed into both the bottom (150g) and top (100g) of each pot, to prevent transfer of labelled soil and AM fungal inoculum between pots. There were five replicates of each treatment. Plants were grown in climate chambers with a 16/8h light/dark cycle at 20/15 °C. Fluorescent daylight lamps (Osram GmbH, Munich, Germany) provided 500 μmol m–2 s–1 photosynthetically active radiation (PAR; 400–700nm). Pots were watered every second day to 60% of water-holding capacity.
Harvesting and physiological analysis
Plants were harvested 37 d after sowing (21 d after transplantation to split-pots). A subsample (~50mg) of fresh shoot material was flash-frozen in liquid N2 and kept at –80 °C. Shoots were dried at 70 °C for 48h and dry weights recorded. Roots were washed, blotted, and weighed, and a weighed subsample for determination of AM colonization was stored in 50% ethanol. Another ~500mg subsample of root material was flash-frozen in liquid N2, crushed, and kept at –80 °C for RNA isolation. The remaining root tissue was dried at 70 °C for 48h and dry weights were determined.
Dried shoot and root samples were oxidized in a 4:1 mixture (v:v) of 65% nitric:70% perchloric acids, and total P was determined by the molybdate blue method using AutoAnalyzer 3 (Bran+Luebbe, Norderstedt, Germany). The 33P in shoot and root tissue was determined in the same digests in a Packard TR 1900 liquid scintillation counter (PerkinElmer, Waltham, MA, USA). Shoot Pi concentrations were analysed after homogenizing ~50mg of frozen sample in 1ml of 1M HCl and centrifugation for 3min at 14 000 g. A 15 μl sample of the supernatant was mixed with 240 μl of malachite green (0.045% w/v)/ammonium molybdate (4.2% w/v) solution in a microplate. After 1min, 30 μl of 34% (w/v) sodium citrate was added and the OD660 was read 5–10min later (modified from Lanzetta et al., 1979). Total N in shoot samples was determined in 2mg of finely homogenized samples on an EA 1110 elemental analyser (Thermo Scientific, Bremen, Germany).
Prior to determining the percentage root length colonized by AM fungi, root samples were cleared in 10% KOH and stained with trypan blue following Kormanik and McGraw (1982). All roots were then assessed for total percentage root length colonized by AM fungi, and root length (Newman, 1966). Additionally, the proportion of the root containing AM structures (arbuscules, vesicles, and/or internal hyphae) was assessed on subsamples of stained roots from pot B of the NM,M pots at P20 only (McGonigle et al., 1990). The presence of hyphae was investigated in root-free HC soil taken from pot B of the NM,M P20 treatments, for both the wild-type and mtpt4 mutant plants, and also in HC soil from the equivalent NM,NM pots. Qualitative information was obtained by stereo microscope inspection of moist soil, while quantitative data were obtained by measuring the length of hyphae collected on membrane filters (Jakobsen et al., 1992).
RNA isolation and real-time qPCR analysis
Total RNA was extracted from ~100mg of root samples of M. truncatula wild type and of mutant mtpt4 grown at 20mg P kg–1 soil, using an miRNeasy Mini Kit (Qiagen Hilden, Germany) with on-column DNase treatment following the manufacturer’s protocol. RNA concentration was measured on a Nanodrop ND-1000 Spectrophotometer (Saveen 1 Werner, Malmö, Sweden). cDNA was synthesized from 200ng of total RNA using dNTP Mix (Qiagen) and Expand Reverse Transcriptase (Roche) including Protector RNase inhibitor (Roche).
The real-time PCR primers (Eurofins MWG operon, Germany) were EF1α (Liu et al., 2008), MtPT1, MtPT2, MtPT3, MtPT4 (Grunwald et al., 2009), and Mt4 (Branscheid et al., 2010). Gene expression analysis was carried out on four replicate plants from each treatment. Real-time PCR analysis was performed using the Rotor Gene 2000 Real Time Cycler (Qiagen). Each 20 μl of PCR contained 8 μl of a 1.25–1 dilution of the reverse transcription reaction (see above), and 12 μl of SYBR Green Master Mix (Fermentas, Thermo Scientific), which included 500nM of each primer. Samples were heated to 95 °C for 10min, followed by 40 cycles of 15 s at 95 °C and 1min at 60 °C. After each PCR, the specificity of the amplification was verified by running a melt curve analysis. The Rotor Gene 2000 software calculated relative amounts of RNA based on PCR cycle threshold values obtained from a dilution series from 2.5–1 to 6.25–3 (each step was a 1:3 dilution in H2O) of a standard reverse transcription sample from a mycorrhizal or non-mycorrhizal plant (depending on the primer of interest). Data were normalized to EF1α mRNA levels.
Calculations
In the NM,NM and M,M control treatments, the shoot P derived from pot A at each soil P level could be directly derived as 50% of the measured shoot P content:
| (1) |
Shoot P derived from pot A could also be estimated from the 33P data:
| (2) |
The numerical ratio corrects for the fact that 33P was mixed with only 600g of the 850g soil in total. While the measured and the estimated P uptake from pot A increased with increasing P level as expected, the two methods produced different results within each P level. Thus the ratios between estimated and measured P uptake were 0.41, 0.77, and 0.88 at P0, P20, and P50, respectively. These ratios are means of very similar values in the NM,NM and M,M treatments at a given soil P level and it was therefore assumed that the observed deviation was not caused by the mycorrhizal status of the roots, but rather by P level-associated differences in isotopic equilibrium between 33P and 31P in the ‘plant available’ soil P pool. Due to the similar ratios for M,M and NM,NM plants, the ratios could be used to calculate the shoot P derived from pot A (i.e. the NM root half) in the NM,M treatments, and at a given P level by dividing the estimated uptake (Equation 2) by one of the three ratios given above.
Shoot uptake of P from pot B was derived as the difference between total shoot P and shoot P derived from pot A, and then the root length-specific (RL spec) P uptake from pot B was calculated. The minimum contribution by the MPU to the root length-specific uptake from pot B in the M,M and NM,M treatments was estimated by subtracting the corresponding NM,NM treatment values. The minimum percentage contribution of mycorrhizas to root length-specific P uptake from pot B was then calculated:
| (3) |
The calculation for P contribution via the MPU in NM,M plants was modified from Smith et al. (2004), due to the disparity between estimated and measured MPU uptake in the mycorrhizal and non-mycorrhizal control plants when using the original equation.
Statistical analysis
Two-way analyses of variance (ANOVA) were performed on the data, with pot configuration and soil P level as factors when analysing the wild-type genotype only, and pot configuration and genotype as factors when comparing the wild-type and mtpt4 mutant genotypes at P20 only (see Supplementary Table S1 available at JXB online for ANOVA outcomes). One-way ANOVA were performed on wild-type distribution data (ratios) only. Where significant (P<0.05) interactions or main effects were found, comparisons were made using Tukey’s honestly significant difference (HSD). Student’s t-tests were performed for certain response variables (specified in the Results). All statistical analyses were performed using JMP 11.2.0 (SAS Institute Inc.).
Results
Root colonization and extraradical hyphal length
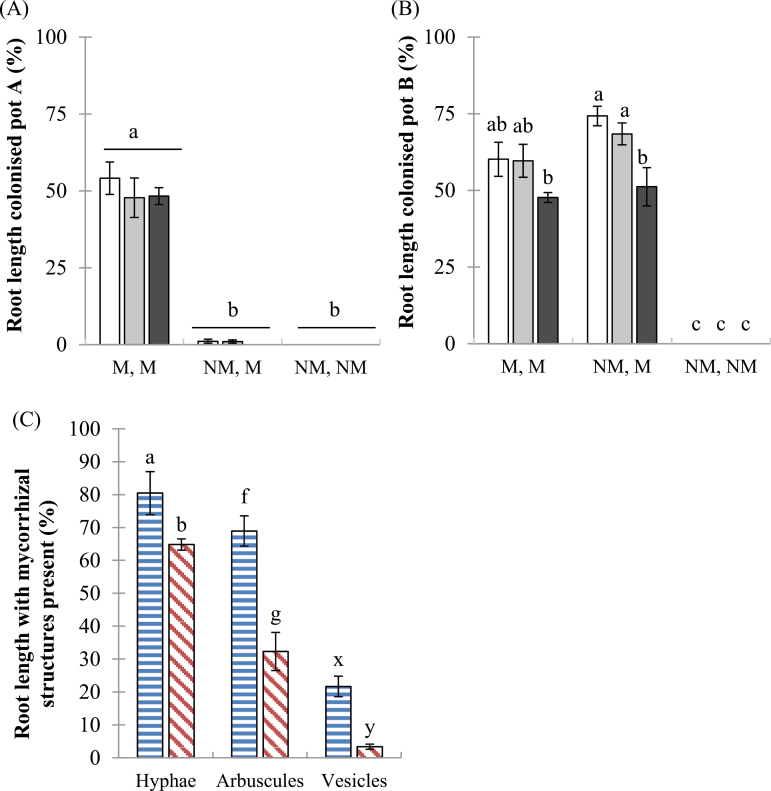
Root length colonized by AM fungi was <1% in all NM pots whereas roots in M pots had 48–74% of their root length colonized (Fig. 2A, B). Mycorrhizal colonization decreased significantly with increasing soil P in the pot B roots of NM,M pots (Supplementary Table S1A at JXB online). Mycorrhizal colonization in the mtpt4 mutant was 32–36% of the root length and was thus lower than in the wild-type genotype (Supplementary Table S2). Furthermore, hyphal, arbuscular, and vesicular colonization in pot B of the NM,M pots were each significantly higher in the wild-type genotype than in the mtpt4 mutant, with the greatest effects on arbuscule and vesicle content (Fig. 2C).
Fig. 2.
Mycorrhizal colonization in wild-type and mtpt4 roots. Root length colonized (%) in the wild-type genotype in (A) pot A and (B) pot B, at three soil P additions: 0 (white bars), 20 (grey bars), and 50 (black bars) mg P kg–1. Values are the mean ±standard error, n=5. Means followed by the same letter were not significantly different at the P<0.05 level (Tukey’s HSD). (C) Presence of different mycorrhizal structures (hyphae, arbuscules, and vesicles) within roots grown at P20 in pot B of NM,M pots only. Wild type, horizontal bars; mtpt4 mutant, diagonal bars. For (C) a different set of letters (ab, fg, xy) indicated a significant difference (at the P<0.05 level, Student’s t-test) between genotypes, for each type of colonization separately. (This figure is available in colour at JXB online.)
External hyphae of AM fungi were found in soil from both colonized wild-type and mtpt4 mutant treatments, although the abundance was significantly lower in the latter (7.03±0.72 and 3.63±0.41 m g–1 dry soil, respectively). Although quantitative analysis did not reveal a significant difference in hyphal length density between mtpt4 mutant soil and NM soil (2.79±0.50 m g–1 dry soil), direct microscopy confirmed the presence of hyphae in moist HC soil from mtpt4 mutant plants, but not from non-mycorrhizal plants.
Plant growth responses to soil P level and mycorrhizal colonization of one or both root halves
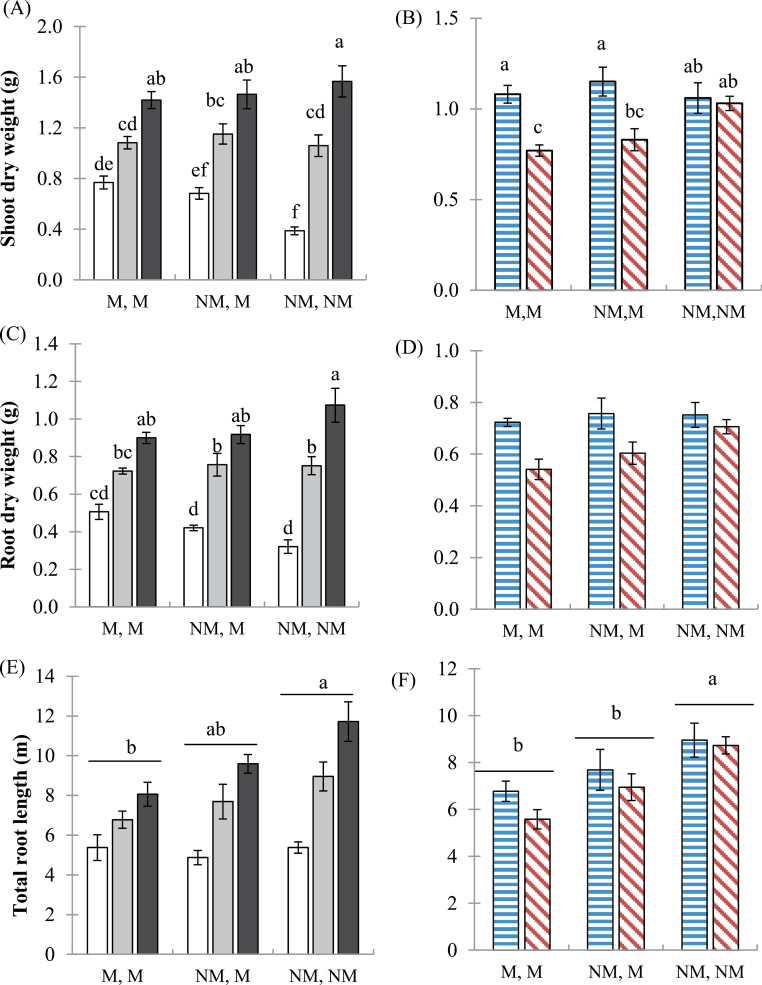
The wild-type plants exhibited strong positive growth responses to formation of mycorrhizal associations and to soil P supply. Shoot and root growth, and total root length of the wild-type genotype increased significantly over three levels of P addition: 0, 20, and 50mg P kg–1 (Fig. 3A, C, E). Furthermore, at low P (P0), shoot growth was significantly higher in the mycorrhizal pots (M,M) than the non-mycorrhizal pots (NM,NM). There was no such pattern at the higher soil P additions. The mtpt4 plants had significantly lower root dry weights than the wild-type plants (pooling pot configuration), and significantly lower shoot dry weights when one or both pots were mycorrhizal (Fig. 3B, D). Total root length in the wild-type plants was significantly lower in the mycorrhizal pots than in the non-mycorrhizal pots, pooling soil P addition treatments (Fig. 3E), but this effect disappeared at 0mg P kg–1 (Supplementary Table S1 at JXB online: significant interaction between P level and pot configuration). Colonization by AM fungi also resulted in reduced root length in mtpt4 mutant plants (Fig. 3F). Importantly, when both pot halves were non-colonized, the wild-type and mtpt4 plants exhibited similar physiological responses, and hence there were no pleiotropic effects of the mtpt4 mutation.
Fig. 3.
Effects of soil P level and AM colonization on plant growth. Shoot and root dry weights and total root length (pot A and pot B) of (A, C, E) the wild-type genotype at three soil P additions: 0 (white bars), 20 (grey bars), and 50 (black bars) mg P kg–1, and (B, D, F) the wild type (horizontal bars) and mtpt4 mutant (diagonal bars) at P20 only. Values are the mean ±standard error, n=5. Means followed by the same letter were not significantly different at the P<0.05 level. (This figure is available in colour at JXB online.)
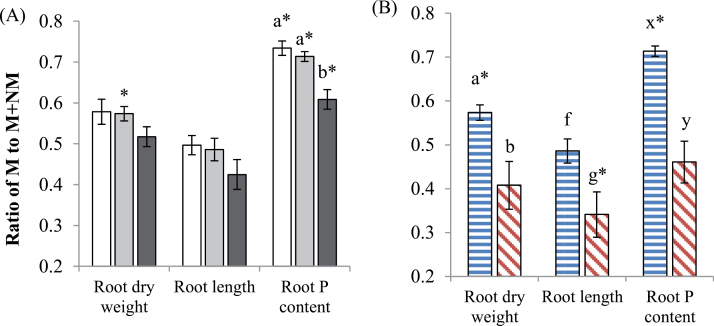
The distribution of resources between colonized and non-colonized root-halves (i.e. NM,M pots) was calculated as ratios of root biomass or root length in the colonized pot (M) to the total biomass or length (NM+M). Importantly, in the NM,NM and M,M pots, the contribution to root biomass and length was equal between both root halves as expected, in both wild-type and mtpt4 plants; that is, the ratio was ~0.5 (data not shown).
In the NM,M-grown wild-type plants, the ratio for both root length and biomass tended to decrease with increasing soil P concentration, but not significantly (P>0.05, one-way ANOVA, Tukey’s HSD, Fig. 4A). This indicated that the AM root half contributed less to root biomass accumulation as the soil P addition increased. At P20, the calculated ratio for root biomass and root length was significantly higher in the wild-type than in the mtpt4 mutant genotype (P<0.05, Student’s t-test, Fig. 4B). In particular, the ratio was <0.5 for both response variables in the mtpt4 genotype, indicating that the AM root half contributed less than half of the total root biomass and length.
Fig. 4.
The distribution of resources between colonized and non-colonized root halves (i.e. NM,M pots) was calculated as the ratios of the colonized pot (M) to the total (NM+M). Ratios for root dry weight, length, and P content in (A) wild-type plants at three soil P additions: 0 (white bars), 20 (grey bars), and 50 (black bars) mg P kg–1, and in (B) the wild type (horizontal bars) and mtpt4 mutant (diagonal bars) at P20 only. Values are the mean ±standard error, n=5. Means followed by the same letter were not significantly different at the P<0.05 level (Tukey’s HSD). For (B) different sets of letters (ab, fg, xy) indicate a significant difference (at the P<0.05 level, Student’s t-test) between genotypes, for different response variables, respectively. Asterisk denotes values that are significantly different from 0.5. (This figure is available in colour at JXB online.)
Responses in plant P nutrition to soil P level and mycorrhizal colonization of one or both root halves
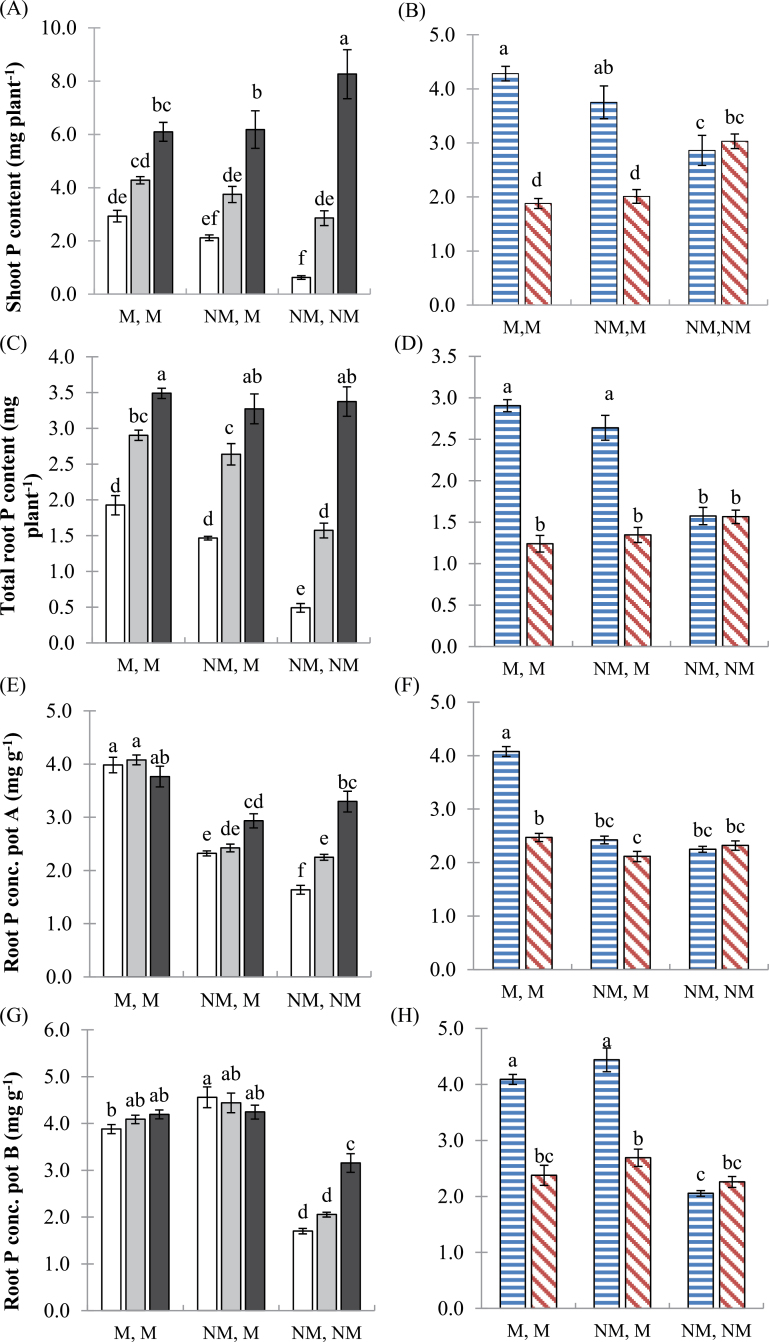
As for biomass, shoot and root P content as well as root P concentration in the wild-type plants responded strongly to AM colonization and soil P. Shoot and root P content increased significantly with increasing soil P addition and, at low P (P0), were significantly higher in the mycorrhizal than in the non-mycorrhizal wild-type plants (Fig. 5A, C). In the mtpt4 genotype at P20, shoot and root P contents were similar to those of the wild type for completely non-mycorrhizal plants, and were otherwise significantly lower than in the wild type (Fig. 5B, D).
Fig. 5.
Effects of increasing soil P and AM colonization on plant P content and concentration. Shoot and root P content (mg plant–1), and root P concentration in pot A and pot B (mg g–1) of (A, C, E, G) the wild-type genotype at three soil P additions: 0 (white bars), 20 (grey bars), and 50 (black bars) mg P kg–1, and (B, D, F, H) the wild type (horizontal bars) and mtpt4 mutant (diagonal bars) at P20 only. Values are the mean ±standard error, n=5. Means followed by the same letter were not significantly different at the P<0.05 level. (This figure is available in colour at JXB online.)
Ratios describing the distribution of P content between the non-mycorrhizal and mycorrhizal root halves of NM,M plants were calculated as for biomass. In the wild-type plants, the allocation of P to the mycorrhizal pot was significantly higher than 0.5 at all soil P addition treatments; however, it decreased significantly as soil P addition increased (P<0.05, one-way ANOVA, Tukey’s HSD, Fig. 4A). In the mtpt4 mutant plants, allocation of P content was even between the mycorrhizal and non-mycorrhizal root halves, and the wild-type plants allocated significantly more P to the mycorrhizal half than the mtpt4 plants (P<0.05, Student’s t-test, Fig. 4B).
Root P concentrations in the colonized wild-type roots were not significantly different at varying soil P additions. Conversely, non-colonized wild-type roots responded positively and significantly to soil P addition (Fig. 5E, G). Concentrations of P in the roots (both pot A and B) of mtpt4 plants were similar between pot configurations, and were significantly lower than those of the mycorrhizal wild-type roots (Fig. 5F, H).
Shoot PO4 concentration increased significantly with increasing soil P concentration in the wild-type plant (pooling pot configuration), and was significantly lower in the non-mycorrhizal (NM,NM) pots than in the mycorrhizal (M,M) pots (pooling soil P addition, Supplementary Fig. S1A at JXB online). At P20, wild-type plants grown in the M,M pots had significantly higher shoot PO4 concentration than wild-type and mtpt4 plants of other treatments, except the non-mycorrhizal wild-type treatment (Supplementary Fig. S1B).
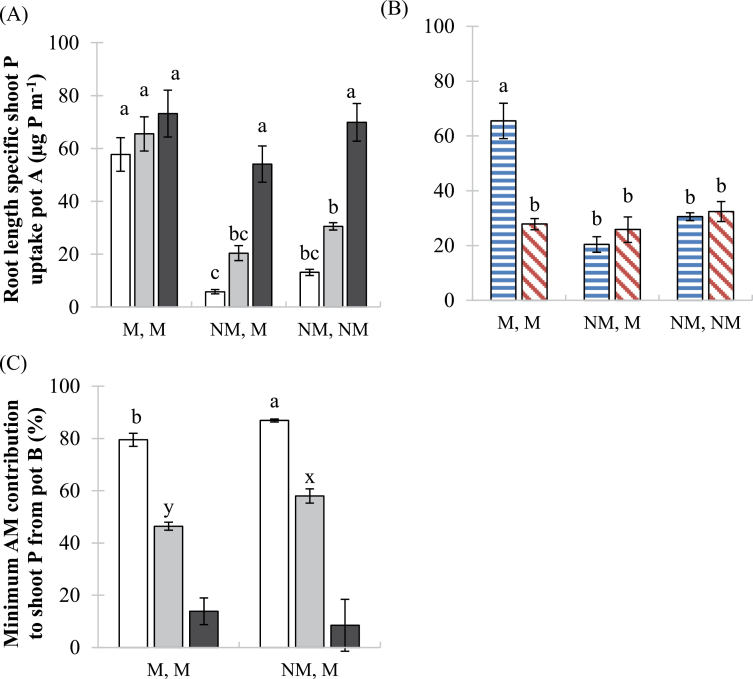
Root length-specific P uptake from pot A increased significantly with increasing soil P addition for the non-mycorrhizal roots (i.e., NM,M and NM,NM plants) (Fig. 6A). Furthermore, root length-specific P uptake from pot A was analysed in more detail at each P level by a Student’s t-test comparing the treatment pots (NM of NM,M) and the NM control (NM,NM). At P0 and P20, root length-specific P uptake was significantly lower in the NM,M than in the NM,NM plants (P<0.05). The wild-type plants had significantly higher root length-specific P uptake at P20 than the mtpt4 plants in the M,M pots only (Fig. 6B).
Fig. 6.
Pi uptake via the direct Pi uptake pathway and the mycorrhizal pathway. (A) Root length-specific shoot Pi uptake from pot A and (C) the minimum contribution by AM fungi from pot B to shoot P of wild-type plants at three soil P additions: 0 (white bars), 20 (grey bars), and 50 (black bars) mg P kg–1. (B) Root length-specific shoot Pi uptake from pot A in the wild type (horizontal bars) and mtpt4 mutant (diagonal bars) at P20 only. Values are mean ±standard error, n=5. Means followed by the same letter were not significantly different at the P<0.05 level (Tukey’s HSD). For (A) data were analysed in more detail at each P level by a Student’s t-test comparing the treatment pots (NM of NM,M) and the NM control (NM,NM), which revealed significantly reduced P uptake from pot A in NM,M plants grown at P0 and P20. For (C) different sets of letters (ab, xy) indicate a significant difference (at the P<0.05 level, Student’s t-test) between M,M and NM,M pots. (This figure is available in colour at JXB online.)
The minimum contribution to shoot P by mycorrhizas in pot B decreased significantly with increasing soil P addition in wild-type plants (Fig. 6C). Furthermore, when Student’s t-tests were used to compare between NM,M and M,M pots at each P level separately, the minimum contribution to shoot P content was significantly higher in the NM,M pots than in the M,M pots, for the P0 and P20 treatments.
Gene expression
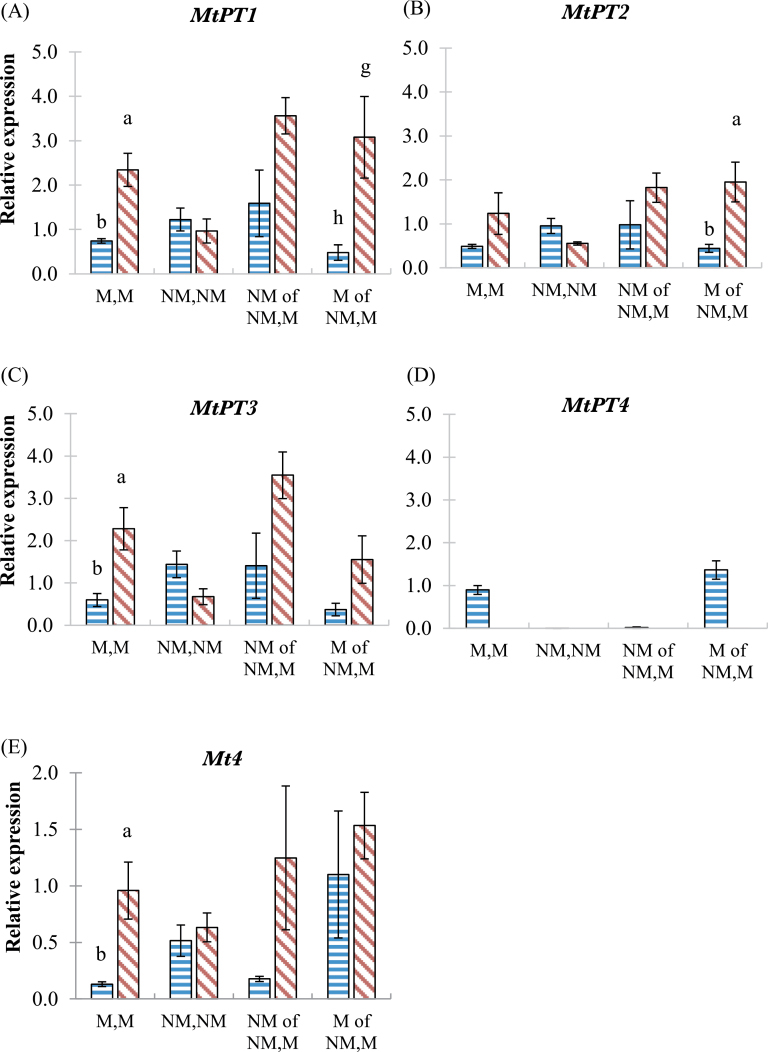
The expression of the direct Pi transporter genes (MtPT1, MtPT2, and MtPT3) in wild-type and mtpt4 genotypes in the colonized root halves at P20 were evaluated by real-time quantitative PCR, then the results were compared using Student’s t-test for each gene. In the M,M pots and in the colonized root half of the NM,M pots, MtPT1, MtPT2, and MtPT3 expression was higher in the mtpt4 roots than in the wild-type roots, in most cases significantly (Fig. 7A–C).
Fig. 7.
Relative gene expression in roots of wild-type and mtpt4 plants. Expression of direct Pi transporter genes (A–C) MtPT1, MtPT2, and MtPT3 mycorrhiza-induced Pi transporter gene (D) MtPT4, and PSI gene (E) Mt4 in the wild type (horizontal bars) and mtpt4 mutant (diagonal bars) at P20 only. Values are the mean ±standard error, n=3 or 4. Letters (ab, gh) denote a significant difference (at the P<0.05 level, Student’s t-test) between genotypes. See main text for further information. (This figure is available in colour at JXB online.)
In the non-mycorrhizal control (NM,NM) and treatment (NM of NM,M) pots, there were no significant differences in any of the Pi transporter genes between genotypes (Fig. 7A–C). In the mtpt4 roots, the expression of MtPT1, MtPT2, and MtPT3 in non-mycorrhizal roots of the NM,M treatment was significantly higher than in the non-mycorrhizal control (NM,NM) treatment, whereas this was not the case in wild-type plants. Furthermore, the mycorrhizal Pi transporter gene MtPT4 was expressed in the mycorrhizal roots of wild-type plants, but not in mtpt4 mutant roots (Fig. 7D).
Expression of Mt4 in the mycorrhizal control pots (M,M) was significantly higher in the mtpt4 genotype than in the wild type. Expression of Mt4 in other pots (NM,NM and NM,M) was not significantly different between the genotypes, but the expression of Mt4 in the non-colonized root half in NM,M pots was substantially higher in the mtpt4 mutant (Fig. 7E).
Discussion
The effect of mycorrhizal colonization of one root half on local and distal DPU expression and activity
This work provides molecular but not physiological confirmation of hypothesis (i) that localized root colonization by AM fungi reduces DPU activity locally but not in a distal, non-colonized patch of root. Root length-specific shoot P uptake from a non-colonized root half was actually lower in NM,M pots than in completely non-colonized wild-type plants. However, the magnitude of this systemic AM-induced suppression of DPU activity was much lower than the local suppression in AM roots, where at least 80% of the P taken up was via the MPU at low soil P levels. Considering that up to 100% of a plant’s P can be delivered via the MPU (Smith et al., 2003, 2004), the actual activity of the MPU in the wild-type plants was probably higher than the estimates presented here, as MPU activity may have been ‘hidden’ due to down-regulation of the DPU in AM roots.
The expression of the DPU transporter genes (MtPT1, MtPT2, and MtPT3) did not concur with the physiological data as wild-type plants grown at P20 had similar levels of gene expression in non-colonized plants to those in the non-colonized half of NM,M plants (also seen at P0; data not shown). This suggests that a colonized root half did not exert a long-distance effect upon DPU transporter expression. In contrast, AM colonization had a strong local effect on the DPU and MPU transporter genes. Here, expression of DPU transporters decreased relative to their expression in non-colonized plants, as reported previously (H. Liu et al., 1998), and the MPU transporter gene was expressed as expected (Harrison et al., 2002; Javot et al., 2007). Effects of root colonization on expression levels differed in the mtpt4 roots, where all DPU transporter genes were expressed similarly in non-mycorrhizal and mycorrhizal roots, but at a higher level than in non-mycorrhizal wild-type roots. The finding that a colonized root half influenced gene expression locally but not at long distance might be related to a local increase in root P concentrations, rather than AM colonization directly. Hence, the effect was absent (and even opposite) in mtpt4 roots that were colonized but did not transfer mycorrhiza-derived P to the plant. These results with mtpt4 confirm hypothesis (ii) that mycorrhiza-induced effects on DPU activity can be separated into P-derived and direct AM effects by comparing the wild-type and mtpt4 genotypes. It was previously suggested by Grønlund et al. (2013) that mycorrhizal colonization has a limited long-distance effect upon DPU, which is in agreement with the present findings in terms of gene expression. Thus, although an active MPU in one half of the root system had some impact on DPU activity in the other half of the system, this was not related to gene expression. There is growing evidence that P transporter gene expression is not always well correlated with P uptake activity (or flux) (C. Liu et al., 1998; Rae et al., 2004; Grace et al., 2009; Smith et al., 2009; Grønlund et al., 2013; Facelli et al., 2014); this could be due to post-transcriptional and/or post-translational regulation of the P uptake pathways.
To further explore the effect of AM colonization on plant P uptake and nutrition, expression of Mt4, a PSI gene in M. truncatula, and PO4 concentration in shoots was investigated. Expression of Mt4 was inversely proportional to the PO4 concentration of shoots, as expected (Burleigh and Harrison, 1997, 1998; Branscheid et al., 2010). Furthermore, expression of Mt4 was similar between the wild-type and mtpt4 mutant genotypes in non-mycorrhizal control pots. In colonized root halves, the Mt4 expression was similar to that in non-mycorrhizal roots in mtpt4 mutant plants, while it was suppressed in the wild-type plants, probably reflecting the local increase in PO4 root concentrations (Burleigh and Harrison, 1998), as shoot PO4 concentrations were similar for wild-type and mtpt4 NM,M plants.
In conclusion, there were both physiological and molecular effects of an inactive mycorrhizal P uptake pathway (mtpt4) upon the DPU, which seems to correspond to local changes of P/Pi concentrations in colonized roots in a functional symbiosis. Interestingly, the expression of the DPU transporter genes in mtpt4 roots was higher in the AM plants, both NM,M and M,M, than in NM,NM plants and in wild-type plants of all treatments. This indicates that the mutant plants compensate for the non-functional MPU, and subsequent loss of contribution of P by the MPU.
The effect of mycorrhizal colonization of one root half on root distribution and P nutrition
This work confirms hypothesis (iii) that colonized and non-colonized patches of root will experience differences in root growth and P nutrition. Specifically, root biomass was preferentially allocated to the colonized root half rather than the non-colonized root half in the wild-type plants where the P concentrations were also higher, but this was not the case in the mtpt4 plants. Previous split-root experiments also found greater root biomass and P concentration in the mycorrhizal side of the pot compared with the non-mycorrhizal side (Grønlund et al., 2013), as well as greater amounts of photosynthate transported to the mycorrhizal half of the split pot from the shoots (Koch and Johnson, 1984). In general terms, mycorrhizal colonization increases root branching and root length (Hodge et al., 2009). The lower root length in mycorrhizal than in non-mycorrhizal plants in this work (M,M versus NM,NM) and in several previous studies (e.g. Ravnskov and Jakobsen, 1995; Olsson et al., 1997) was probably caused by fungal competition for plant carbon in the absence of a mycorrhizal growth response, but a high root colonization, at 20mg and 50mg P kg–1.
In contrast to the wild-type, root biomass and root length in the mtpt4 mutant plants was higher in the non-colonized than in the colonized root halves. The higher expression of Mt4 and DPU MtPT genes in colonized roots compared with non-mycorrhizal roots suggests an increased sensing of Pi starvation in the colonized mtpt4 root half, although P concentrations were the same for all non-mycorrhizal and mycorrhizal root halves, which fits with only the DPU being active.
Although the colonized mtpt4 roots did not take up Pi via the MPU, the plant still supported colonization by AM fungi, which would have come at a carbon cost to the plant (Smith et al., 2010), and may explain the observed reduction in shoot and root growth in colonized mtpt4 plants compared with the wild type. Furthermore, the mtpt4 plants may have reduced investment in the colonized root half in favour of the non-colonized root half, which would be providing a return on the investment (in terms of Pi uptake).
Proper functioning of MtPT4 is critical to mycorrhiza-mediated Pi uptake, and arbuscules of colonized mtpt4 roots experience premature degeneration and death when MtPT4 is inactivated (Javot et al., 2007). However, premature arbuscule death can be suppressed by N limitation (Javot et al., 2011). The mtpt4 plants in this study had shoot N concentrations >3% dry weight in all treatments (data not shown), which suggested that they were not N limited (Marschner and Marschner, 2012). The arbuscules may therefore have been experiencing premature degeneration in the mtpt4 roots, and this is supported by the lower percentage of arbuscules in the mutant compared with wild-type roots. However, the colonized mtpt4 roots, although defective in MPU Pi uptake, were still producing external hyphae to a greater extent than previously reported (Javot et al., 2007), although at lower densities than the wild-type. This phenotype observed in the present study will require further work to be confirmed in light of the differences with the work of Javot et al. (2007).
In conclusion, partial colonization of roots by AM fungi had marked effects on the root growth distribution and on Pi uptake locally in the colonized root system. This highlights the plasticity of roots in response to AM colonization, where most research on root plasticity has focused on it in response to soil nutrient patches. Root plasticity in response to AM colonization could confer greater competitive ability for the plant, in terms of increased Pi uptake, and more effective allocation of root biomass and length.
There was physiological evidence that colonization by AM fungi had a limited, long-distance impact upon DPU activity, but this was not supported by gene expression data. This highlights the growing disconnect between gene expression data and actual activity, and, thus, the need to include both physiological and molecular methods, and for further studies on up- and downstream regulatory events.
Comparison of the results from wild-type and mtpt4 mutant plants indicated that a major factor in the effect of AM colonization on the DPU could rely on the local changes in root P concentrations in an active symbiosis, as mtpt4 mycorrhizal roots behaved mostly like non-mycorrhizal roots, although they were colonized. Furthermore, the mtpt4 plants seemed to have additional sensing of the carbon cost of the non-functional AM symbiosis, as photosynthates were allocated to the non-colonized root half.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Inorganic phosphate (PO4) concentration in shoots.
Table S1. ANOVA summary table for all response variables.
Table S2. Mycorrhizal colonization (% of root length) of mtpt4 mutant genotype, grown at 20mg P kg-1.
Acknowledgements
The authors wish to thank Professor Maria Harrison for providing access to the M. truncatula seeds and for valuable discussions, and Professors Sally Smith and Andrew Smith for valuable discussions. We also acknowledge Mette Flodgaard for her excellent technical assistance. SJWW wishes to acknowledge support received from the Monash University Postgraduate Publications Award. TRC gratefully acknowledges the Australian Research Council for support (FT120100463).
References
- Balzergue C, Puech-Pagès V, Bécard G, Rochange SF. 2011. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. Journal of Experimental Botany 62, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible W-R, Krajinski F. 2010. Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Molecular Plant-Microbe Interactions 23, 915–926. [DOI] [PubMed] [Google Scholar]
- Breuillin F, Schramm J, Hajirezaei M, et al. 2010. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. The Plant Journal 64, 1002–1017. [DOI] [PubMed] [Google Scholar]
- Bucher M. 2007. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist 173, 11–26. [DOI] [PubMed] [Google Scholar]
- Bucher M, Rausch C, Daram P. 2001. Molecular and biochemical mechanisms of phosphorus uptake into plants. Journal of Plant Nutrition and Soil Science 164, 209–217. [Google Scholar]
- Burleigh SH, Harrison MJ. 1997. A novel gene whose expression in Medicago truncatula roots is suppressed in response to colonization by vesicular-arbuscular mycorrhizal (VAM) fungi and to phosphate nutrition. Plant Molecular Biology 34, 199–208. [DOI] [PubMed] [Google Scholar]
- Burleigh SH, Harrison MJ. 1999. The down-regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiology 119, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh SJ, Harrison MJ. 1998. Characterization of the Mt4 gene from Medicago truncatula. Gene 216, 47–53. [DOI] [PubMed] [Google Scholar]
- Chiou T-J, Liu H, Harrison MJ. 2001. The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. The Plant Journal 25, 281–293. [DOI] [PubMed] [Google Scholar]
- Christophersen HM, Smith FA, Smith SE. 2009. Arbuscular mycorrhizal colonization reduces arsenate uptake in barley via downregulation of transporters in the direct epidermal phosphate uptake pathway. New Phytologist 184, 962–974. [DOI] [PubMed] [Google Scholar]
- Cordell D, Drangert JO, White S. 2009. The story of phosphorus: global food security and food for thought. Global Environmental Change-Human and Policy Dimensions 19, 292–305. [Google Scholar]
- Epstein E, Bloom AJ. 2005. Mineral nutrition of plants: principles and perspectives. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Facelli E, Duan T, Smith SE, Christophersen HM, Facelli JM, Smith FA. 2014. Opening the black box: outcomes of interactions between arbuscular mycorrhizal (AM) and non-host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply. Plant, Cell and Environment 37, 1382–1392. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, González E, Bustos R, Linhares F, Leyva A, Paz-Ares J. 2004. The transcriptional control of plant responses to phosphate limitation. Journal of Experimental Botany 55, 285–293. [DOI] [PubMed] [Google Scholar]
- Gilbert N. 2009. Environment: the disappearing nutrient. Nature 461, 716–718. [DOI] [PubMed] [Google Scholar]
- Glassop D, Smith S, Smith F. 2005. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 222, 688–698. [DOI] [PubMed] [Google Scholar]
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE. 2009. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytologist 181, 938–949. [DOI] [PubMed] [Google Scholar]
- Grønlund M, Albrechtsen M, Johansen IE, Hammer EC, Nielsen TH, Jakobsen I. 2013. The interplay between P uptake pathways in mycorrhizal peas: a combined physiological and gene-silencing approach. Physiologia Plantarum 149, 234–248. [DOI] [PubMed] [Google Scholar]
- Grunwald U, Guo W, Fischer K, Isayenkov S, Ludwig-Müller J, Hause B, Yan X, Küster H, Franken P. 2009. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treated Medicago truncatula roots. Planta 229, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. 2005. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology 59, 19–42. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J. 2002. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. The Plant Cell 14, 2413–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. 2009. Plant root growth, architecture and function. Plant and Soil 321, 153–187. [Google Scholar]
- Jakobsen I, Abbott LK, Robson AD. 1992. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L 1. Spread of hyphae and phosphorus inflow into roots. New Phytologist 120, 371–380. [Google Scholar]
- Javot H, Penmetsa RV, Breuillin F, Bhattarai KK, Noar RD, Gomez SK, Zhang Q, Cook DR, Harrison MJ. 2011. Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. The Plant Journal 68, 954–965. [DOI] [PubMed] [Google Scholar]
- Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ. 2007. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences, USA 104, 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE, Johnson CR. 1984. Photosynthate partitioning in split-root citrus seedlings with mycorrhizal and nonmycorrhizal root systems. Plant Physiology 75, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormanik P, McGraw A. 1982. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In: Schenck N, ed. Methods and principles of mycorrhizal research. St Paul, MN: American Phytopathological Society, 37–45. [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. 2010. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 334, 11–31. [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA. 1979. An improved assay for nanomole amounts of inorganic phosphate. Analytical Biochemistry 100, 95–97. [DOI] [PubMed] [Google Scholar]
- Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG. 1998. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiology 116, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Trieu AT, Blaylock LA, Harrison MJ. 1998. Cloning and characterization of two phosphate transporters from Medicago truncatula roots: regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Molecular Plant-Microbe Interactions 11, 14–22. [DOI] [PubMed] [Google Scholar]
- Liu J, Versaw WK, Pumplin N, Gomez SK, Blaylock LA, Harrison MJ. 2008. Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. Journal of Biological Chemistry 283, 24673–24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L. 2014. Phosphate nutrition: improving low-phosphate tolerance in crops. Annual Review of Plant Biology 65, 95–123. [DOI] [PubMed] [Google Scholar]
- Marschner H, Dell B. 1994. Nutrient uptake in mycorrhizal symbiosis. Plant and Soil 159, 89–102. [Google Scholar]
- Marschner H, Marschner P. 2012. Marschner’s mineral nutrition of higher plants. New York: Academic Press. [Google Scholar]
- Martín AC, Del Pozo JC, Iglesias J, Rubio V, Solano R, De La Peña A, Leyva A, Paz-Ares J. 2000. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. The Plant Journal 24, 559–567. [DOI] [PubMed] [Google Scholar]
- McGonigle T, Miller M, Evans D, Fairchild G, Swan J. 1990. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist 115, 495–501. [DOI] [PubMed] [Google Scholar]
- Merrild MP, Ambus P, Rosendahl S, Jakobsen I. 2013. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytologist 200, 229–240. [DOI] [PubMed] [Google Scholar]
- Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M. 2009. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytologist 181, 950–959. [DOI] [PubMed] [Google Scholar]
- Newman EI. 1966. A method of estimating total length of root in a sample. Journal of Applied Ecology 3, 139–145. [Google Scholar]
- Olsen S, Cole C, Watanabe F, Dean L. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington, DC: USDA Circular 939. [Google Scholar]
- Olsson PA, Baath E, Jakobsen I. 1997. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Applied and Environmental Microbiology 63, 3531–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. 2002. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences, USA 99, 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science 16, 442–450. [DOI] [PubMed] [Google Scholar]
- Poulsen KH, Nagy R, Gao LL, Smith SE, Bucher M, Smith FA, Jakobsen I. 2005. Physiological and molecular evidence for Pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytologist 168, 445–453. [DOI] [PubMed] [Google Scholar]
- Rae AL, Jarmey JM, Mudge SR, Smith FW. 2004. Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Functional Plant Biology 31, 141–148. [DOI] [PubMed] [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology 50, 665–693. [DOI] [PubMed] [Google Scholar]
- Ravnskov S, Jakobsen I. 1995. Functional compatibility in arbuscular mycorrhizas measured as hyphal P transport to the plant. New Phytologist 129, 611–618. [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. 2010. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Molecular Plant 3, 288–299. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiology 116, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüßler A, Walker C. 2010. The Glomeromycota. A species list with new families and new genera. The Royal Botanic Garden Edinburgh. [Google Scholar]
- Smith FA, Grace EJ, Smith SE. 2009. More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist 182, 347–358. [DOI] [PubMed] [Google Scholar]
- Smith SE, Facelli E, Pope S, Smith FA. 2010. Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil 326, 3–20. [Google Scholar]
- Smith SE, Jakobsen I, Grønlund M, Smith FA. 2011. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiology 156, 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. New York: Academic Press. [Google Scholar]
- Smith SE, Smith FA, Jakobsen I. 2003. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiology 133, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Smith FA, Jakobsen I. 2004. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytologist 162, 511–524. [Google Scholar]
- Xiao K, Liu J, Dewbre G, Harrison M, Wang Z. 2006. Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula. Plant Biology 8, 439–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.