Abstract
Introduction
The current surgical management of acute complicated diverticulitis has seen a major paradigm shift from routine operative intervention to a more conservative approach. This has been made possible by the widespread availability of computed tomography (CT) to enable stratification of the disease severity of acute complicated diverticulitis. The aim of this study was to retrospectively validate a CT grading system for acute complicated diverticulitis in the prediction of the need for operative or percutaneous intervention.
Methods
Hospital and radiology records were reviewed to identify patients with acute complicated diverticulitis confirmed by CT. A consultant gastrointestinal radiologist, blinded to the clinical outcomes of patients, assigned a score according to the CT grading system.
Results
Three hundred and sixty-seven patients (34.6%) had CT performed for acute diverticulitis during the study period. Forty-four patients (12.0%) had acute complicated diverticulitis (abscess and/or free intraperitoneal air) confirmed on CT. There were 22 women (50%) and the overall median age was 59 years (range: 19–92 years). According to the CT findings, there was one case with grade 1, eighteen patients with grade 2, four with grade 3 and twenty-one with grade 4 diverticulitis. Three patients with grade 2, three patients with grade 3 and ten patients with grade 4 disease underwent acute radiological or surgical intervention.
Conclusions
The use of a CT grading system for acute complicated diverticulitis did not predict the need for acute radiological or operative intervention in this small study. Decision making guided by the patient’s clinical condition still retains a primary role in the management of acute complicated diverticulitis.
Keywords: Diverticulitis, Grading, Intervention
The prevalence of diverticular disease is increasing, affecting a third of patients above 45 years of age.1 Symptomatic diverticulitis occurs in 10–25% of patients1 and diverticular perforation has a population incidence of 4 cases per 100,000.2 Additionally, diverticular perforation that requires operative intervention is associated with mortality rates of between 12% and 36%.3–6
The current surgical management of complicated acute diverticulitis has seen a major paradigm shift from routine operative intervention to a more conservative approach.7–9 This change in practice reflects our increasing understanding of the morbidity and mortality associated with emergency surgery for complicated diverticular disease as well as subsequent interventions attempting to deal with the consequences of the emergency surgery such as stoma complications, incisional hernias and stoma reversal. Historically, surgical intervention was indicated for sepsis source control, which often required laparotomy and resection of the perforated segment of colon and end colostomy formation (Hartmann’s procedure). This operation is known to be associated with a significant mortality rate and complication profile, and up to 65% of end colostomies were not reversed.10–14
Traditionally, the Hinchey classification for diverticulitis (developed in 1978) was used with several subsequent modifications15 to assist surgeons in decision making during the management of complicated diverticular disease but these classifications are based largely on the intraoperative findings. Consequently, even though these classification systems helped to guide treatment, they did not reduce the rate of operative interventions and their serious consequences.
The increasing adoption of a more conservative approach in complicated diverticular disease has only been possible because of the advances in antibiotic therapy, nutritional support, critical care and interventional radiology.16 However, it was mainly the widespread availability and accessibility of computed tomography (CT) in the assessment of the acute surgical abdomen that played a major role in the current trends in the management of acute diverticulitis. CT has enabled accurate diagnosis of complicated diverticular disease as well as stratifying disease severity and could therefore help to identify patients who may benefit from non-operative therapy.17,18 Furthermore, the use of radiologically guided percutaneous drainage of diverticular abscesses might avoid the need for acute operative intervention.
Increasingly, surgical intervention is reserved for patients who have failed conservative treatment, have generalised peritonitis or are haemodynamically unstable. Laparoscopy and lavage may represent a feasible alternative to open surgery in the management of acute diverticulitis, with the avoidance of colonic resection (and probably colostomy) during the acute illness.19–21
As a result, the role of CT has become crucial in decision making for patients with acute diverticulitis, and CT grading systems were developed to stratify disease severity and guide management.16,22 Nevertheless, most of the published literature uses CT grading in combination with strict management protocol and patients are often offered surgical intervention based on the outcome of the CT grading rather than on clinical assessment.16 In theory, this could lead to some patients having ‘premature’ surgical intervention and some of them could possibly have responded to aggressive medical treatment.
The aim of this study was to retrospectively validate a CT grading system16 for acute complicated diverticulitis to determine its ability to predict the need for operative or percutaneous intervention in a group of patients who had their management and the decision for surgical or radiological intervention based on clinical assessment.
Methods
Patients with a diagnosis of diverticulosis were identified retrospectively by review of computerised hospital records using the International Statistical Classification of Diseases code for ‘diverticular disease of intestine’ (K57). The radiology database was then searched to identify patients who had acute complicated diverticulitis confirmed by CT. Complicated diverticulitis in this study was defined as abscess and/or free intraperitoneal gas on admission CT. The hospital record search was limited to the period January 2010 to August 2011 inclusive.
A consultant gastrointestinal radiologist, blinded to the outcome of the clinical management, reviewed the CT and assigned a score according to the CT grading system (Table 1).16 This was correlated with the patient’s clinical outcome to determine the grading system’s predictive value. There were no exclusion criteria and the main inclusion criterion was the availability of CT for review.
Table 1.
Perforated diverticulitis computed tomography grading system16
| Grade | Definition |
|---|---|
| 1 | Localised free air (pericolonic) without abscess |
| 2 | Small (<2cm) collections of distant free air OR small (<4cm) abscess |
| 3 | Large (>2cm) collections of distant free air OR large (>4cm) abscess |
| 4 | Free air with non-loculated free fluid in the peritoneal cavity |
Patient demographic data and the outcomes of failed non-operative management were entered into Excel® (Microsoft, Redmond, WA, US) for analysis.
Results
A total of 1,060 patients with a discharge diagnosis of diverticulosis were identified after review of the hospital records. Of these, 365 patients (34.6%) had CT performed for acute diverticulitis during the study period. Forty-four patients (12.0%) had acute complicated diverticulitis (abscess and/or free intraperitoneal gas) confirmed on CT. There were 22 women (50%) and the overall median age was 59 years (range: 19–92 years).
CT grading
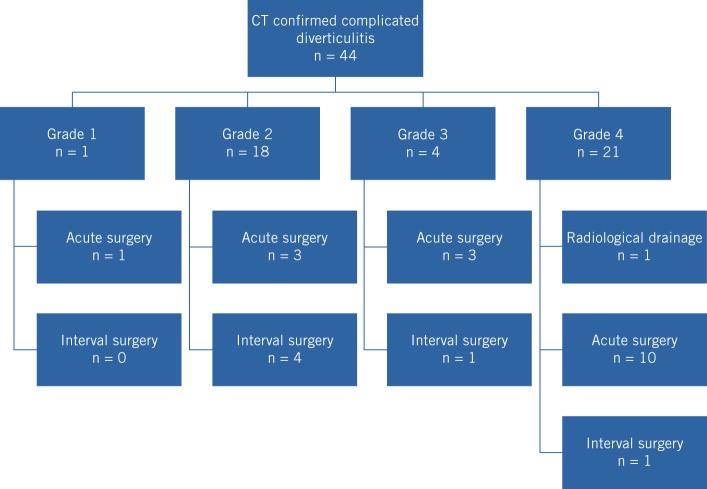
According to the CT findings, there was one patient with grade 1, eighteen with grade 2, four with grade 3 and twenty-one with grade 4 diverticulitis. Three patients with grade 2, three patients with grade 3 and ten patients with grade 4 disease underwent acute radiological or surgical intervention (Fig 1). The indications for acute surgical intervention were failure of conservative management and anatomical factors that precluded safe radiological drainage. The median duration from admission until acute intervention was 1 day (range: 0–7 days). The median length of hospital stay was 4 (grade 2), 12 (grade 3) and 9 (grade 4) days.
Figure 1.
Number of patients in each grade of diverticulitis as confirmed by computed tomography (CT) and subsequent management
Acute interventions
The cases that required acute intervention are summarised in Table 2. The one patient with grade 1 diverticulitis according to CT underwent an open sigmoid colectomy for ongoing symptoms of pain during non-operative treatment. Interestingly, this patient had had a previous diverticular resection with colostomy and subsequent restoration of the gastrointestinal tract. Intraoperatively, there was minimal intraperitoneal contamination and conditions were appropriate for a primary anastomosis. Three patients with CT findings of grade 2 disease underwent an emergency Hartmann’s procedure.
Table 2.
Demographics and outcomes of acute surgical management
| Age / sex | CT grade of diverticulitis | Indication for acute surgical intervention | Intervention | Length of stay | Complications | Pathology |
|---|---|---|---|---|---|---|
| 62 M | 1 | Ongoing pain with non-operative treatment | Open sigmoid colectomy | 11 days | Superficial surgical site infection | Diverticulosis |
| 43 M | 2 | Failed non-operative management | Hartmann’s procedure | 36 days | Postoperative chest sepsis and intensive care unit admission | Diverticular abscess |
| 52 F | 2 | Failed non-operative management | Open subtotal colectomy (due to unhealthy looking caecum at laparotomy) | 20 days | Adhesive small bowel obstruction 11 months after index operations, requiring operative intervention | Diverticular abscess |
| 76 F | 2 | Failed non-operative management | Hartmann’s procedure | 19 days | Postoperative myocardial infarct; admission to coronary care unit and angiography | Perforated diverticulitis |
| 57 M | 3 | Unsuitable for radiological drainage | Laparoscopic washout and drain insertion | 7 days | Nil | N/A |
| 69 F | 3 | Large bowel obstruction | Hartmann’s procedure | 10 days | Nil | Perforated diverticulitis |
| 88 M | 3 | Significant co-morbidities precluding major surgery | Open left paracolic drainage | 14 days | Nil | N/A |
| 50 F | 4 | Generalised peritonitis following head and neck surgery | Hartmann’s procedure | 37 days | Nil | Diverticulitis |
| 51 M | 4 | Small bowel obstruction | Hartmann’s procedure | 29 days | Full-thickness wound dehiscence requiring return to theatre | Diverticular abscess |
| 53 M | 4 | Generalised peritonitis | Hartmann’s procedure | 17 days | Nil | Diverticular abscess |
| 55 F | 4 | Generalised peritonitis | Hartmann’s procedure | 9 days | Superficial surgical site infection | pT4 N0 Dukes’ B moderately differentiated adenocarcinoma, completely excised |
| 63 M | 4 | Generalised peritonitis | Hartmann’s procedure | 18 days | Nil | Diverticulitis |
| 63 M | 4 | Generalised peritonitis | Open subtotal colectomy (owing to involvement of the caecum in the inflammatory mass) | 16 days | Nil | Diverticular abscess |
| 63 M | 4 | Generalised peritonitis | Hartmann’s procedure | 20 days | Nil | Perforated diverticulitis |
| 65 F | 4 | Failed non-operative management | Hartmann’s procedure | 22 days | Ischaemic colostomy and return to theatre on day 1 postoperatively for stoma refashion | Diverticulitis |
| 71 F | 4 | Generalised peritonitis 8 days following cardiac surgery | Hartmann’s procedure | 96 days | Postoperative multiorgan failure | Diverticular abscess |
| 85 F | 4 | Failed non-operative management | Hartmann’s procedure | 14 days | Death on postoperative day 14 from multiorgan failure | Perforated diverticulitis |
CT = computed tomography; N/A = not applicable
Among those patients identified by CT as having grade 3 diverticulitis, one patient had laparoscopic washout and drainage of collections that were not amenable to radiological drainage. One elderly patient underwent open surgical drainage of a left paracolic diverticular colocutaneous abscess via a left flank incision under general anaesthesia as the abscess was pointing and easily accessible for open drainage. This patient was not offered elective diverticular resection in view of his significant cardiac co-morbidities.
Among those patients who had grade 4 diverticulitis according to CT, one patient had successful radiological drainage of the diverticular abscess. This patient proceeded to have an elective open left hemicolectomy one month after the acute admission. Histology of the resected specimen showed chronic diverticulitis. All ten of the patients who underwent acute surgical intervention required a Hartmann’s procedure. The clinical indications for surgery were generalised peritonitis (n=7), worsening sepsis after trial of non-operative management (n=2) and small bowel obstruction secondary to severe diverticular sepsis (n=1).
Postoperative complications included two patients who returned to theatre; one patient required refashioning of an ischaemic colostomy and the other had resuturing of a full-thickness wound dehiscence. Additionally, one patient had a superficial surgical site infection managed with intravenous antibiotics and two patients had multiorgan failure, of which one did not survive.
The resection specimen of a 55-year-old woman whose CT results suggested she had grade 4 diverticulitis revealed a perforated adenocarcinoma with associated incidental diverticular disease. The final pathological staging was of pT4 N0 Dukes’ B moderately differentiated adenocarcinoma. The tumour was excised completely and the patient referred to the oncologists for adjuvant treatment.
Follow-up
Six patients had elective colonic resections after recovery from acute complicated diverticulitis. A left-sided colectomy was performed in four patients with CT confirmed grade 2 diverticulitis, one patient with grade 3 and one patient with grade 4. Three patients had an open procedure, two had a laparoscopic resection and one had a laparoscopic sigmoid colectomy that was converted to an open procedure. The latter patient developed an anastomotic leak, which required a return to theatre, takedown of the colorectal anastomosis and a Hartmann’s procedure. Two patients had reversal of the Hartmann’s procedure at follow-up. It is worth noting that no malignancy was detected in the resection specimens (Table 3).
Table 3.
Demographics and outcomes of elective diverticular resections
| Age / sex | CT grade of diverticulitis | Large bowel investigations | Intervention | Length of stay for acute admission | Complications | Pathology |
|---|---|---|---|---|---|---|
| 46 F | 2 | Flexible sigmoidoscopy and CT colonography | Open anterior resection | 10 days | Nil | Diverticulosis |
| 52 M | 2 | Flexible sigmoidoscopy | Open sigmoid colectomy | 4 days | Nil | Diverticulosis |
| 60 F | 2 | Barium enema | Laparoscopic sigmoid colectomy | 4 days | Nil | Diverticulosis |
| 61 F | 2 | Nil | Laparoscopic sigmoid colectomy | 3 days | Nil | Diverticulosis |
| 57 M | 3 | Nil | Laparoscopic to open sigmoid colectomy | 7 days | Anastomotic leak, conversion to Hartmann’s procedure; subsequently had colostomy reversal | Diverticulosis |
| 54 F | 4 | Nil | Open left hemicolectomy | 5 days | Nil | Chronic diverticulitis |
CT = computed tomography
Mortality
There were three deaths in this series of patients. There was a perioperative death in an 85-year-old woman who had grade 4 diverticulitis as identified by CT. She underwent a Hartmann’s procedure after four days of failed non-operative management. She developed pulmonary oedema that was refractory to maximal medical therapy and died 14 days after surgery.
One elderly female patient whose CT results suggested grade 4 diverticulitis was managed conservatively and an informed decision was made not to escalate her treatment to include operative intervention. She died eight days into her hospital admission.
One elderly patient with CT confirmed grade 3 diverticulitis that was managed conservatively owing to her co-morbidities died four months later from an unrelated illness (intracerebral haemorrhage).
Discussion
The role of emergency surgery in acute complicated diverticulitis has diminished even in the presence of diverticular abscesses and free intra-abdominal gas.23 The change in treatment principles in acute diverticulitis was a consequence of the increasing availability and diagnostic accuracy of CT, which has enabled rapid stratification of the acute surgical abdomen and acute diverticulitis disease severity. CT can confirm the diagnosis, identify the presence of diverticular abscesses or collections, and determine the feasibility of radiologically guided drainage.24,25 This strategy allows for sepsis control without resorting to major surgical intervention.
Several classification systems exist for complicated diverticulitis. These systems aim to determine the severity of acute diverticulitis, predict mortality and guide surgical decisions. Hinchey categorised perforated diverticulitis into four categories, dependent on intraoperative findings.26 The Hinchey classification has since undergone various modifications to incorporate clinical and radiological information, with the aim of improving its utility as a decision making tool.27–29 Other commonly used acute diverticulitis classification systems include the Mannheim peritonitis index30 (patient and disease related factors) and the Charlson co-morbidity index31 (patient related factors).
Pasternak et al evaluated the predictive values of six scoring systems in the decision making process for acute diverticulitis.32 They concluded that scoring systems based on patient related co-morbidities (eg Charlson co-morbidity index) had greater predictive value for mortality than scores based on locoregional factors (eg Hinchey classification). Nevertheless, no scoring system can as yet replace close and repeated clinical assessment combined with clinical acumen in predicting which patients with complicated acute diverticulitis will require intervention.33,34
Several groups have investigated the value of preoperative CT in the prediction of the need for operative intervention in acute diverticulitis. Gielens et al retrospectively compared the CT Hinchey classification with intraoperative Hinchey staging.35 The sensitivity of CT in Hinchey IV disease was 100% but decreased to 42% in Hinchey III acute diverticulitis. The explanation provided by the authors for this finding was the potential understaging of Hinchey II disease (as staged by CT) in the presence of minimal pus found only at operation (Hinchey III). Preoperative CT Hinchey grading also had low predictive value at differentiating intraoperative Hinchey III and IV diverticulitis.
The CT grading system for acute diverticulitis used in this study was based on the work by Siewert et al22 and Dharmarajan et al.16 The data of Siewert et al showed that patients with diverticular abscesses of <4cm could be treated successfully using antibiotics without the need for acute surgical intervention.22 On the other hand, patients with diverticular abscesses of >4cm were candidates for radiologically guided drainage. Dharmarajan et al adapted the results from Siewert’s study, from which they derived a simple CT grading system for acute perforated diverticulitis.16 This grading system was used in our study for its ease of use and simplicity.
In our patient group, there was a low operative intervention rate during the acute presentation of complicated diverticulitis, even in the presence of small intra-abdominal abscess (grade 2) or free air (grade 4). This finding was in agreement with the conclusions from Costi et al,23 which showed that conservative management was feasible for patients with free intra-abdominal gas secondary to perforated acute diverticulitis. In their study group of 39 haemodynamically stable patients with perforated diverticulitis, non-operative management was successful in 36 patients (92.3%). Seven of these patients (19.4%) required radiologically guided abscess drainage. The mortality rate from their series was 0%.
In our study, all the patients with grade 4 diverticulitis as identified by CT who proceeded to urgent surgery underwent a Hartmann’s procedure. This surgical decision was made in the presence of widespread intra-abdominal contamination. Alternative treatment options have been reported in literature, including suture repair of the diverticular perforation, omentoplasty, laparoscopic lavage, proximal diversion, resection of the perforated colon and primary anastomosis.21,36–39 However, our unit practice was to mostly perform Hartmann’s procedure for these patients. A total of 12 Hartmann’s procedures were performed in this study, of which 3 cases (25%) had elective reversals. This was consistent with the reported rates of Hartmann’s reversal in the literature.14
The operative intervention rate in cases with CT results for grade 3 diverticulitis was 100%. This was secondary to failure of conservative management, with one case each of large bowel obstruction, worsening sepsis in a co-morbid elderly patient and failure to drain an abscess under radiological guidance. Despite this, the small absolute numbers of patients suggested to have grade 3 diverticulitis did not allow for valid conclusions to be made about these data.
The absolute postoperative complication rate in this series for acute surgery was 52.9% (9/17) with two patients returning to theatre for either resuturing of abdominal wound dehiscence or refashioning of ischaemic colostomy (Table 2). Elective sigmoid resection was also associated with significant complications, with one anastomotic leak after open sigmoid colectomy, requiring conversion to Hartmann’s procedure (Table 3). Indeed, the published data have shown that the morbidity and mortality of elective diverticular resections was greater than for elective malignant colorectal resection.40
Our study indicates that despite the increasing interest in radiological scoring systems in predicting the need for either radiological or surgical intervention, decision making should continue to be based on clinical judgement owing to the limitations of the currently available scoring systems. Our data highlight the need for a robust radiological scoring tool to help guide clinicians in their management of complicated diverticular disease.
Study limitations
This study could be criticised for its retrospective nature, lack of strict treatment protocol to streamline patients’ management according to the CT staging and also because patients were treated by a group of surgeons with diverse experience, subspecialty interests and views on the management of complicated acute diverticulitis. However, we do believe that these points could be part of the strength of this study. It is increasingly recognised that the natural history of diverticular disease is poorly understood and could be changing. Factors to account for such change include increasing incidence of the disease in the younger population,22 the availability of a wider range of antibiotics, better management of acutely ill patients16 and increasing use of the minimally invasive approach.7
All these factors have led to progressive change in policy towards less aggressive treatment. This study adds to the existing understanding of the natural history of this condition as decision making was based on patients’ clinical response to treatment with no strict protocols that might lead to ‘avoidable’ surgical intervention. The involvement of a diverse group of surgeons with different subspecialty interests reflects accurately the outcomes in this group of patients with no selection or treatment preference bias.
We also acknowledge that the absolute number of patients was relatively small but the outcome of this study adds to the increasing evidence that surgical intervention in patients with acute complicated diverticulitis should rely on clinical judgement rather than the current scoring systems. In addition, we appreciate that only one consultant radiologist with a specialist interest in gastrointestinal imaging reviewed the CT and carried out the retrospective scoring, and this interest and expertise might not be available in routine practice for emergency CT reporting. Nevertheless, the radiologist was blinded to both the original CT reports and the patients’ clinical outcomes.
There have been major changes in the indications for subsequent elective resection of complicated diverticular disease following successful conservative management. Over the last decade, several publications have challenged the previous practice parameters of the main associations of colorectal surgery (the Association of Coloproctology of Great Britain and Ireland, and the American Society of Colon and Rectal Surgeons) that elective surgery should be offered after two episodes of uncomplicated acute diverticulitis or after one episode in young patients.41–43
The current literature suggests that there is good clinical and experimental evidence against the previous recommendations for elective resection. Presently, it is believed that expectant management is associated with lower mortality and stoma formation, and entails lower costs. Furthermore, there is no clear evidence that younger patients presenting with acute diverticulitis exhibit a more aggressive form of the disease.41 These observations indicate our increasing understanding of the natural history of diverticular disease in the elective resection setting and it could be that this is now the time to revisit the natural history of the disease in acute presentation as well.
Conclusions
The use of this simple CT grading system for acute complicated diverticulitis did not predict the need for acute radiological or operative intervention in this small study. Patients with grade 4 diverticulitis as identified by CT (free gas and intra-abdominal free fluid) could still be managed conservatively. Decision making guided by the patient’s clinical condition retains a primary role in the management of acute complicated diverticulitis.
Acknowledgements
The material in this paper was presented at the European Society of Coloproctology annual meeting held in Vienna, September 2012, and published as an abstract in: Colorectal Dis 2012; 14(Suppl 2): 19.
References
- 1.Chapman J, Davies M, Wolff B et al. Complicated diverticulitis. Ann Surg 2005; 242: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart AR, Kennedy HJ, Stebbings WS, Day NE. How frequently do large bowel diverticula perforate? An incidence and cross-sectional study. Eur J Gastroenterol Hepatol 2000; 12: 661–665. [DOI] [PubMed] [Google Scholar]
- 3.Schwesinger WH, Page CP, Gaskill HV et al. Operative management of diverticular emergencies: strategies and outcomes. Arch Surg 2000; 135: 558–562. [DOI] [PubMed] [Google Scholar]
- 4.Biondo S, Ramos E, Deiros M et al. Prognostic factors for mortality in left colonic peritonitis: a new scoring system. J Am Coll Surg 2000; 191: 635–642. [DOI] [PubMed] [Google Scholar]
- 5.Zeitoun G, Laurent A, Rouffet F et al. Multicentre, randomized clinical trial of primary versus secondary sigmoid resection in generalized peritonitis complicating sigmoid diverticulitis. Br J Surg 2000; 87: 1,366–1,374. [DOI] [PubMed] [Google Scholar]
- 6.Elliott TB, Yego S, Irvin TT. Five-year audit of the acute complications of diverticular disease. Br J Surg 1997; 84: 535–539. [PubMed] [Google Scholar]
- 7.Daniels L, de Korte N, Winter D et al. Overtreatment of sigmoid diverticulitis: plea for a less aggressive approach. Dig Dis 2012; 30: 86–91. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen J, Lange JF. Treatment of perforated diverticulitis with generalized peritonitis: past, present, and future. World J Surg 2010; 34: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh S, Krukowski ZH. Outcome of a conservative policy for managing acute sigmoid diverticulitis. Br J Surg 2007; 94: 876–879. [DOI] [PubMed] [Google Scholar]
- 10.Tokode OM, Akingboye A, Coker O. Factors affecting reversal following Hartmann’s procedure: experience from two district general hospitals in the UK. Surg Today 2011; 41: 79–83. [DOI] [PubMed] [Google Scholar]
- 11.David GG, Al-Sarira AA, Willmott S et al. Use of Hartmann’s procedure in England. Colorectal Dis 2009; 11: 308–312. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen J, Coene PP, Van Hout NM et al. Restoration of bowel continuity after surgery for acute perforated diverticulitis: should Hartmann’s procedure be considered a one-stage procedure? Colorectal Dis 2009; 11: 619–624. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee S, Leather AJ, Rennie JA et al. Feasibility and morbidity of reversal of Hartmann’s. Colorectal Dis 2005; 7: 454–459. [DOI] [PubMed] [Google Scholar]
- 14.Maggard MA, Zingmond D, O’Connell JB, Ko CY. What proportion of patients with an ostomy (for diverticulitis) get reversed? Am Surg 2004; 70: 928–931. [PubMed] [Google Scholar]
- 15.Klarenbeek BR, de Korte N, van der Peet DL, Cuesta MA. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis 2012; 27: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharmarajan S, Hunt SR, Birnbaum EH et al. The efficacy of nonoperative management of acute complicated diverticulitis. Dis Colon Rectum 2011; 54: 663–671. [DOI] [PubMed] [Google Scholar]
- 17.Lohrmann C, Ghanem N, Pache G et al. CT in acute perforated sigmoid diverticulitis. Eur J Radiol 2005; 56: 78–83. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser AM, Jiang JK, Lake JP et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005; 100: 910–917. [DOI] [PubMed] [Google Scholar]
- 19.Karoui M, Champault A, Pautrat K et al. Laparoscopic peritoneal lavage or primary anastomosis with defunctioning stoma for Hinchey 3 complicated diverticulitis: results of a comparative study. Dis Colon Rectum 2009; 52: 609–615. [DOI] [PubMed] [Google Scholar]
- 20.Alamili M, Gögenur I, Rosenberg J. Acute complicated diverticulitis managed by laparoscopic lavage. Dis Colon Rectum 2009; 52: 1,345–1,349. [DOI] [PubMed] [Google Scholar]
- 21.Myers E, Hurley M, O’Sullivan GC et al. Laparoscopic peritoneal lavage for generalized peritonitis due to perforated diverticulitis. Br J Surg 2008; 95: 97–101. [DOI] [PubMed] [Google Scholar]
- 22.Siewert B, Tye G, Kruskal J et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: size matters. Am J Roentgenol 2006; 186: 680–686. [DOI] [PubMed] [Google Scholar]
- 23.Costi R, Cauchy F, Le Bian A et al. Challenging a classic myth: pneumoperitoneum associated with acute diverticulitis is not an indication for open or laparoscopic emergency surgery in hemodynamically stable patients. A 10-year experience with a nonoperative treatment. Surg Endosc 2012; 26: 2,061–2,071. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosetti P. Acute diverticulitis of the left colon: value of the initial CT and timing of elective colectomy. J Gastrointest Surg 2008; 12: 1,318–1,320. [DOI] [PubMed] [Google Scholar]
- 25.Brandt D, Gervaz P, Durmishi Y et al. Percutaneous CT scan-guided drainage vs. antibiotherapy alone for Hinchey II diverticulitis: a case–control study. Dis Colon Rectum 2006; 49: 1,533–1,538. [DOI] [PubMed] [Google Scholar]
- 26.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg 1978; 12: 85–109. [PubMed] [Google Scholar]
- 27.Wasvary H, Turfah F, Kadro O, Beauregard W. Same hospitalization resection for acute diverticulitis. Am Surg 1999; 65: 632–635. [PubMed] [Google Scholar]
- 28.Köhler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. Surg Endosc 1999; 13: 430–436. [DOI] [PubMed] [Google Scholar]
- 29.Hansen O, Graupe F, Stock W. Prognostic factors in perforating diverticulitis of the large intestine. Chirurg 1998; 69: 443–449. [DOI] [PubMed] [Google Scholar]
- 30.Linder MM, Wacha H, Feldmann U et al. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg 1987; 58: 84–92. [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 32.Pasternak I, Dietrich M, Woodman R et al. Use of severity classification systems in the surgical decision-making process in emergency laparotomy for perforated diverticulitis. Int J Colorectal Dis 2010; 25: 463–470. [DOI] [PubMed] [Google Scholar]
- 33.Markus PM, Martell J, Leister I et al. Predicting postoperative morbidity by clinical assessment. Br J Surg 2005; 92: 101–106. [DOI] [PubMed] [Google Scholar]
- 34.Hartley MN, Sagar PM. The surgeon’s ‘gut feeling’ as a predictor of post-operative outcome. Ann R Coll Surg Engl (Suppl) 1994; 76: 277–278. [PubMed] [Google Scholar]
- 35.Gielens MP, Mulder IM, van der Harst E et al. Preoperative staging of perforated diverticulitis by computed tomography scanning. Tech Coloproctol 2012; 16: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naraynsingh V, Maharaj R, Hassranah D et al. Perforated left-sided diverticulitis with faecal peritonitis: is the Hinchey classification the best guide for surgical decision making? Tech Coloproctol 2011; 15: 199–203. [DOI] [PubMed] [Google Scholar]
- 37.Breitenstein S, Kraus A, Hahnloser D et al. Emergency left colon resection for acute perforation: primary anastomosis or Hartmann’s procedure? A case-matched control study. World J Surg 2007; 31: 2,117–2,124. [DOI] [PubMed] [Google Scholar]
- 38.Regenet N, Pessaux P, Hennekinne S et al. Primary anastomosis after intraoperative colonic lavage vs Hartmann’s procedure in generalized peritonitis complicating diverticular disease of the colon. Int J Colorectal Dis 2003; 18: 503–507. [DOI] [PubMed] [Google Scholar]
- 39.Gooszen AW, Tollenaar RA, Geelkerken RH et al. Prospective study of primary anastomosis following sigmoid resection for suspected acute complicated diverticular disease. Br J Surg 2001; 88: 693–697. [DOI] [PubMed] [Google Scholar]
- 40.Bokey EL, Chapuis PH, Pheils MT. Elective resection for diverticular disease and carcinoma. Comparison of postoperative morbidity and mortality. Dis Colon Rectum 1981; 24: 181–182. [DOI] [PubMed] [Google Scholar]
- 41.Biondo S, Lopez Borao J, Millan M et al. Current status of the treatment of acute colonic diverticulitis: a systematic review. Colorectal Dis 2012; 14: e1–e11. [DOI] [PubMed] [Google Scholar]
- 42.Hogan A, Winter D. Management of acute diverticulitis: is less more? Dis Colon Rectum 2011; 54: 126–128. [DOI] [PubMed] [Google Scholar]
- 43.Mäkelä JT, Kiviniemi HO, Laitinen ST. Spectrum of disease and outcome among patients with acute diverticulitis. Dig Surg 2010; 27: 190–196. [DOI] [PubMed] [Google Scholar]