Abstract
Introduction
Selective conservatism for thoracic trauma is well established but the emergence of new technologies may cause management strategies to continue to evolve.
Methods
A retrospective study was conducted on thoracic trauma patients managed in a single institution in South Africa over a 4-year period to determine the appropriateness of our current policy of selective conservatism.
Results
A total of 1,239 patients were included in the study; 112 required an emergency thoracotomy, 125 were admitted for observation and 1,002 required a tube thoracostomy (TT). Ninety-one per cent of the patients were male and the median age was 24 years. Seventy-five per cent of the cases were penetrating trauma and the remaining were blunt trauma. The indications for TT were pneumothorax (PTX) (n=382, 38%), haemothorax (HTX) (n=300, 30%) and haemopneumothorax (HPTX) (n=320, 32%).
A total of 13% (127/1,002) of all chest x-rays (CXR) following tube removal demonstrated residual pathologies that precluded immediate discharge: 32 (8%) in Group A (PTX), 44 (15%) in Group B (HTX) and 51 (16%) in Group C (HPTX). All 32 patients in Group A were simply observed and did not require further intervention. In Group B, 17 patients required repeat TTs and 27 required video assisted thoracoscopic surgery (VATS) for clearance of residual HTX. Twenty-nine patients in Group C required repeat TTs and twenty-two required VATS.
Conclusions
The vast majority of patients with thoracic trauma can be managed conservatively with TT alone. Residual pathology appeared to be lowest in patients with a PTX, which seldom requires treatment, while only a minority of patients required repeat TTs or VATS for a retained HTX. Selective conservatism is still appropriate in the current era in a developing world setting.
Keywords: Selective conservatism, Thoracic trauma, Tube thoracostomy
South Africa has a long tradition of the use of selective conservatism in the management of trauma.1 This has been in direct response to chronic resource constraints and a huge burden of trauma. The pioneers of this approach worked in an era prior to the rapid advances in imaging technology and minimally invasive surgery.2–5 Over the last two decades, a number of new technologies have impacted on trauma care and have become widely available.6–8 These include 64-slice computed tomography (CT), interventional radiological techniques, focused sonography for trauma, video assisted thoracoscopic surgery (VATS) and laparoscopy.6–8
Although each of these new technologies offers new diagnostic and therapeutic opportunities and potential benefits, there is a risk that they will be adopted in an ad hoc pattern in response to local conditions and availability.1 This phenomenon of ‘organic evolution’ may lead to management algorithms changing and morphing without there being a formal directive. For this reason, ongoing audit is essential to ensure new technology adopted is applied appropriately.1,10,11 In light of this, our management of thoracic trauma was reviewed to examine whether selective conservatism is still appropriate and to identify subgroups of patients in whom new technologies may provide benefit. This study provides an overview of thoracic trauma managed at a high volume trauma service in South Africa.
Methods
This was a retrospective study undertaken at the Pietermaritzburg Metropolitan Trauma Service (PMTS). A retrospective review of our prospectively maintained regional trauma registry was conducted over a four-year period from January 2010 to December 2013. Ethics approval for this study and to maintain this registry was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (reference BE 207/09). The PMTS is a high volume trauma service that provides definitive trauma care to the western part of KwaZulu-Natal province and covers a catchment population of over three million people. The annual trauma volume is approximately 3,000 admissions with over 40% being due to penetrating trauma.12 This is a direct reflection of the high incidence of interpersonal violence and serious crime experienced throughout the region.
Indications for operative exploration
We use the following criteria in conjunction with a patient’s overall clinical picture as indications for emergency operative exploration: ongoing haemodynamic instability;2 massive haemothorax (HTX) with initial chest tube output of >1,500ml blood, not responding to resuscitation;3 ongoing tube thoracostomy (TT) drainage of blood of >200ml per hour, with no signs of clinical improvement (or worsening clinical status);4 clinical evidence of cardiac tamponade in any shocked patients;5 and imminent cardiac arrest from any penetrating mechanism. We have facilities for autotransfusion and we make use of this on a regular basis in patients who undergo operative exploration.
Indications for tube thoracostomy
Patients who do not meet our criteria for immediate operative exploration are managed by a detailed clinical assessment followed by chest radiography (CXR). Our criteria for TT are a pneumothorax (PTX) with a visible rim of >2cm measured from the apex to the cupola and/or a fluid level above the seventh rib on CXR. Figure 1 shows an example of a typical young male patient with a significant HTX following a stab to the right chest that required a TT.
Figure 1.
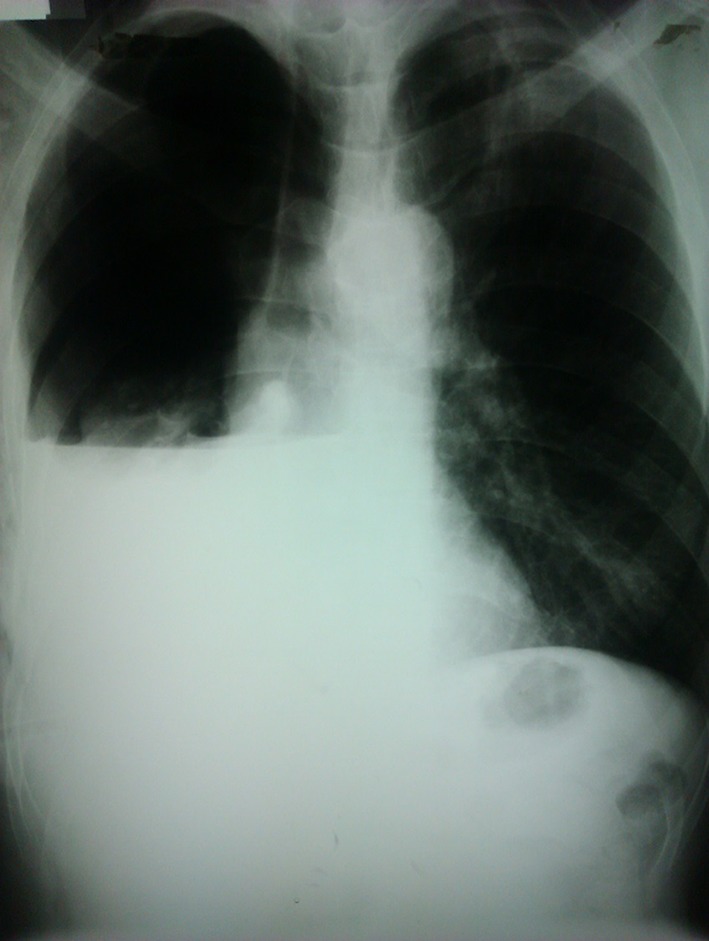
A significant haemothorax following a stab to the right chest that required a tube thoracostomy
Indications for observation only
Asymptomatic patients with a small PTX of <2cm in size (measured from the apex of the lung to the highest point of the cupola) are admitted for observation. All such patients are actively observed by the senior surgical registrars for evidence of clinical deterioration such as respiratory distress, tachypnoea and desaturation. A follow-up CXR is routinely performed 12 hours following admission in these patients to determine the need for further intervention. Figure 2 shows an example of a small PTX in a typical patient following a stab injury to the right thorax.
Figure 2.
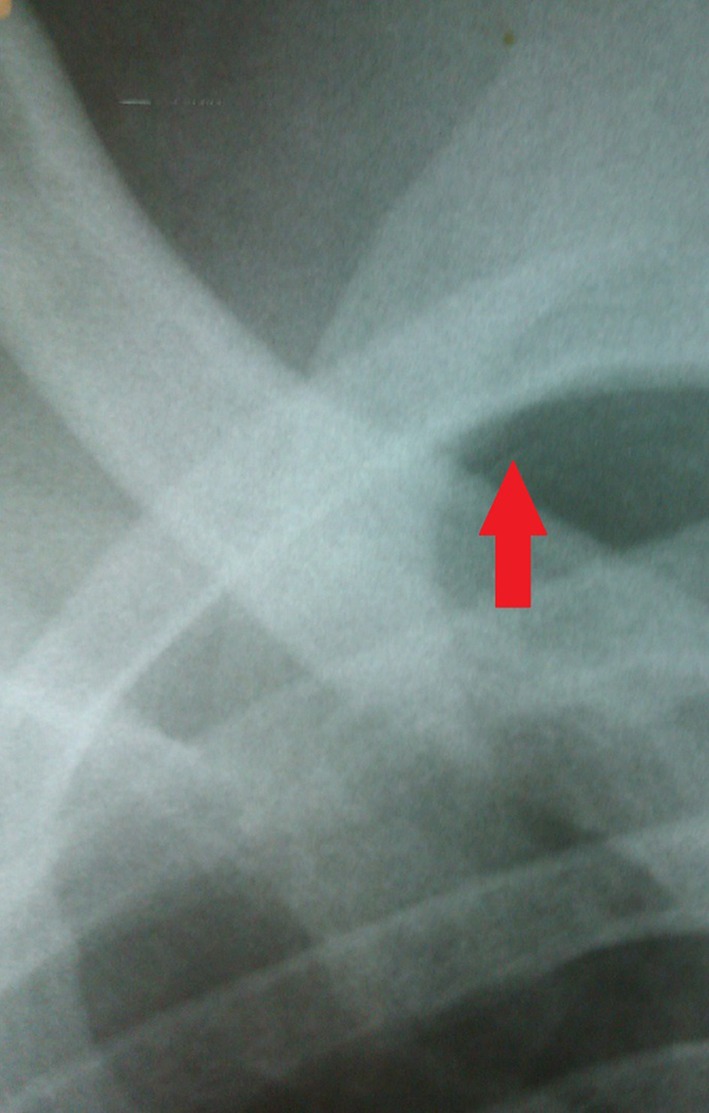
A small pneumothorax (arrow) following a stab injury to the right thorax
Technique of tube thoracostomy
All TTs are performed in the trauma unit under local anaesthesia using the Advanced Trauma Life Support® blunt dissection technique with the tube inserted into the fifth intercostal space,13 connected to a simple underwater seal bottle filled with 500ml of saline. Following tube insertion, a routine CXR is obtained to ascertain the position of the tube.13,14 Patients are then admitted directly to our designated trauma ward for active clinical observation unless they require transfer to the operating theatre or the intensive care unit.
Management of tube thoracostomy
Patients are encouraged repeatedly to mobilise vigorously and daily visits by physiotherapists are routine. The subsequent assessment of a patient is based primarily on clinical findings. A tube is removed only if the following clinical criteria are met: patient is clinically stable, not in distress, with normal respiratory parameters;2 tube fluid is not swinging;3 and tube fluid drainage is <50ml per day. Tubes are removed with the patient performing a valsalva manoeuvre by blowing into a glove and an occlusive dressing is placed immediately over the defect to achieve maximal seal. All patients are sent for a routine CXR within six hours of tube removal. All CXRs are reviewed by the senior trauma registrar or the consultant trauma surgeon, who dictates further management based on the CXR findings.
Management of residual pathology
Residual pathology is defined as incomplete evacuation of a PTX or HTX that is still visible on CXR following tube removal. Patients with residual collections are subjected to a lateral decubitus CXR. If there is a ‘run off’, then a second TT is inserted. If there is no ‘run off’, ultrasonography is performed to confirm the presence of a pleural collection rather than pulmonary consolidation. Patients with a significant retained collection undergo VATS. In patients with complicated collections or equivocal findings on ultrasonography, CT may be obtained prior to intervention.
Patient selection
Data collected included basic demographics, mechanism of injury and side of injury. Further details on the initial thoracic pathologies identified were documented. These were divided into PTX, HTX or haemopneumothorax (HPTX). The initial management and the proportion of patients managed with TT alone were reviewed. All data were extracted from the registry on to an Excel® spreadsheet (Microsoft, Redmond, WA, US) for processing. Patients were separated into the following broad cohorts: those who required urgent operative exploration, those with small PTXs for observation only, those managed by TT alone (Group A: PTX, Group B: HTX and Group C: HPTX) and those with residual pathology following tube removal
Results
During the 4-year study period, 1,239 patients with thoracic trauma were managed by the PMTS. Ninety-one per cent were male and the median age for all patients was 24 years. A total of 112 patients required emergency thoracotomy and were not considered further in this study. One hundred and twenty-five patients had a small PTX and were admitted for observation. The remaining 1,002 patients were managed by TT alone. Figure 3 summarises the patient groups managed according to our protocol.
Figure 3.
Patient groups managed according to our protocol
Of the 1,002 patients who were managed with TT alone, 755 (75%) sustained penetrating trauma while the remaining 247 (25%) sustained blunt trauma. Of those with penetrating trauma, 569 (75%) had stab wounds (SWs) and 186 (25%) had gunshot wounds (GSWs). Of those with blunt trauma, 135 (55%) were from road traffic accidents (RTAs), 97 (39%) from assaults and 15 (6%) from falls.
Small pneumothorax group
Of the 125 patients with a small PTX admitted for observation, all sustained their injuries as a result of SWs. The vast majority (n=125,97%) were managed successfully without TT and only four patients eventually required TT.
Pathologies requiring tube thoracostomy
Of the 1,002 patients who were managed with TT alone, 382 (38%) had a PTX, 300 (30%) had a HTX and 320 (32%) had a HPTX. A total of 899 patients (90%) had a CXR performed before tube insertion, prior to TT confirming the pathology. The remaining 103 patients (10%) had TT performed on clinical grounds only. All 1,002 patients had a CXR following tube insertion as well as following removal.
Overall yield of CXR following tube removal
Overall, 127 CXRs (13%) performed following tube removal demonstrated residual pathologies that precluded immediate discharge of the patient. Of the 127 CXRs with residual pathologies, 32 (25%) were from Group A, 44 (35%) from Group B and 51 (40%) from Group C.
Subgroup analysis: Group A
Of the 382 PTX patients, 92% were male and the median age was 24 years. In this group, 347 patients (91%) had penetrating injuries, all of which were SWs. The remaining 35 (9%) had blunt injuries: 22 RTAs, 11 assaults and 2 falls.
Thirty-two (8%) of the CXRs performed following tube removal in this group demonstrated residual pathologies that ultimately influenced patient management. All 32 patients had CXR findings of a residual PTX of <2cm and their observation period was extended for a further 24 hours. None of these patients required repeat TTs and they were all eventually discharged. The remaining 350 patients (92%) were discharged from the trauma service.
Subgroup analysis: Group B
Of the 300 HTX patients, 86% were male and the median age was 25 years. In this group, 109 cases (36%) were due to penetrating injuries, comprising 79 SW cases and 30 GSW cases. The remaining 191 patients (64%) sustained blunt trauma: 102 RTAs, 76 assaults and 13 falls.
Forty-four (15%) of the CXRs performed following tube removal in this group ultimately influenced patient management. Seventeen (6%) of these patients had persistent HTXs that were above the seventh rib and required repeated TTs for evacuation of the residual HTX. All 17 were managed successfully with TT alone. The remaining 27 (7%) underwent VATS. The 256 patients (85%) whose management was not influenced were discharged.
Subgroup analysis: Group C
Of the 320 HPTX patients, 94% were male and the median age was 25 years. In this group, 299 patients (93%) had penetrating injuries, comprising 143 SWs and 156 GSWs. The remaining 21 cases (7%) were blunt injuries: 11 RTAs and 10 assaults.
Fifty-one (16%) of the CXRs performed following tube removal in this group ultimately influenced patient management. Twenty-nine (9%) of these patients had persistent HTXs that required repeat TTs for complete evacuation. The remaining 22 (7%) underwent VATS. The 269 patients (84%) whose management was not influenced were discharged.
Discussion
Our current protocols for the management of thoracic trauma continue to be based on a policy of selective conservatism that is heavily dependent on clinical assessment.1,10,11 Although a number of new modalities have emerged, they have complemented our philosophy and do not completely replace it. We have shown that most small pleural collections can be safely observed and this finding supports previous work from our institution from over three decades ago.3,5 Furthermore, there is good evidence to suggest that the vast majority of small PTXs can be managed safely without the need for TT.3,5,15
Patients who require TT generally do well and only 13% will have a residual pathology detected on routine CXR following tube removal. The yield was lowest in those in whom TT was performed for PTX. CXR following tube removal appears to merely confirm the resolution of PTX. The yield was slightly higher for those with HTX but was highest in patients with HPTX. Over half of the HPTX cases were caused by GSWs while none of the PTX cases were secondary to a GSW. This probably reflects the higher energy transfer of GSWs, which results in greater destruction of lung tissues than for stab injuries.16
VATS is well established in our environment and has found its place in selective conservative management algorithms.7,8,17,18,19 We found that less than 5% of patients who require TT for HTX or HPTX will ultimately undergo either subsequent TT or VATS. From our current study, it would appear that the use of a selective conservative approach to thoracic trauma remains appropriate in our developing world environment.
Conclusions
Selective conservatism remains a valid strategy for the management of thoracic trauma in a high volume developing world setting. Modern modalities complement the pre-existing algorithms but do not completely replace them.
References
- 1.Clarke DL, Thomson SR, Madiba TE, Muckart DJ. Selective conservatism in trauma management: a South African contribution. World J Surg 2005; 29: 962–965. [DOI] [PubMed] [Google Scholar]
- 2.Hegarty MM. A conservative approach to penetrating injuries of the chest. Experience with 131 successive cases. Injury 1976; 8: 53–59. [DOI] [PubMed] [Google Scholar]
- 3.Muckart DJ, Luvuno FM, Baker LW. Penetrating injuries of the pleural cavity. Thorax 1984; 39: 789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetriades D, Rabinowitz B, Markides N. Indications for thoracotomy in stab injuries of the chest: a prospective study of 543 patients. Br J Surg 1986; 73: 888–890. [DOI] [PubMed] [Google Scholar]
- 5.Weigelt JA, Aurbakken CM, Meier DE, Thal ER. Management of asymptomatic patients following stab wounds to the chest. J Trauma 1982; 22: 291–294. [DOI] [PubMed] [Google Scholar]
- 6.Tayal VS, Beatty MA, Marx JA et al. FAST (focused assessment with sonography in trauma) accurate for cardiac and intraperitoneal injury in penetrating anterior chest trauma. J Ultrasound Med 2004; 23: 467–472. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo EH, Schmacht DC, Gable DR et al. Thoracoscopy in the management of posttraumatic persistent pneumothorax. J Am Coll Surg 1998; 186: 636–639. [DOI] [PubMed] [Google Scholar]
- 8.Oosthuizen GV, Clarke DL, Laing GL et al. Introducing video-assisted thoracoscopy for trauma into a South African township hospital. World J Surg 2013; 37: 1,652–1,655. [DOI] [PubMed] [Google Scholar]
- 9.O’Malley E, Boyle E, O’Callaghan A et al. Role of laparoscopy in penetrating abdominal trauma: a systematic review. World J Surg 2013; 37: 113–122. [DOI] [PubMed] [Google Scholar]
- 10.Clarke DL, Quazi MA, Reddy K, Thomson SR. Emergency operation for penetrating thoracic trauma in a metropolitan surgical service in South Africa. J Thorac Cardiovasc Surg 2011; 142: 563–568. [DOI] [PubMed] [Google Scholar]
- 11.Clarke DL, Gall TM, Thomson SR. Double jeopardy revisited: clinical decision making in unstable patients with, thoraco-abdominal stab wounds and, potential injuries in multiple body cavities. Injury 2011; 42: 478–481. [DOI] [PubMed] [Google Scholar]
- 12.Laing GL, Skinner DL, Bruce JL et al. Understanding the burden and outcome of trauma care drives a new trauma systems model. World J Surg 2014; 38: 1,699–1,706. [DOI] [PubMed] [Google Scholar]
- 13.American College of Surgeons. ATLS® Student Course Manual. 9th edn. Chicago: ACS; 2012. [Google Scholar]
- 14.Laws D, Neville E, Duffy J. BTS guidelines for the insertion of a chest drain. Thorax 2003; 58 Suppl 2: ii53–ii59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knottenbelt JD, van der Spuy JW. Traumatic pneumothorax: a scheme for rapid patient turnover. Injury 1990; 21: 77–80. [DOI] [PubMed] [Google Scholar]
- 16.Madiba TE, Thomson SR, Mdlalose N. Penetrating chest injuries in the firearm era. Injury 2001; 32: 13–16. [DOI] [PubMed] [Google Scholar]
- 17.Davis JW, Mackersie RC, Hoyt DB, Garcia J. Randomized study of algorithms for discontinuing tube thoracostomy drainage. J Am Coll Surg 1994; 179: 553–557. [PubMed] [Google Scholar]
- 18.Maritz D, Wallis L, Hardcastle T. Complications of tube thoracostomy for chest trauma. S Afr Med J 2009; 99: 114–117. [PubMed] [Google Scholar]
- 19.Alrahbi R, Easton R, Bendinelli C et al. Intercostal catheter insertion: are we really doing well? ANZ J Surg 2012; 82: 392–394. [DOI] [PubMed] [Google Scholar]



