Abstract
Background
Interferon-α (INF-α) therapy is frequently associated with disabling depression, fatigue, and related neuropsychiatric effects. While depression in major depressive disorder is associated with low serotonin transporter binding, animal models suggest that interferon-associated mood effects are linked to increased presynaptic serotonin transporter binding. This study tested the hypotheses that interferon administration to human subjects increases presynaptic serotonin binding activity, and that this effect correlates with incident depression symptoms.
Methods
Positron Emission Tomography (PET) scans using [11C]-DASB were obtained for 9 Hepatitis C patients before and after INF-a treatment for 8 weeks. Serotonin transporter binding was estimated using the Likelihood Estimation in Graphical Analysis model (LEGA) and measured as the volume of distribution (VT) divided by the free fraction of ligand (fp). Depression was measured with the Structured Clinical Interview for DSM-IV Diagnosis (SCID) and the Hamilton Rating Scale for Depression (HAM-D).
Results
Compared to pre-interferon treatment values, changes in serotonin transporter binding and depression symptoms were not significant. There was no correlation between changes in serotonin transporter binding and depression symptoms.
Limitations
The study is limited by small sample size, minimal effect on observed mood symptoms within the sample, and brief duration of follow-up.
Conclusion
These findings do not support the hypothesis of an interferon-induced change in serotonin transporter function as the cause of incident depressive symptoms in patients treated with INF-α. Additional study of these possible relationships should be of longer duration and include more subjects with more pronounced changes in mood.
Keywords: Interferon, Serotonin, Imaging, Depression, Transporter, Binding
Introduction
Because of its antiviral and immunomodulatory properties, the inflammatory cytokine interferon-α (INF-α) has become a standard treatment for hepatitis C, melanoma, and renal carcinoma. With its widespread use has come the recognition of its significant adverse effect profile, including fatigue, depression, and related neuropsychiatric symptoms, which are common, emerge over several weeks of treatment, and tend to be persistent (Raison et al., 2005a; Raison et al., 2005b). Thus, Kraus et al reported that 14 of 121 patients receiving INF-α developed depression, as measured by the Hospital Anxiety and Depression Scale (HADS) (Kraus et al., 2002). Similarly, using the Montgomery Asberg Depression Rating Scale (MADRS), Bonaccorso et al followed 30 patients receiving 3 MIU INF-α three times/week, and found that 40.7% of patients suffered from depression at the study endpoint, after three months of treatment (Bonaccorso et al., 2002). Castera et al reported that among 33 patients receiving 3 MIU INF-α three times/week, 8 patients developed depressive symptoms; of these, 4 (12%) developed major depressive episodes (Castera et al., 2002). These side effects have clinical significance, as they are the primary reason for discontinuation of INF treatment (Dusheiko, 1997).
Similar effects have been reproduced repeatedly in animal models, in which experimental administration of pro-inflammatory cytokines produces a syndrome called “sickness behavior” that closely resembles major depressive disorder (MDD) in humans (Anisman and Merali, 1999; Dantzer, 2001; PlataSalaman et al., 1996; Yirmiya, 1996; Yirmiya, 2000). According to the DSM-IV, these episodes would be diagnosed as a mood disorder secondary to a substance (interferon), rather than major depressive disorder.
In major depressive disorder (MDD), major depressive episodes (MDE) are associated with lower pre-synaptic serotonin transporter binding activity in some studies, as measured by Positron Emission Tomography (PET) scanning, an effect that is not a consequence of antidepressant treatment (Parsey et al., 2006). The effect of lower transporter binding is strongest in midbrain (MID), anterior cingulate (ACN), ventral striatum (VST), and hippocampus (HIP) (Ogden et al., 2007). Several studies have suggested that IFN-induced changes in tryptophan metabolism as well as functional polymorphisms in the serotonin transporter gene may be a basis of IFN-α induced depression in humans (Capuron et al., 2003; Bull et al., 2009). However, a rodent study postulated that the depression induced by INF-α could possibly result from a novel mechanism, with reduced intra-synaptic levels of serotonin resulting from induction of increased serotonin transporter binding. Specifically, increased levels of serotonin transporter mRNA were detected in the midbrain of mice treated with IFN-α, suggesting that increased transcription of serotonin transporter results in enhanced uptake of serotonin at serotonergic synapses, causing rapid termination of transmission (Morikawa et al., 1998).
To investigate this possible mechanism of induction of depression in humans, we examined the effects of interferon-α treatment on serotonin transporter binding in Hepatitis C patients. Specifically, we measured serotonin transporter binding using PET and [11C]-DASB, a selective serotonin transporter ligand (Houle et al., 2000; Wilson et al., 2000), in four brain regions of interest, the midbrain (MID), anterior cingulate (ACN), ventral striatum (VST), and hippocampus (HIP), before and after 8 weeks of INF-α treatment. We hypothesized that serotonin transporter binding would be increased by receiving INF-α treatment, and that inter-individual variation in changes in depression symptoms would be positively correlated with increase in serotonin transporter binding.
Materials and Methods
The study was conducted with the approval of the New York State Psychiatric Institute and Columbia University Medical Center Institutional Review Boards.
Subjects
Subjects with hepatitis C were recruited from the patient census of the Center for Liver Disease and Transplantation of the New York Presbyterian Hospital Columbia University Medical Center. Patients with melanoma were also eligible, but none were enrolled. Inclusion criteria were: (1) age 18–65 years, (2) capacity to provide written informed consent, (3) diagnosis of melanoma or Hepatitis C, with plans to begin interferon-a therapy. Exclusion criteria were: (1) less than one year of stable remission from alcohol or drug dependency, (2) liver cirrhosis classified as Child’s Pugh B/C, (3) a significant medical condition expected to limit survival to less than six months, (4) a significant physical illness with potential to affect the brain/serotonergic system; (5) co-infection with Hepatitis B or HIV, (6) diagnosis of current major depressive disorder, dysthymia, current or past bipolar disorder, current anxiety disorder, impulse control disorder, or mental retardation, (7) suicidal intent, (8) treatment with antidepressants, or treatment within the past 21 days with other psychotropic or other medications with possible effects on the serotonergic system, (9) use of herbal medication with psychotropic effects, (10) chronic opioid medication use, (11) pregnancy or risk of pregnancy, or (12) Mini-Mental State Examination (MMSE) score less than 24.
Inclusion criteria were verified by the following methods: Structured Clinical Interview for DSM IV (SCID), history, urine toxicology, blood test, MMSE, and physical examination. Depression symptoms were assessed with the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960).
Study Design
Subjects meeting eligibility criteria and giving informed consent for participation were assessed using the SCID and HAM-D. Baseline PET imaging was obtained within two weeks of this assessment and before the initiation of interferon-α treatment, which was administered according to the standard protocols of the Center for Liver Disease as pegylated interferon, 120–180 mcg per week. During treatment, subjects met with a research psychiatrist (PAS) biweekly to monitor depression symptoms, and the HAM-D was repeated. Since none of the subjects developed a major depressive episode during treatment, they were followed until the end of the 8th week of INF therapy, after which repeat PET imaging was obtained.
Magnetic Resonance Imaging
Magnetic resonance images (MRIs) were acquired on a 3-T Signa Advantage system (GE Healthcare, Waukesha, WI), as previously described (Ogden et al., 2007). The final voxel size was 1.02 × 1.02 × 1.00 mm, with an acquisition time of 11 minutes.
PET Imaging
The anterior cingulate, hippocampus, midbrain and ventral striatum were designated as regions of interest. Changes in serotonin transporter binding were most obvious in these areas in our previous study of MDD (Parsey et al., 2006). It was assumed that any alterations in binding would be most noticeable in these regions in the Hepatitis C patients.
Radiosynthesis of [11C]-DASB was done using our previously published method (Ogden et al., 2007). 20 mCi or less of [11C]-DASB was administered intravenously. The average injected dose was 17.1 ± 0.9 mCi and 16.3 ± 3.0 mCi for baseline and follow-up scans respectively. The average injected mass was 4.89 ± 2.04 μg and 5.49 ± 2.42 μg for baseline and follow-up scans respectively. PET images were acquired using an ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN) (63 slices covering an axial field of view of 15.5 cm, axial sampling of 2.4 mm, in 3D mode).
An arterial line was placed to collect blood samples for calculation of an arterial input function. A venous catheter was utilized for [11C]-DASB administration. The subject was placed in a polyurethane head holder to limit head motion. PET imaging was performed using a Siemens ECAT EXACT HR+. A 10-minute transmission scan was performed and followed by intravenous administration of [11C]-DASB. Emission data was obtained for 110 minutes as 19 frames of increasing frame durations (3 at 20s, 3 at 1 min, 3 at 2 mins, 2 at 5 min, and 8 at 10 min).
Image Analysis
Statistical Parametric Mapping 5 (SPM5, Wellcome Department of Cognitive Neurology) was used to segment the brain into gray and white matter regions. Freesurfer (http://surfer.nmr.mgh.harvard.edu/) was used for skull stripping. Four regions of interest (ROIs): anterior cingulate, hippocampus, midbrain and ventral striatum were drawn automatically using a set of 18 template brains (normal subject MRIs). These template brains were previously created by an expert manually drawing ROIs based on atlases and published reports (Duvernoy, 1991; Kates et al., 1997; Killiany et al., 1997; Talairach and Tournoux, 1988) as described (Milak et al., 2010). Each of the 18 template brains was then nonlinearly warped to the target brain using the Automated Registration Toolbox (Ardekani et al., 2005; Klein et al., 2009). The probability that a voxel of the target brain is described by a particular ROI was computed by taking percent of the warped template brains with that voxel labeled as that particular ROI. Cortical regions were then further processed by gray-matter masking.
In order to apply the anatomically defined ROIs to the PET image, PET and MRI images were co-registered. FLIRT (FMRIB Linear Image Registration Tool version 5.0; FMRIB Image Analysis Group) was used to calculate eight potential transformations between the PET image and the MRI image for each subject (using the various MRI and PET derived sources and targets (DeLorenzo et al., 2009). Resulting coregistrations were automatically ranked based on mutual information. The optimal coregistration was then manually inspected to verify the optimal coregistration was chosen (DeLorenzo et al., 2009).
Time-activity curves were calculated by taking the average PET activity within an ROI in each time frame. These averages were based on the probabilistic ROIs (PET activity was multiplied by the probability of belonging to the ROI.) Binding measures were calculated from the time activity curves as described below.
Modeling
Arterial blood samples were obtained at 31 time points. For the first two minutes, samples were collected every 10 seconds. From two to four minutes, samples were collected every 20 seconds. Then, samples were taken at 4, 6, 8, 12, 16, 20, 30, 40, 50, 60, 80, 90, and 100 minutes. Radioactivity of 200-μL aliquots of centrifuged plasma samples was measured in a 1480 Wizard 3M automatic γ counter (Wallac, Turku, Finland). Six arterial samples, taken at 2, 12, 20, 50, 80, and 100 minutes were further analyzed using a high-pressure liquid chromatography assay to calculate the percentage of parent compound. These values were fit with a bi-exponential function describing the percentage of parent compound over time (Ogden et al., 2007). The metabolite-corrected arterial input function was then obtained by multiplying the calculated plasma counts by the percentage of parent compound, and the resulting points were fit by a straight line and sum of three exponentials, before and after the peak respectively (Ogden et al., 2007).
Binding estimates were obtained using the Likelihood Estimation in Graphical Analysis model (LEGA) (Ogden et al., 2007). Our primary outcome measure was total volume of distribution divided by free fraction in plasma (VT/fP). Using this measure, estimated binding in the brain is not influenced by the free fraction of ligand available for binding. The binding potential relative to the free tracer in the plasma BPF was not used as the primary outcome measure, because it requires a reference region in the brain devoid of specific binding, and the cerebellum, which is most commonly used for that purpose, does have some specific binding of [11C]-DASB (Parsey et al., 2006). To facilitate comparison of results to other published studies we also report serotonin transporter binding as BPF (Bmax/KD) and BPND (BPF x fND, where Bmax is the total number of available transporters, KD= is the affinity of the tracer for the transporters and fND is the concentration of free radiotracer in the brain), and modeling by the one-tissue compartment model (Innis et al., 2007).
Voxel Analysis
For parametric analysis, VT/fP was calculated at every voxel using Bayesian estimation in graphical analysis (Zanderigo et al., 2010), a fully automatic approach that incorporates LEGA VT estimation in a Bayesian framework. Voxel images were placed in standard space as follows: Each subject’s MRI was nonlinearly warped to a high resolution MRI template (Holmes et al., 1998) using the Automatic Registration Toolbox (ART, Ardekani et al. 2005). Voxel maps (VT/fP) were then warped to this space using the PET-to-MRI coregistration and the ART-derived nonlinear transformation.
A voxel-wise comparison of VT/fP using Statistical Parametric Mapping (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK) was performed. Voxel maps were smoothed using an 8 mm Gaussian kernel. A paired t-test was used to assess differences before and after interferon. No global normalization or grand mean scaling was used. Analysis was confined to grey matter. Significant differences between the groups were set at α = 0.05 after Familywise Error (FWE) correction.
Results
Subjects
Of 13 screened subjects, 4 were excluded due to unstable mood disorder past history, recent abstinence from alcohol or drug abuse, chronic opioid use (i.e., methadone maintenance), and antidepressant use. Nine subjects with hepatitis C enrolled and completed PET imaging before and after interferon treatment.
Depression
Before beginning interferon treatment, none of the subjects met DSM-IV diagnostic criteria for major depressive disorder. HAM-D mood symptoms were minimal before interferon (mean 3.1, SD = 3.1), and remained low after interferon therapy (mean 5.4, SD = 3.6). (Table 1) The overall effect of interferon-alpha therapy on HAM-D was not significant (paired t test, t = −1.98, p = .08), but for seven of nine subjects there was an increase in HAM-D score of 1–5 points, while one subject had no change in HAM-D and one subject (subject 6) had a 5 point fall in HAM-D score after interferon therapy (sign test, p =.08), indicating a trend towards increased HAM-D after interferon treatment. The impact of interferon on mood was modest - only one subject (subject 8) developed a HAM-D score above 10 during interferon therapy. No subject met criteria for diagnosis of a major depressive episode during the follow-up period.
Table 1.
HAM-D Scores Pre-Interferon and Post-Interferon Treatment
| Subject # | HAM-D before interferon alpha therapy | HAM-D after 8 weeks of interferon alpha therapy |
|---|---|---|
| 1 | 6 | 7 |
| 2 | 8 | 13 |
| 3 | 0 | 4 |
| 4 | 2 | 2 |
| 5 | 1 | 7 |
| 6 | 6 | 1 |
| 7 | 0 | 3 |
| 8 | 0 | 6 |
| 9 | 5 | 6 |
Serotonin transporter binding activity
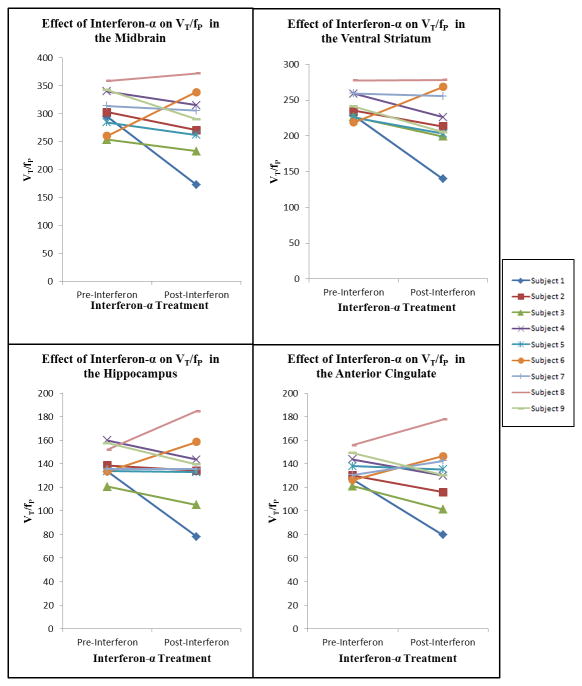
Serotonin transporter binding activity before and after interferon therapy is reported in Tables Two and Three, and illustrated in Figures 1 and 2. The effect of interferon on VT/fP was not significant in any of the four regions of interest. For 6 subjects, VT/fP values declined in all four regions of interest after interferon therapy. For one subject (subject 6), VT/fP increased in all regions of interest after interferon therapy, and for one subject (subject 8) VT/fP increased in anterior cingulate, HIP, and MID, and was unchanged in VST. In the VST, seven subjects had reduced VT/fP, one subject had no change, and one subject had increased VT/fP after interferon treatment (sign test, p < .04). In the anterior cingulate, HIP, and MID, most subjects had reduced VT/fP, but the effect of interferon on VT/fP in these regions was not statistically significant.
Figure 1.
PET [11C]-DASB VT/fP values before and after Interferon-α treatment: Midbrain, Ventral Striatum, Hippocampus, and Anterior Cingulate.
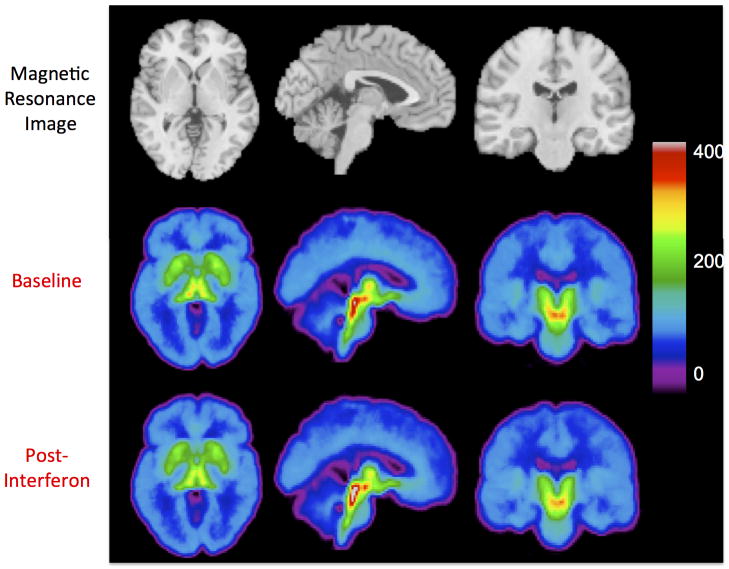
Figure 2.
Axial, sagittal, and coronal views of [11C]DASB binding before and after interferon therapy. For each subject (n=9), VT/fp (volume of distribution divided by the free fraction) was calculated at every voxel. The top row shows a magnetic resonance image template, for anatomical reference. The middle and bottom rows show the corresponding views of the mean [11C]DASB VT/fp images. The VT/fp value associated with each color is indicated by the color bar.
A voxel-based analysis on the parametric images was used to explore brain regions beyond the four ROIs. Parametric VT/fP maps were calculated for each subject (Figure 2). A significant binding change (positive or negative) in response to treatment was not observed using the voxel-wise analysis.
There were also no significant effects of interferon therapy in any region in analyses using BPF and BPND as outcome measures, and one-tissue compartment modeling (29).
Correlation of changes in depression symptoms with changes in serotonin transporter binding activity
Overall, there were no significant correlations between degree of change in depression severity during interferon therapy and changes in serotonin transporter binding in any of the regions of interest. Inspection of Figure 1 and Table 1 indicates that the only subject (subject 6) whose depression symptoms were lower after interferon therapy than at baseline was also the only subject in whom VT/fP rose in all four regions of interest during interferon therapy. In contrast, however, subject 8, in whom VT/fP rose in three of four regions of interest during interferon therapy, had increased depression symptoms after interferon therapy.
Discussion
To our knowledge, this is the first study of the effect of interferon-α on brain serotonin transporter binding using PET imaging in human subjects. We had hypothesized that, in humans, interferon-α induces an increase in presynaptic serotonin transporter binding, and that this effect contributes to an increase in depression symptoms. Our hypotheses were not supported. We found that, after eight weeks of INF-α treatment, there was no significant change from baseline [11C]-DASB VT/fP, in any of four regions of interest examined in nine subjects, and there was no evidence of a correlation between change in VT/fP and change in depression symptoms. In the majority of subjects, depression symptoms worsened, and VT/fP decreased, but both effects were small and not statistically significant. It is of potential interest that the direction of change in VT/fP was congruent with lower serotonin transporter binding seen in major depressive disorder in human subjects (Parsey et al., 2006; Oquendo et al., 2007) and not the increased serotonin binding activity seen in rat models of interferon-induced sickness behavior (Morikawa et al., 1998). However, since none of our subjects developed MDD, the results do not permit the conclusion that depression arising in interferon-treated human subjects is associated with lower presynaptic serotonergic binding as reported by some in major depressive disorder. Of note the alternative outcome measures of transporter binding also did not indicate and significant change.
Strengths of this study include standardized, prospective observation of subjects as they began and throughout the course of interferon treatment, the use of observer-scored, structured ratings for depression symptoms, and the use of a PET ligand with specifically high affinity for the serotonin transporter protein.
Our findings are limited by the fact that, although we observed a trend toward a rise in depression symptoms following interferon therapy (i.e., seven of nine subjects had higher HAM-D after interferon than before), the overall effect of interferon on mean HAM-D was not significant, and none of our subjects developed a major depressive episode. It is possible that a larger or more diverse sample, or greater duration of follow-up, would have permitted us to identify subjects with a larger range of changes in HAM-D, including development of major depressive episodes, might have given us greater statistical power to identify an interferon effect. In order to avoid confounding factors affecting the PET imaging of serotonin binding activity, we avoided including patients who had hepatic cirrhosis, were on methadone maintenance, were taking antidepressants, or who had not achieved stable long-term abstinence from alcohol or drug dependence. These restrictions significantly reduced the number of possible subjects referred for screening, and reduced by one third the number of eligible subjects from among those screened. It is possible that these “medical” exclusions, by selecting relatively healthy subjects for the study, inadvertently ruled out from inclusion in the study exactly those hepatitis patients most prone to interferon-related mood effects.
As for the duration of the study, it is possible that the 8-week duration of interferon treatment was too short to observe an effect on serotonergic function that would be evident at a later time. Other studies have measured depression over a longer time frame (Bonaccorso et al., 2002; Kraus et al., 2003; Hunt et al., 1997). Hunt et al saw changes in depression at the six-month mark after initiation of interferon treatment (Hunt et al., 1997), while Kraus et al saw Hospital and Anxiety Depression Scale scores peak significantly at the 3–4 month mark (Kraus et al., 2003). It is possible that a serotonin transporter binding effect would be more evident after a longer period of INF-a treatment.
Summary
Our study did not find an increase in serotonin transporter binding activity in Hepatitis C patients treated with Interferon-a. However, to fully explore the effect on interferon on brain serotonergic function, and the relation of these possible serotonergic effects with interferon-associated depression, will require a study of a larger sample with a greater impact on mood.
Table 2.
VT/fP Before and After Interferon-α Treatment
| Subject | Pre/Post Interferon Treatment | ACN VT/fP | HIP VT/fP | MID VT/fP | VST VT/fP |
|---|---|---|---|---|---|
| 1 | Pre | 126.783 | 133.324 | 294.7308 | 228.3075 |
| Post | 79.5807 | 78.0952 | 172.74046 | 139.9158 | |
| 2 | Pre | 130.220 | 138.780 | 302.6649 | 235.4069 |
| Post | 115.904 | 134.1295 | 270.5235 | 213.236 | |
| 3 | Pre | 121.0099 | 120.714 | 253.415 | 226.651 |
| Post | 101.2485 | 105.223 | 232.876 | 199.198 | |
| 4 | Pre | 143.8536 | 159.9958 | 339.7898 | 258.9127 |
| Post | 129.8679 | 143.52949 | 315.113 | 226.2867 | |
| 5 | Pre | 137.9915 | 133.7639 | 284.406 | 225.949 |
| Post | 135.3246 | 132.9005 | 262.0083 | 203.2436 | |
| 6 | Pre | 126.4058 | 133.5308 | 259.9748 | 219.019 |
| Post | 146.5706 | 158.6685 | 338.5418 | 268.4038 | |
| 7 | Pre | 130.5066 | 135.407 | 313.8189 | 259.418 |
| Post | 142.5038 | 135.4208 | 305.358 | 255.7457 | |
| 8 | Pre | 155.9676 | 152.10556 | 358.5816 | 277.7547 |
| Post | 177.674 | 184.7648 | 371.983 | 278.3538 | |
| 9 | Pre | 149.326 | 157.524 | 342.3268 | 241.251 |
| Post | 130.2867 | 139.5967 | 290.264 | 205.350 |
ACN: anterior cingulate; HIP: hippocampus; MID: midbrain; VST: ventral striatum
Table 3.
Outcome Measures for the LEGA Model Comparing [11C]-DASB Binding Before and After Treatment
| LEGA Model | |||
|---|---|---|---|
| Variable/ROI | Pre INF | Post INF | P |
| BPND AVG | |||
| ACN | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.730 |
| HIP | 0.4 ± 0.1 | 0.5± 0.1 | 0.503 |
| MID | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.725 |
| VST | 1.5± 0.2 | 1.4 ± 0.2 | 0.137 |
| BPF AVG | |||
| ACN | 37.8 ± 5.7 | 36.5 ± 11.3 | 0.688 |
| HIP | 42.6 ± 9.2 | 42.4 ± 13.9 | 0.964 |
| MID | 207.5 ± 31.5 | 192.1 ± 42.5 | 0.281 |
| VST | 143.4 ± 15.8 | 128.8 ± 26.5 | 0.092 |
| VT/fP AVG | |||
| ACN | 135.8 ± 11.8 | 128.77 ± 28.0 | 0.372 |
| HIP | 140.6 ± 13.1 | 134.7 ± 30.1 | 0.513 |
| MID | 305.5 ± 36.7 | 284.4 ± 59.0 | 0.266 |
| VST | 241.4 ± 19.7 | 221.1 ± 42.4 | 0.133 |
ACN: anterior cingulate; HIP: hippocampus; MID: midbrain; VST: ventral striatum
Table 4.
Outcome Measures Calculated with a One-Tissue Compartment Model of [11C]-DASB Binding Before and After Treatment.
| One Tissue Compartment Model | |||
|---|---|---|---|
| Variable/ROI | Pre INF | Post INF | p |
| BPND AVG | |||
| ACN | 0.4 ± .1 | 0.4 ± .1 | 0.944 |
| HIP | 0.5 ± .1 | 0.5 ± .1 | 0.535 |
| MID | 2.3 ± .3 | 2.2 ± .3 | 0.718 |
| VST | 1.6 ± .2 | 1.5 ± .2 | 0.110 |
| BPF AVG | |||
| ACN | 37.6 ± 5.6 | 35.0 ± 10.2 | 0.361 |
| HIP | 42.8 ± 8.8 | 41.6 ± 13.4 | 0.768 |
| MID | 212.6 ±33.0 | 193.7± 43.0 | 0.182 |
| VST | 146.5 ± 17.8 | 128.3 ± 25.8 | 0.031 |
| VT/fP AVG | |||
| ACN | 131.1 ± 12.1 | 122.1 ± 25.9 | 0.204 |
| HIP | 136.3 ± 13.6 | 128.8 ± 28.5 | 0.352 |
| MID | 306.2 ± 38.8 | 280.8 ± 58.5 | 0.169 |
| VST | 240.0 ± 22.6 | 215.4 ± 40.8 | 0.057 |
ACN: anterior cingulate; HIP: hippocampus; MID: midbrain; VST: ventral striatum
Table 5.
Correlation Coefficients Between VT/fP Binding Changes and Hamilton Score Changes
| ROI | Correlation Coefficient |
|---|---|
| ACN | −0.032 |
| HIP | 0.041 |
| MID | −0.296 |
| VST | −0.299 |
ACN: anterior cingulate; HIP: hippocampus; MID: midbrain; VST: ventral striatum
Acknowledgments
Supported by NIMH grant, number R21MH067093
References
- 1.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005a;66(1):41–8. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005b;19(1):23–7. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 5.Castera L, Zigante F, Bastie A, Buffet C, Dhumeaux D, Hardy P. Incidence of interferon alfa-induced depression in patients with chronic hepatitis C. Hepatology. 2002;35:978–979. doi: 10.1053/jhep.2002.32104. [DOI] [PubMed] [Google Scholar]
- 6.Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:112S–121S. doi: 10.1002/hep.510260720. [DOI] [PubMed] [Google Scholar]
- 7.Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann NY Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 9.PlataSalaman CR, Sonti G, Borkoski JP, Wilson CD, FrenchMullen JMH. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- 10.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 11.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmächer T. Illness, cytokines, and depression. Ann NY Acad Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 12.Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–324. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 15.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 16.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14(12):1095–104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- 18.Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio)araalkylamines. J Med Chem. 2000;43:3103–3110. doi: 10.1021/jm000079i. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duvernoy H. The Human Brain: Surface, Three-dimensional Sectional Anatomy and MRI. New York: Springer-Verlag Wien; 1991. [Google Scholar]
- 21.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 22.Killiany RJ, Moss MB, Nicholson T, Jolesz F, Sandor T. An interactive procedure for extracting features of the brain from magnetic resonance images: the lobes. Hum Brain Mapp. 1997;5:355–363. doi: 10.1002/(SICI)1097-0193(1997)5:5<355::AID-HBM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain: Three-Dimensional Proportional System: An Approach of Cerebral Imaging. New York: Theime Medical Publisher; 1988. [Google Scholar]
- 24.Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med. 2010;51:1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, Parsey RV. A new method for assessing PET-MRI coregistration. Proceedings of SPIE - Medical Imaging. 2009;7258:72592W-7–72592W-8. [Google Scholar]
- 28.Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ. Acute occupancy of brain serotonin transporter by sertraline as measured by [C-11]DASB and positron emission tomography. Biol Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knutti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 30.Zanderigo F, Ogden RT, Bertoldo A, Cobelli C, Mann JJ, Parsey RV. Empirical Bayesian estimation in graphical analysis: a voxel-based approach for the determination of the volume of distribution in PET studies. Nucl Med Biol. 2010;37:443–451. doi: 10.1016/j.nucmedbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 32.Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 34.Hunt CM, Dominitz JA, Bute BP, Waters B, Blasi U, Williams DM. Effect of interferon-alpha treatment of chronic hepatitis C on health-related quality of life. Dig Dis Sci. 1997;42:2482–2486. doi: 10.1023/a:1018852309885. [DOI] [PubMed] [Google Scholar]