Abstract
Introduction
The need to manage an open abdomen is becoming more common in general surgical practice and a variety of methods of temporary abdominal closure (TAC) are available. The evidence for the efficacy of the various forms of TAC as well as the subsequent definitive fascial closure (DFC) rates and complications comes mainly from large trauma series in the US, which represent a different patient population to those in the UK in whom TAC is usually required.
Methods
All cases of open abdomen management in our hospital over a five-year period were reviewed to ascertain the methods of TAC used, our success in achieving DFC and the applicability of managing such cases in a district hospital environment.
Results
Nineteen patients underwent TAC, with two deaths (10.5%) and an overall DFC rate at hospital discharge of 12/17 (70.6%). The median lengths of critical care and hospital stays were 19.5 and 38.0 days respectively. Thirteen out of seventeen survivors had at least one significant complication.
Conclusions
The management of the open abdomen can be achieved safely in a district general hospital setting with acceptable outcomes for the non-trauma patients commonly seen in UK practice but it is a resource intensive and expensive undertaking.
Keywords: Ventral hernia, Surgical mesh, Negative pressure wound therapy
Ogilivie first described a method of temporary abdominal closure (TAC) during the Second World War1 but it has come to prominence with the wider recognition of the harmful effects of abdominal compartment syndrome (ACS)2 and the role of damage control surgery.3 The management of the open abdomen necessitates some form of TAC, which may change over time, and may include a plan to progress to definitive abdominal fascial closure or default to a planned ventral hernia (Table 1).
Table 1.
Definition of terms related to open abdomen management (adapted from Zarzaur et al)4
| Term | Definition |
|---|---|
| Open abdomen | Any abdomen in which definitive fascial closure cannot be achieved |
| Temporary abdominal closure | Any method used to control the egress of abdominal contents without closure of the abdominal wall fascia |
| Definitive (primary) fascial closure | Fascia-to-fascia closure of abdominal defect with or without prosthetic repair material within the initial hospitalisation |
| Planned ventral hernia | An open abdominal wound that is allowed to granulate and covered with a skin graft before patient discharge with the intention to perform definitive repair in 6–12 months or an abdomen in which deliberate skin-only closure is performed |
Patients with open abdomens that do not achieve definitive fascial closure (DFC) by the time of hospital discharge have prolonged worsened physical and mental wellbeing4 and are responsible for significant societal and healthcare costs.5 The Open Abdomen Advisory Panel therefore recommends: ‘… all appropriate efforts to attempt definitive closure of the abdominal defect within the initial hospitalization’.6 The evidence for open abdomen management (OAM) is derived largely from post-trauma laparotomies, predominantly from the US.7 The literature on OAM following septic catastrophe, the situation usually encountered in European countries, is more scarce8–12 and reports from the UK total fewer than 150 patients.13–18 Only two of these reports are not from university teaching hospitals. We present the outcomes of a consecutive series of patients who underwent OAM in a UK district general hospital (DGH) serving a population of approximately 275,000 people.
Methods
In the absence of specific clinical codes for OAM, after registration with the hospital’s clinical effectiveness department, the theatre logs for January 2007 to December 2011 were searched to find all potential cases. Operations described as ‘relook laparotomy’, ‘abdominal washout’, ‘resuture of abdomen’, ‘burst abdomen’ and ‘temporary abdominal closure’ or variations thereof were identified, as were any operations where it was recorded that gauze had been retained in the patient. The notes of all such patients were retrieved to exclude those who did not undergo OAM. The remaining cases were analysed to provide demographic, surgical and outcome data.
Results
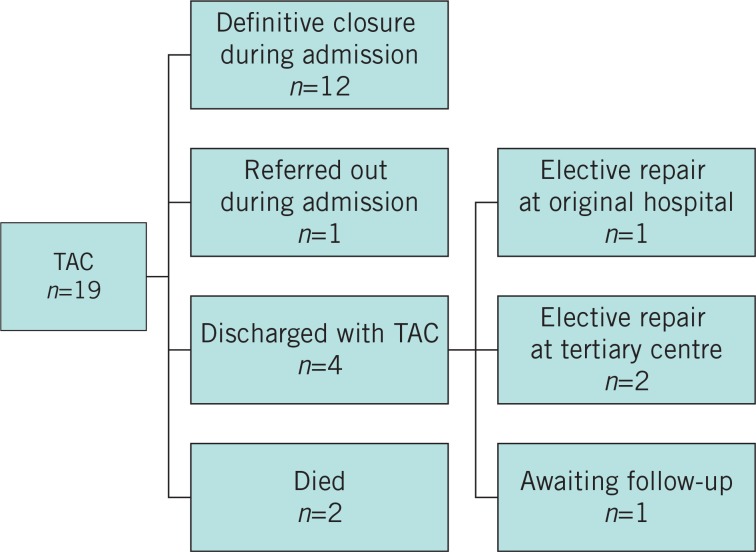
For the 60-month study period, 65 potential open abdomen cases were identified. Of these, 46 cases were not relevant to our study, leaving 19 cases for review (Fig 1). There were 11 male patients and the median age was 62.5 years (range: 19–75 years). Ten open abdomens followed complications of elective surgery, eight cases followed complications of emergency surgery and one was managed as an open abdomen from the initial operation (Table 2).
Figure 1.
Outcome of 19 open abdomen cases. TAC = temporary abdominal closure.
Table 2.
Demographics and surgical details of 19 open abdomen cases
| Features | DFC achieved (n=12) | DFC not achieved (n=7) |
|---|---|---|
| Median age (range) | 62.5 (19–75) | 63.0 (36–69) |
| Co-morbidities | ||
| Ischaemic heart disease | 4 (33%) | 3 (43%) |
| Malignancy | 6 (50%) | 1 (14%) |
| Hypertension | 2 (17%) | 1 (14%) |
| Hypothyroidism | 1 (8%) | 2 (29%) |
| Stroke | 1 (8%) | 1 (14%) |
| Indication for TAC | ||
| Intra-abdominal sepsis | 12 (100%) | 5 (71%) |
| Planned relook | 5 (42%) | 5 (71%) |
| Concerns over ACS | 3 (25%) | 0 (0%) |
| Index TAC technique | ||
| Bogota bag | 6 (50%) | 1 (14%) |
| OpSite® sheet/sandwich | 4 (33%) | 5 (71%) |
| NPWT | 0 (0%) | 0 (0%) |
| MMFT | 1 (8%) | 1 (14%) |
| Loose gauze packing | 1 (8%) | 0 (0%) |
| Secondary TAC technique | ||
| Bogota bag | 4 (33%) | 0 (0%) |
| OpSite® sheet/sandwich | 0 (0%) | 3 (43%) |
| NPWT | 5 (42%) | 3 (43%) |
| MMFT | 3 (25%) | 1 (14%) |
| Complications | ||
| Death | 0 (0%) | 2 (29%) |
| Pneumonia | 3 (25%) | 2 (29%) |
| Multiorgan dysfunction | 1 (8%) | 2 (29%) |
| Small bowel fistula | 0 (0%) | 1 (14%) |
DFC = definitive fascial closure; TAC = temporary abdominal closure; ACS = abdominal compartment syndrome; NPWT = negative pressure wound therapy; MMFT = mesh mediated fascial traction.
Intra-abdominal sepsis was the reason for TAC in 17/19 cases, planned relook laparotomy in 10/19 cases and worry over ACS in 3/19 cases. The decision in each case was made by a consultant surgeon either in attendance or after telephone discussion. OpSite® (Smith and Nephew, London, UK) sheet coverage was the most common method of initial TAC, followed by use of a cut open sterile saline bag sutured to the fascia or skin (Bogota bag). Although there was no increase in the frequency of OAM over the study period, mesh mediated fascial traction (MMFT) was used more frequently as surgeons with a specific interest in managing the abdominal wall became increasingly involved in all of the unit’s open abdomen cases.
All 19 patients required admission to the intensive care unit (ICU), (median: 8.5 days, range: 1–51 days). Two patients died from overwhelming sepsis on days 1 and 10 of their ICU stays. Of the 17 survivors, 14 also required high dependency unit care for a median 6.5 days (range: 1–22 days).
Thirteen of the survivors had complications. Pneumonia (n=5) and multiorgan dysfunction (n=3) were the most common, with others including single cases of myocardial infarction, pulmonary embolism, acute kidney injury and small bowel fistulation. Sixteen patients had nasogastric/jejunal feeding and one patient had distal limb feeding through his stoma. Twelve patients required total parenteral nutrition. A median number of two (range: 0–10) further operations were needed following the institution of TAC and twelve patients had DFC at discharge, equating to 63% of all OAM patients or 71% of survivors (12/17). Fascial closure was achieved using continuous size 1 nylon or polydioxanone sutures, supported on occasion by full-thickness deep tension sutures. There were no significant differences in demographic, disease or operative characteristics between those who achieved DFC and those who did not. The average length of hospital stay for survivors was 54 days (range: 16–154 days).
Of the seven patients who did not achieve DFC, two died, three were discharged with ventral hernias and were later reconstructed (one in our hospital and two in a tertiary referral plastic surgery unit), one patient was transferred to a tertiary referral centre during the acute episode and the final patient is awaiting maturation of his laparostomy before reconstruction of a planned ventral hernia. Five out of eight survivors treated with negative pressure wound therapy (NPWT) achieved DFC, with one enterocutaneous fistula. Four out of four treated with MMFT achieved DFC although one dehisced while in hospital and was discharged with a ventral hernia. Four out of four patients treated with Bogota bag closure were closed at the first or second laparotomy after institution of TAC. The four patients who achieved fascial closure after the Bogota bag TAC had fewer returns to theatre (mean: 1.75) than the nine patients who were closed after NPWT or MMFT (mean 3.635) although this did not reach statistical significance (p=0.14, Student’s t-test for independent means).
Discussion
OAM has been credited with improving outcomes after trauma, and its acceptance has been linked with the growing realisation of the role of damage control surgery and the consequences of ACS.19 The literature is predominantly derived from major US trauma centres or tertiary referral centres in Europe and the evidence of UK practice is sparse. Guidelines for OAM reflect US practice and offer no guidance as to what facilities should be available in a UK hospital managing the open abdomen.4,20
Two key features of OAM will facilitate DFC: the prevention of visceral adhesions to the underside of the anterior abdominal wall and maintenance of domain as the forces on the divided wall pull the fascial edges laterally.4,17 Much of the debate regarding TAC centres on which technique achieves these aims in the most reliable and complication free manner.
Temporary abdominal closure techniques
Measures such as the Bogota bag prevent evisceration and increase the abdominal domain by a silo effect but do not facilitate fascial reapproximation. Placement of a Bogota bag at the first operation at which the abdomen is left open is common but in cases where no other TAC is used, DFC rates are low.21 The OpSite® sandwich is another simple technique and has a DFC rate of 91%.15
The most common method employed currently applies negative pressure therapy to the abdominal wound either by homemade systems with various modifications22 or commercially available kits. Application of negative pressure manages the exudate from the abdomen and maintains inward traction on the fascia while the use of a plastic sheet prevents visceral adhesions, allowing direct fascial suture in due course. DFC rates of 100% have been reported.23 Acceptance of abdominal NPWT is hindered by worries over enterocutaneous fistulation rates of up to 15%24 although it is considered safe by the Open Abdomen Advisory Panel.4 Enterocutaneous fistulation in an open abdomen rarely closes spontaneously, and increases mortality and morbidity.25 The UK’s National Institute of Health and Care Excellence commented in 2009 that evidence supporting NPWT was ‘inadequate in both quantity and quality’,26 and the results of an 18-month countrywide prospective audit were published in 2013.27
The Wittmann Patch® (Starsurgical, Burlington, WI, US) is a patented hook and burr device that is sutured to the abdominal wall fascia, providing inward traction to regain and maintain the abdominal domain.28 The fascia is closed once apposition is adequately regained. The technique has been modified by the insertion of a fenestrated sheet inside the abdominal cavity and a vacuum pack above the patch to control fluid egress.29 The rate of DFC varies between studies from 82% to 93%, with a low rate of complications.28,30
The final commonly employed method of TAC is MMFT, where a fenestrated plastic sheet is placed under a non-absorbable mesh sutured to the edges of the fascial defect. The mesh is tightened sequentially in the midline by reefing with towel clips or suture. If access to the abdominal cavity is required, the mesh is divided, trimmed and sutured back in the midline; inward traction of the mesh continues until the fascial edges are reapproximated and then closed. It, too, can be modified by the use of topical negative pressure to control fluid egress. Outcomes are good with DFC rates of 77–100%31,32 and minimal rates of fistulation.
Although doubtless significant, the overall financial impact of OAM is poorly quantified. However, in one study, hospital charges for those managed with NPWT totalled approximately $25,000 per patient.33
Reviews of the literature focus on the rates of DFC as well as complications such as mortality and enterocutaneous fistulation as major endpoints.7,19,34 The aim of our study was to assess whether the outcomes from our DGH were comparable with those published. These patients are extremely resource intensive with prolonged critical care and overall hospital stays, requirements for multiple trips to theatre and ongoing nutritional support. Our hospital and ICU length of stay, complication rates and mortality rates are comparable with other reported data, and we only suffered one enterocutaneous fistula in a patient in whom multiple enterotomies were exteriorised. Our DFC rate of 71% compares favourably with the 22–88% closure rates from other series of open abdomens for abdominal sepsis,8–10,12,14,21 particularly as it was an unselected group of patients treated by different surgeons using a variety of techniques.
Studies have shown that a defined algorithm for OAM using NPWT35 or the Wittmann Patch®36 improves the rate of DFC. Broadly, the published protocols involve repeated trips to theatre every 2–5 days where approximation is attempted sequentially until DFC or until dressing changes interfere with patient recovery. A similar protocol has been introduced in our hospital based around MMFT.
This study is limited by its retrospective nature and relatively small, heterogeneous cohort of patients but it does highlight the lack of consistency of practice of OAM and adds to the literature on OAM in the UK (Table 3). Two Canadian studies have also demonstrated a wide variation in practice in OAM,37,38 and a survey of Norwegian surgical units revealed that only 39% of surgeons would attempt DFC after TAC and would refer patients to a tertiary centre.39
Table 3.
UK series of open abdomen management
| Authors | Study period | n | Indication | Method | Mortality | DFC | EFC | Comments |
|---|---|---|---|---|---|---|---|---|
| Subramonia13 | 2006–2007 | 19* | Abdominal sepsis (10), dehiscence (9) | VAC | 7/51 (14%) | 31/51 (61%) | 2/51 (4%) | Specific data for the 19 open abdomens not reported |
| Amin14 | 2005–2008 | 20 | Abdominal sepsis | VAC | 0/20 (0%) | 15/20 (75%) |
2/20 (10%) |
|
| Horwood15 | 2003–2008 | 27 | Abdominal sepsis | VAC | 10/27 (35%) | 5/17 (29%) |
2/17 (11%) |
|
| Wilde16 | 36 months | 11 | Abdominal sepsis (8), ischaemia (2), haemorrhage (1) | OpSite® sandwich | 0/11 (0%) | 10/11 (91%) |
2/11 (18%) |
|
| Rao17 | 2003–2005 | 29 | Peritonitis (20), oedema (5); ACS (4) | VAC | 10/29 (34%) | 0/29 (0%) |
6/29 (21%) |
DFC not attempted |
| Anderson18 | 2003–2009 | 29 | Abdominal sepsis | VAC | 9/29 (31%) | 0/29 (0%) |
9/29 (31%) |
DFC not attempted. Later reconstruction in 14/18 patients. |
VAC = vacuum assisted closure; ACS = abdominal compartment syndrome; DFC = delayed fascial closure (number of fascial closures achieved on index hospital admission in survivors); EFC = enterocutaneous fistula.
51 patients reported of which 32 had partial-thickness abdominal wound dehiscence rather than an open abdomen.
It is likely that no matter which TAC is used, a proportion of these patients will be discharged without DFC and it is important to realise that discharge from hospital is not the end of the patients’ healthcare requirements; they should, if at all possible, be offered abdominal wall reconstruction. That, in itself, is a considerable undertaking and requires a surgeon experienced in a variety of reconstructive techniques including component separation.8
Conclusions
The role of OAM is increasingly well established in trauma, ACS and the catastrophically septic abdomen. DFC has both individual and healthcare system benefits, and is the ultimate aim of such management. A variety of techniques for TAC exist and the success rates of achieving DFC vary widely; OAM for intra-abdominal infection results in a lower rate of DFC than in trauma, regardless of which technique is used. This report demonstrates that open abdomens can be managed safely outside of tertiary referral centres, and acceptable fascial closure and complication rates can be achieved. However, managing these patients is resource intensive and expensive, and is best undertaken by an interested surgeon.
References
- 1.Ogilvie WH. The late complications of abdominal war-wounds. Lancet 1940; 236: 253–257. [Google Scholar]
- 2.Stawicki SP, Brooks A, Bilski T et al The concept of damage control: extending the paradigm to emergency general surgery. Injury 2008; 39: 93–101. [DOI] [PubMed] [Google Scholar]
- 3.Rotondo MF, Zonies DH. The damage control sequence and underlying logic. SurgClin North Am 1997; 77: 761–777. [DOI] [PubMed] [Google Scholar]
- 4.Zarzaur BL, DiCocco JM, Shahan CP et al Quality of life after abdominal wall reconstruction following open abdomen. J Trauma 2011; 70: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RS, Morris JA, Diaz JJ et al Complications after 344 damage-control open celiotomies. J Trauma 2005; 59: 1,365–1,371. [DOI] [PubMed] [Google Scholar]
- 6.Campbell A, Chang M, Fabian T et al Management of the open abdomen: from initial operation to definitive closure. Am Surg 2009; 75: S1–S22. [PubMed] [Google Scholar]
- 7.Diaz JJ, Cullinane DC, Dutton WD et al The management of the open abdomen in trauma and emergency general surgery: part 1 – damage control. J Trauma 2010; 68: 1,425–1,438. [DOI] [PubMed] [Google Scholar]
- 8.Verdam FJ, Dolmans DE, Loos MJ et al Delayed primary closure of the septic open abdomen with a dynamic closure system. World J Surg 2011; 35: 2,348–2,355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmelzle M, Alldinger I, Matthaei H et al Long-term vacuum-assisted closure in open abdomen due to secondary peritonitis: a retrospective evaluation of a selected group of patients. Dig Surg 2010; 27: 272–278. [DOI] [PubMed] [Google Scholar]
- 10.Wondberg D, Larusson HJ, Metzger U et al Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis: low primary closure rate. World J Surg 2008; 32: 2,724–2,729. [DOI] [PubMed] [Google Scholar]
- 11.Agalar F, Eroglu E, Bulbul M et al Staged abdominal repair for treatment of moderate to severe secondary peritonitis. World J Surg 2005; 29: 240–244. [DOI] [PubMed] [Google Scholar]
- 12.Bosscha K, Hulstaert PF, Visser MR et al Open management of the abdomen and planned reoperations in severe bacterial peritonitis. EurJ Surg 2000; 166: 44–49. [DOI] [PubMed] [Google Scholar]
- 13.Subramonia S, Pankhurst S, Rowlands BJ, Lobo DN. Vacuum-assisted closure of postoperative abdominal wounds: a prospective study. World J Surg 2009; 33: 931–937. [DOI] [PubMed] [Google Scholar]
- 14.Amin AI, Shaikh IA. Topical negative pressure in managing severe peritonitis: a positive contribution? World J Gastroenterol 2009; 15: 3,394–3,397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwood J, Akbar F, Maw A. Initial experience of laparostomy with immediate vacuum therapy in patients with severe peritonitis. Ann R Coll Surg Engl 2009; 91: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde JM, Loudon MA. Modified Opsite® sandwich for temporary abdominal closure: a non-traumatic experience. Ann R Coll Surg Engl 2007; 89: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007; 9: 266–268. [DOI] [PubMed] [Google Scholar]
- 18.Anderson O, Putnis A, Bhardwaj R et al Short- and long-term outcome of laparostomy following intra-abdominal sepsis. Colorectal Dis 2010; 13: e20–e32. [DOI] [PubMed] [Google Scholar]
- 19.Regner JL, Kobayashi L, Coimbra R. Surgical strategies for management of the open abdomen. World J Surg 2012; 36: 497–510. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan M, Banwell P, Orgill DP et al Guidelines for the Management of the Open Abdomen. Malvern, PA: HMP Communications; 2005. [Google Scholar]
- 21.Kirshtein B, Roy-Shapira A, Lantsberg L, Mizrahi S. Use of the ‘Bogota bag’ for temporary abdominal closure in patients with secondary peritonitis. Am Surg 2007; 73: 249–252. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AM, Kuhn WP, Barker P. Vacuum-assisted closure of the open abdomen in a resource-limited setting. S Afr J Surg 2010; 48: 114–115. [PubMed] [Google Scholar]
- 23.Cothren CC, Moore EE, Johnson JL et al One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg 2006; 192: 238–242. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira PG, Salim A, Inaba K et al A prospective look at the current state of open abdomens. Am Surg 2008; 74: 891–897. [DOI] [PubMed] [Google Scholar]
- 25.Schein M, Decker GA. Gastrointestinal fistulas associated with large abdominal wall defects: experience with 43 patients. Br J Surg 1990; 77: 97–100. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence. Negative Pressure Wound Therapy for the Open Abdomen. London: NICE; 2009. [Google Scholar]
- 27.National Institute for Health and Care Excellence. Negative Pressure Wound Therapy for the Open Abdomen. London: NICE; 2013. [PubMed] [Google Scholar]
- 28.Wittmann DH. Staged abdominal repair: development and current practice of an advanced operative technique for diffuse suppurative peritonitis. ActaChir Austriaca 2000; 32: 171–178. [Google Scholar]
- 29.Fantus RJ, Mellett MM, Kirby JP. Use of controlled fascial tension and an adhesion preventing barrier to achieve delayed primary fascial closure in patients managed with an open abdomen. Am J Surg 2006; 192: 243–247. [DOI] [PubMed] [Google Scholar]
- 30.Tieu BH, Cho SD, Luem N et al The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma 2008; 65: 865–870. [DOI] [PubMed] [Google Scholar]
- 31.Vertrees A, Greer L, Pickett C et al Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg 2008; 207: 801–809. [DOI] [PubMed] [Google Scholar]
- 32.Petersson U, Acosta S, Bjӧrck M. Vacuum-assisted wound closure and mesh-mediated fascial traction – a novel technique for late closure of the open abdomen. World J Surg 2007; 31: 2,133–2,137. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan M. Managing the open abdomen: acknowledging the risks, utilizing the technology. OstomyWound Manage 2004; 50(Suppl 11A): S20–S25. [Google Scholar]
- 34.Boele van Hensbroek P, Wind J, Dijkgraaf MG et al Temporary closure of the open abdomen: a systematic review on delayed primary fascial closure in patients with an open abdomen. World J Surg 2009; 33: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg 2004; 239: 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg JA, George RL, Griffin RL et al Closing the open abdomen: improved success with Wittmann Patch staged abdominal closure. J Trauma 2008; 65: 345–348. [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick AW, Laupland KB, Karmali S et al Spill your guts! Perceptions of Trauma Association of Canada member surgeons regarding the open abdomen and the abdominal compartment syndrome. J Trauma 2006; 60: 279–286. [DOI] [PubMed] [Google Scholar]
- 38.Karmali S, Evans D, Laupland KB et al To close or not to close, that is one of the questions? Perceptions of Trauma Association of Canada surgical members on the management of the open abdomen. J Trauma 2006; 60: 287–293. [DOI] [PubMed] [Google Scholar]
- 39.Groven S, Nӕss PA, Trondsen E, Gaarder C. A national survey on temporary and delayed abdominal closure in Norwegian hospitals. Scand J Trauma Resusc Emerg Med 2011; 19: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]