Abstract
Introduction
The aim of this study was to evaluate the influence of flurodeoxyglucose positron emission tomography computed tomography (FDG PET-CT), as an adjunct to conventional CT staging, in the detection of extrahepatic disease in patients with potentially resectable colorectal liver metastasis.
Methods
Overall, 133 consecutive patients with colorectal liver metastases staged with CT and PET-CT referred to the East Lancashire regional hepatobiliary multidisciplinary team over a two-year period were included in this study. Abnormal findings on PET-CT were correlated with follow-up imaging and/or histology. All imaging was reviewed by specialist hepatobiliary radiologists for the presence/absence of extrahepatic disease. The influence of the PET-CT findings was categorised for each patient in relation to operability and other significant findings.
Results
PET-CT had a major impact on staging of extra hepatic disease in 20% of patients, in comparison with the initial CT. Six per cent of patients were upstaged from operable CT findings to inoperable findings on PET-CT because of the discovery of inoperable occult extrahepatic disease. Five per cent had operable local regional nodal disease detected on PET-CT. A further 3% had premalignant colorectal lesions detected on PET-CT. Six per cent of patients were downstaged from indeterminate or suspected inoperable CT findings to operable findings on PET-CT.
Conclusions
The use of PET-CT in this setting may prevent futile operations, guide the resection of local regional nodal disease and downstage a number of patients thought to have extrahepatic disease on conventional imaging. This study has shown similar results to other recent studies and supports the use of PET-CT as a necessary staging modality in patients with potentially resectable colorectal liver metastases.
Keywords: Hepatectomy, Colorectal cancer, Positron emission tomography, Liver neoplasm
Colorectal cancer is one of the most common cancers in the UK with more than 40,000 new cases diagnosed annually.1 It is the second most common cancer in women after breast cancer and the third most common in men after prostate and lung cancer.
Liver resection for colorectal liver metastasis (CLM) is an established treatment and offers a realistic chance of disease free survival. Five-year survival rates following liver resection have been reported at around 30% with a perioperative mortality rate of <3%.2,3 The most common site of recurrent metastases following liver resection is the liver, followed by the lung.4
Identifying the group of patients who would benefit from a liver resection for CLM is paramount. The patient selection process is multifactorial with the main factors being local resectability of the liver lesions, patient fitness for major surgery, the presence of extrahepatic disease that would preclude successful curative liver resection and patient choice.
Radiological imaging plays a hugely important role in the determination of local resectability of the liver disease and in identifying the presence of extrahepatic disease that would preclude curative resection. Magnetic resonance imaging (MRI) of the liver using liver specific agents and diffusion weighted sequences is the modality of choice for imaging the local resectability of liver metastasis as well as locating previously undetected liver metastasis. Many studies have compared the best modality for imaging hepatic metastases5–10 with pooled sensitivity on a per-lesion basis of 88% for MRI compared with 74% for computed tomography (CT) and 79% for positron emission tomography (PET-CT).11
PET using flurodeoxyglucose (FDG) combined with non-contrast CT produces a hybrid image containing the functional information of increased glucose uptake and the anatomical detail of CT. The CT component of the PET-CT is used for attenuation correction and anatomic reference. It is therefore of less diagnostic quality than standard contrast enhanced staging CT. This is due to the lack of use of intravenous contrast medium, which would distort the attenuation correction applied to the PET component of the acquisition although modern PET-CT machines are capable of producing diagnostic quality CT.
Following contrast enhanced CT of the chest, abdomen and pelvis, PET-CT is the current modality of choice for the detection of extrahepatic disease. Standard PET (without the CT component) has been shown to have a higher sensitivity and specificity (91.5% and 95.4% respectively) than contrast enhanced CT (60.9% and 91.1% respectively)12 although this has not been assessed to the same extent with the use of PET-CT.13 The aim of this study was to evaluate the influence of FDG PET-CT, as an adjunct to conventional CT staging, in the detection of extrahepatic disease in patients with potentially resectable CLM.
Methods
All patients referred to the hepatobiliary multidisciplinary team at East Lancashire Hospitals NHS Trust for consideration of liver resection for CLM between 1 January 2010 and 1 January 2012 were identified. The following inclusion criteria were then applied for the study:
> Liver metastasis had to be from colorectal adenocarcinoma.
> Patients must have undergone both contrast enhanced CT of the chest, abdomen and pelvis and FDG PET-CT as part of their workup with less than 90 days between the two imaging modalities.
> If there were positive or indeterminate findings on PET-CT, these must have confirmatory evidence with either histology or follow-up imaging.
If the follow-up imaging or histology was lacking or unavailable, the patient was excluded from the study.
Contrast enhanced CT technique
The contrast enhanced CT was performed at the local referring hospital to a set protocol. In addition, all imaging was performed on modern multislice machines according to recommendations by the Royal College of Radiologists for cross-sectional imaging in cancer management.14
PET-CT technique
All patients were imaged at the Preston PET CT Centre located at Lancashire Teaching Hospitals NHS Foundation Trust on a 64-slice VCT PET-CT scanner (GE Healthcare, Chalfont St Giles, UK). A standard protocol was used for all patients. Following an injection of up to 400MBq of FDG, patients were rested for one hour before imaging. Prior to imaging, patients were asked to empty their bladder. Supine imaging was performed with the patients’ arms extended above their head and the CT was carried out using 140kV with a modulated 80mAs charge. The field of view extended from the base of the orbits to midthigh level. The emission time per bed acquisition for the PET was 2 minutes and 40 seconds with an 11-slice overlap, with approximately 7 bed positions acquired in the average scan depending on the patient’s height.
Imaging review
Imaging was reviewed using our local picture archiving and communication system (PACS) as well as the PACS at the regional PET centre and at other hospitals using the Northwest PACS web portal. All contrast enhanced CT staging was reviewed by two specialist hepatobiliary radiologists to ensure the same level of scrutiny for each image. Discordant imaging interpretations were settled by discussion to reach a consensus decision. PET-CT results were obtained from the report made by the imaging specialist at the regional centre.
Each patient was categorised into negative, indeterminate or suspicious for the presence of extrahepatic disease on staging CT first and then categorised according to the subsequent PET-CT findings. Patients were organised into groups as to their potential operability on the basis of the CT and on the basis of the combined imaging. They were further categorised as to whether the addition of the PET-CT had a major impact, a negative impact, a minor negative impact or no impact at all on the overall imaging findings. These groups are summarised in Table 1.
Table 1.
Image findings for extrahepatic disease and patient categorisation
| CT findings | CT group | PET-CT findings | PET influence | Impact of PET |
|---|---|---|---|---|
| Negative for EHD | Potentially operable | Positive inoperable EHD | Upstaged to inoperable | Major impact |
| Negative for EHD | Potentially operable | No inoperable EHD; new operable regional nodal disease detected | Upstaged but remained potentially operable | Major impact |
| Suspicious or indeterminate for EHD | Inoperable | No inoperable EHD | Downstaged to potentially operable | Major impact |
| Negative for EHD | Potentially operable | No inoperable EHD; occult new premalignant lesion detected | Remained potentially operable; additional significant lesion detected | Major impact |
| Suspicious for EHD | Inoperable | Positive inoperable EHD | None | No impact |
| Negative for EHD | Potentially operable | No inoperable EHD | None | No impact |
| Indeterminate for EHD | Indeterminate | Indeterminate for EHD | None | No impact |
| Indeterminate or suspicious for EHD | Inoperable / indeterminate | False negative for inoperable EHD | Falsely downstaged EHD | Negative impact |
| Negative | Potentially operable | False indeterminate findings | Prompted biopsy | Minor negative impact |
| Negative | Potentially operable | False positive inoperable EHD | Prompted biopsy | Minor negative impact |
CT = computed tomography; PET = positron emission tomography; EHD = extrahepatic disease
Results
Overall, 175 patients were referred during the 2-year study period. Of these, 133 met the inclusion criteria. Forty-two patients were excluded from the study. The indications for exclusion were:
> more than 90 days between CT and PET-CT (n=10)
> no PET-CT performed (n=15)
> incomplete CT including high resolution or non-contrast CT instead of standard contrast enhanced staging CT of the chest (n=10)
> non-colorectal metastasis (n=1, sarcoma)
> incomplete follow-up available for positive or indeterminate lesions (n=6)
The mean patient age was 67 years (range: 43–88 years) and there was a male-to-female ratio of 88:45. The mean time interval between contrast enhanced CT and PET-CT was 35 days (range: 3–90 days). The mean duration of imaging follow-up for intermediate or suspicious lesions that did not have excision with histopathological diagnosis was 335 days.
Upstaged – major impact
Of the 133 patients included in the study, 18 (14%) had correct new significant positive findings on PET-CT. PET-CT was considered to have had a major impact on the imaging findings in these patients.
Eight patients (6%) were correctly upstaged from ‘potentially operable’ on contrast enhanced CT to ‘inoperable’ on PET-CT. Occult extrahepatic disease was located in: vertebrae (n=1, Fig 1), pancreas (n=1, Fig 2), lung (n=1), vagina (n=1), colorectal (n=1) and multiple sites (n=2). In one further patient, a new oesophageal cancer was also detected.
Figure 1.
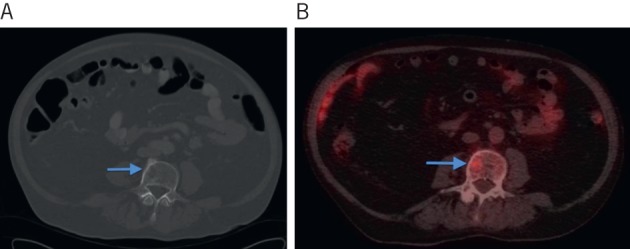
Computed tomography (CT) showing subtle abnormality in the L3 vertebral body (A) and positron emission tomography CT showing increased avidity in the same region in keeping with a metastasis (B).
Figure 2.
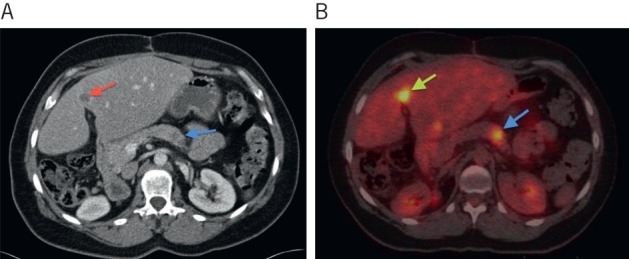
Computed tomography (CT) showing a subtle hypoattenuating lesion in the pancreas (white arrow) (A) and positron emission tomography CT showing the pancreatic lesion as highly avid in keeping with a metastasis (white arrow) (B). A liver metastasis is also shown on both studies (black arrows).
Four patients (3%) were found to have unexpected premalignant disease. All of these were colonic lesions confirmed as tubulovillous adenomas on subsequent biopsy.
Six patients (5%) were upstaged owing to regional and isolated retroperitoneal nodal disease. However, they remained potentially operable. The location of the disease was: portocaval (n=3), perigastric (n=1), precaval (n=1) and aortocaval (n=1).
Downstaged – major impact
Eight patients (6%) had indeterminate or suspicious inoperable extrahepatic disease on CT but were downstaged by PET-CT (adrenal: n=3, lung: n=2, pelvic nodes: n=2, local rectal recurrence: n=1). PET-CT had a major impact in the imaging findings in these patients.
False positive results – minor negative impact
There were three false positive results (2%) from PET-CT after normal contrast enhanced CT. All were areas of increased uptake in the colon or rectum, which were subsequently found to be benign on biopsy. PET-CT had a minor negative impact on the management of these patients.
False indeterminate results – minor negative impact
Eight patients (6%) had indeterminate lesions on PET-CT that had been negative on CT. These were located in: tonsils (n=2), parotid gland (n=1), thyroid gland (n=1), skin (n=1), breast (n=1), lung (n=1) and colorectal recurrence (n=1). All were benign on biopsy and PET-CT was considered to have a minor negative impact on these patients.
False negative results – negative impact
There were three false negative results (2%) from PET-CT. These were located in: abdominal wall (n=1), iliac node (n=1) and spleen (n=1, Fig 3). All of these lesions had been considered indeterminate on CT, were not avid on PET-CT but proved subsequently to be metastases on follow-up imaging. In these patients, PET-CT had a negative impact on the imaging outcome and a potential major negative impact on patient management.
Figure 3.
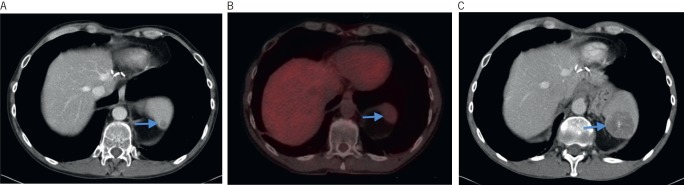
Computed tomography (CT) showing subtle lesion in the spleen (arrow) (A). Lesion was not avid on positron emission tomography CT (arrow) (B). Follow-up CT demonstrating the lesion had increased in size, consistent with a metastasis from the colorectal mucinous primary (arrow) (C).
No difference between modalities – no impact
PET-CT had no impact in 93 patients (70%). Seventy-three patients (55%) were clear from extrahepatic disease on both contrast enhanced CT and PET-CT. Ten (8%) had indeterminate findings on contrast enhanced CT that remained indeterminate after PET-CT (lung: n=7, precaval node: n=1, local rectal recurrence: n=1, presacral soft tissue: n=1). Ten patients (8%) had suspicious contrast enhanced CT findings confirmed by PET-CT (colorectal recurrence: n=5, lung: n=3, duodenum: n=1, para-aortic node: n=1).
Discussion
In this study, PET-CT had a major impact in 20% (26/133) of patients. Most importantly, 6% of patients were upstaged from potentially operable CT findings to inoperable findings, based on the detection of extrahepatic disease by PET-CT. Six per cent of patients were correctly downstaged from suspected or indeterminate CT evidence of inoperable extrahepatic disease to potentially operable by PET-CT.
The false positive rate was 2%. All these patients had benign histology on biopsy shortly after the PET-CT. The false negative rate was 2% and comparable with other contemporaneous studies.15,16
A systematic review in 2005 by Weiring et al looked at 32 separate studies and showed FDG PET to have a higher sensitivity than CT for the detection of extrahepatic metastasis (91.5% vs 60.9%).12 Most of the studies reviewed focused on the clinical impact or change in management due to the FDG PET, with change in 20–32% of cases.
Not all previous studies have been so positive about the use of FDG PET. In 2005 Truant et al reported the sensitivity of FDG PET versus CT for extrahepatic abdominal sites of malignancy using histological correlation to be 63% and 25% respectively.17 However, patient numbers were low and the 9% of patients for whom PET provided additional information was tempered by a 6% false positive rate.
In 2004 Fernandez et al looked at five-year survival rates for patients undergoing liver resection for CLM and found that the addition of FDG PET to the workup of patients increased the postoperative five-year survival rates from 33% to 58.6% by successfully selecting out those patients who were likely to have little or no survival benefit from surgery.18 In 2001 Strasburg et al demonstrated a three-year survival rate of 60% for patients selected for surgery with CT and FDG PET in comparison with 40% for CT alone from previous series.19
More recently, in 2011 Briggs et al reported PET-CT to have a major impact on management in 30% of patients with potentially resectable colorectal liver or lung metastases.15 Occult inoperable extrahepatic disease was uncovered by PET-CT in 9% of cases, in comparison with 6% in our study. In 2008 Kong et al reported unexpected extrahepatic disease detected by PET-CT in 17% of patients who had not shown this on CT.20
Improving and changing technology has added to the uncertainty of the influence of PET-CT, with many of the original studies carried out without the benefit of the anatomical detail conveyed by hybrid machines. In addition, the ability of modern CT to detect more subtle disease that would not have been possible on older machines has improved staging by CT. As a result, the gap in diagnostic sensitivity and specificity is altering as technology advances.
In economically challenging times, the potential positive impact of PET-CT has to be balanced against its cost and current relative limited availability in the UK. Only 40,000 PET scans are carried out by the National Health Service each year.21 Our study and others demonstrate that in the majority of patients with CLM, PET-CT does not alter the extrahepatic imaging findings or management.15,16
A number of predictive models exist that are designed to improve selection for liver surgery in patients with CLM. Engledow et al looked at the use of the Fong clinical risk score22 in the context of PET-CT in CLM.16 They found that PET-CT changed management in 34% of patients and that there was a trend towards PET-CT being more influential in patients categorised as low risk by Fong criteria (44%) in comparison with the high risk patients (14%) although this failed to reach statistical significance. The study by Engledow et al raises the question of how patients might best be selected for PET-CT. However, more research is needed to determine a successful way to select those patients in whom PET-CT has the most impact.
Conclusions
This study provides confirmation of the value of PET-CT in the staging of potentially operable CLM, using modern equipment and standardised protocols.
Acknowledgements
The authors would like to thank you the staff at Preston PET CT Centre for their help with the manuscript.
References
- 1.Bowel Cancer. NHS Choices. http://www.nhs.uk/Conditions/Cancer-of-the-colon-rectum-or-bowel/ (cited January2014).
- 2.Rees M, Tekkis PP, Welsh FK et al Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008; 247: 125–135. [DOI] [PubMed] [Google Scholar]
- 3.Simmonds PC, Primrose JN, Colquitt JL et al Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006; 94: 982–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes KS, Simon R, Songhorabodi S et al Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986; 100: 278–284. [PubMed] [Google Scholar]
- 5.Wiering B, Ruers TJ, Krabbe PF et al Comparison of multiphase CT, FDG-PET and intra-operative ultrasound in patients with colorectal liver metastases selected for surgery. Ann Surg Oncol 2007; 14: 818–826. [DOI] [PubMed] [Google Scholar]
- 6.Bipat S, van Leeuwen MS, Comans EF et al Colorectal liver metastases: CT, MR imaging, and PET for diagnosis – meta-analysis. Radiology 2005; 237: 123–131. [DOI] [PubMed] [Google Scholar]
- 7.Mainenti P, Mancini M, Mainolfi et al Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging 2010; 35: 511–521. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjya S, Bhattacharjya T, Baber S et al Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. Br J Surg 2004; 91: 1,361–1,369. [DOI] [PubMed] [Google Scholar]
- 9.Rappeport ED, Loft A, Berthelsen AK et al Contrast-enhanced FDG-PET/CT vs SPIO-enhanced MRI vs FDG-PET vs CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol 2007; 48: 369–378. [DOI] [PubMed] [Google Scholar]
- 10.Floriani I, Torri V, Rulli E et al Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging 2010; 31: 19–31. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence. Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer. London: NICE; 2011. p93. [Google Scholar]
- 12.Wiering B, Krabbe PF, Jager GJ et al The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 2005; 104: 2,658–2,670. [DOI] [PubMed] [Google Scholar]
- 13.Selzner M, Hany TF, Wildbrett P et al Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg 2004; 240: 1,027–1,034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royal College of Radiologists. Recommendations for Cross-Sectional Imaging in Cancer Management. London: RCR; 2006. [Google Scholar]
- 15.Briggs RH, Chowdhury FU, Lodge JP, Scarsbrook AF. Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin Radiol 2011; 66: 1,167–1,174. [DOI] [PubMed] [Google Scholar]
- 16.Engledow AH, Skipworth JR, Pakzad F et al The role of 18FDG PET/CT in the management of colorectal liver metastases. HPB 2012; 14: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truant S, Huglo D, Hebbar M et al Prospective evaluation of the impact of [18F]fluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg 2005; 92: 362–369. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez FG, Drebin JA, Linehan DC et al Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004; 240: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strasberg SM, Dehdashti F, Siegel BA et al Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg 2001; 233: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong G, Jackson C, Koh DM et al The use of 18F-FDG PET/CT in colorectal liver metastases – comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging 2008; 35: 1,323–1,329. [DOI] [PubMed] [Google Scholar]
- 21.PET Scan. NHS Choices. http://www.nhs.uk/Conditions/PET-scan/ (cited January2014).
- 22.Fong Y, Fortner J, Sun RL et al Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]


