Abstract
The free solution mobilities of single- and double-stranded DNA molecules with variable charge densities have been measured by capillary electrophoresis. DNA charge density was modified either by appending positively or negatively charged groups to the thymine residues in a 98 base pair (bp) DNA molecule, or by replacing some of the negatively charged phosphate internucleoside linkers in small single-or double-stranded DNA oligomers with positively charged phosphoramidate linkers. Mobility ratios were calculated for each data set by dividing the mobility of a charge variant by the mobility of its unmodified parent DNA. Mobility ratios essentially eliminate the effect of the background electrolyte on the observed mobility, making it possible to compare analytes measured under different experimental conditions. Neutral moieties attached to the thymine residues in the 98-bp DNA molecule had little or no effect on the mobility ratios, indicating that bulky substituents in the DNA major groove do not affect the mobility significantly. The mobility ratios observed for the thymine-modified and linker-modified DNA charge variants increased approximately linearly with the logarithm of the fractional negative charge of the DNA. Mobility ratios calculated from previous studies of linker-modified DNA charge variants and small multi-charged organic molecules also increased approximately linearly with the logarithm of the fractional negative charge of the analyte. The results do not agree with the Debye-Hückel-Onsager theory of electrophoresis, which predicts that the mobility of an analyte should depend linearly on analyte charge, not the logarithm of the charge, when the frictional coefficient is held constant.
Keywords: Capillary electrophoresis, DNA charge variants, Fractional charge, Free solution mobility, Small multi-charged organic molecules
1 Introduction
Capillary electrophoresis (CE) is a useful probe of the electrostatics of DNA and other polyelectrolytes in solution because the Debye-Hückel-Onsager theory suggests that the free solution mobility of an analyte should be directly proportional to its effective charge and inversely proportional to its frictional coefficient [1, 2]. However, the effective charge of DNA is lower than expected from the number of negatively charged phosphate groups because the polyion is surrounded by a dense layer of condensed counterions that effectively decrease the net charge [3]. Various gel and CE experiments have indicated that DNA and its condensed counterions migrate as a single kinetic unit in the electric field [4–12]. Hence, electrophoresis can be used to probe counterion condensation as well as DNA electrostatics.
In most CE experiments carried out to date, the DNA charge density has been held constant and the properties of the background electrolyte (BGE) have been varied [4, 13–15]. However, two previous CE studies have explored the effect of variations in DNA charge density on the observed mobility. In the first study, a 118 base pair (bp) DNA restriction fragment was modified by replacing up to 9 negatively charged phosphate internucleoside linkers with cationic phosphoramidate linkers [16]. The second study characterized the mobility of small single-stranded (ss) DNA oligomers containing 16 nucleotides with up to ten phosphate linkers replaced by neutral phosphoramidate linkers [17]. In both studies, the free solution mobilities of the modified DNAs decreased with the increasing number of neutral or positively charged linkers, as expected, due to the decrease in the net charge of the DNA.
To broaden the types of DNA charge variants that have been characterized, we have now modified DNA charge density either by appending neutral or charged groups to the thymine residues in a 98 bp dsDNA molecule or by replacing some of the negatively charged phosphate linkers in small ss- or dsDNA oligomers with positively charged phosphoramidate linkers. Mobility ratios were calculated for each data set by dividing the mobility of each charge variant by the mobility of its fully charged, unmodified parent DNA. Mobility ratios minimize the contribution of the physical properties of the BGE to the observed mobility, making it possible to compare analytes measured under different experimental conditions [15].
The mobilities and mobility ratios observed for DNAs with neutral substituents attached to thymine were essentially equal, indicating that bulky substituents in the DNA major groove have little or no effect on the mobility. However, the mobility ratios of the DNA charge variants depended on the fractional negative charge of the DNA. Regardless of whether the fractional charge was varied by appending charged substituents to the thymine residues or by replacing some of the phosphate internucleoside linkers with positively charged phosphoramidate linkers, the mobility ratios increased approximately linearly with the logarithm of the fractional negative charge of the DNA. Mobility ratios calculated from previous CE studies of DNA charge variants [16, 17] exhibit the same dependence on the logarithm of the fractional negative charge, as do mobility ratios calculated from previous studies of small multi-charged organic molecules [18, 19]. The results cannot be explained by the Debye-Hückel-Onsager theory of electrophoresis [1, 2, 20, 21], which posits that the mobility of an analyte should increases linearly, not logarithmically, with analyte charge when the frictional coefficient is held constant.
2 Materials and Methods
2.1 Thymine-modified DNAs
The sequence of the unmodified 98-bp parent DNA molecule used in this work is:
5′-AGCCTAGCCT ATGACATGAC ACGTTACGAC CAGACCAGCT GCACTGCAGA CTGGACTGAC GCTAGCTGAC TGTACTGTAT GCAATGCAAC CTCACCTC-3′
3′ -TCGGATCGGA TACTGTACTG TGCAATGCTG GTCTGGTCGA CGTGACGTCT GACCTGACTG CGATCGACTG ACATGACATA CGTTACGTTG GAGTGGAG-5′
The sequence contains approximately equal numbers of A·T and G·C base pairs, with no A-tracts or long runs G/C residues. The 36 thymine residues indicated in bold were modified at the C5 position by various substituents, as indicated by R in Figure 1. The acronyms used to designate the various thymine-modified DNAs are indicated in the figure; the acronyms and chemical sequences of the R substituents are given in first two columns of Table 1. The preparation of the thymine-modified DNAs and their characterization are described elsewhere [22].
Figure 1.
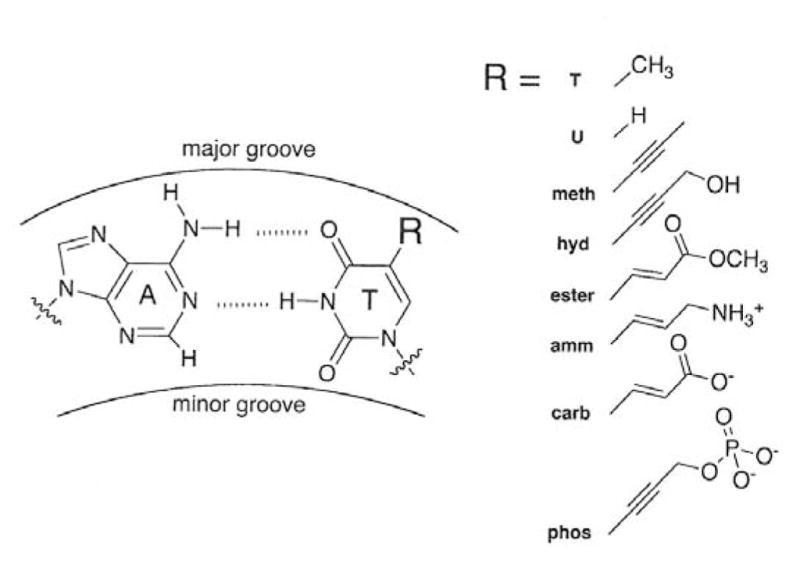
The structure of an A/T base pair (left) with the site of thymine modification indicated by R. The structures of the R substituents and the acronyms used to denote the thymine-modified DNAs are given on the right.
Table 1.
Acronyms, chemical structures, and properties of the R groups in the thymine-modified DNA molecules.
| Acronym | R | Net Charge | Mobility in NH41+, m.u.1 | Mobility Ratio2 in NH41+ |
|---|---|---|---|---|
| T | -CH3 (thymine) | −196 | 2.963 | 1.000 |
| U | -H (uracil) | −196 | 2.960 | 0.999 |
| meth | -C≡ C-CH3 | −196 | 2.958 | 0.998 |
| hyd | -C≡C-CH2OH | −196 | 2.958 | 0.998 |
| ester | -CH=CH-CO2CH3 | −196 | 2.941 | 0.993 |
| amm | -CH=CH-CH2-NH31+ | −160 | 2.786 | 0.940 |
| carb | -CH=CH-CO21− | −2233 | 3.031 | 1.023 |
| carb/2 | only 1 strand modified | −2103 | 3.006 | 1.014 |
| phos | -C≡C-CH2-OPO32− | −2323 | 3.0464 | 1.0284 |
| phos/2 | only 1 strand modified | −2143 | 2.9964 | 1.0114 |
Normalized mobility (1 m.u. = 1 × 10−4 cm2/Vs). The average standard deviation of the mobilities was ±0.003 m.u.
Mobility ratio = mobility (charge variant) / mobility (sample T).
pH-corrected net charge (see text).
Measured without a marker (see text).
2.2 Linker-modified DNAs
The ss- and dsDNA oligomers contained 20 or 23 nucleotides or base pairs and were modified by replacing one to six of their negatively charged phosphate linkers with cationic N,N′-diethylethylenediamine phosphoramidate linkers (called DEED for brevity). The DEED linkers have the formula O=P(O-ribose)2NHCH2CH2N(CH2CH3)2 and have a single positive charge at neutral pH. The preparation and characterization of the DEED-modified oligomers are described elsewhere [16, 23]. The acronyms and sequences of the DEED-modified oligomers are given in the first two columns of Table 2. The acronyms indicate whether the oligomer is single- or double-stranded and the number of nucleotides or base pairs in the oligomer, followed by a slash and a number indicating the number of cationic DEED internucleoside linkers. The double-stranded charge variants were prepared by heating ss20/1 or ss23/1 with its complement in 100 mM Tris-Cl buffer for 5 minutes at 95°C, followed by gradual cooling to room temperature. The DEED-modified samples were a kind gift from John Dagle (University of Iowa, Departments of Biochemistry and Pediatrics). The unmodified oligomers were purchased from IDT (Coralville, IA).
Table 2.
Acronyms, sequences, and properties of linker modified DNA oligomers.
| Acronym1 | DNA Sequence2 | Net Charge | Mobility, m.u.3 | Mobility Ratio4 |
|---|---|---|---|---|
| ss20/1 | GCCTATGAAAGGTCTCGACC | −17 | 3.115 | 0.966 |
| ss20/4 | TTTGTGTCCCCCTCTCAGGT | −11 | 2.59 | 0.804 |
| ss20/6 | CACAAACCTGTTCTTGGCAG | −7 | 1.58 | 0.490 |
| ss20/6 | TCGGACTCTGAGTTTCGGTT | −7 | 1.64 | 0.509 |
| ss20/0 | GCCTATGAAAGGTCTCGACC | −19 | 3.22 | 1.000 |
| ss23/1 | TTGGCCTATGAAAGGTCTCTCTAAT | −20 | 3.07 | 0.948 |
| ss23/0 | TTGGCCTATGAAAGGTCTCTCTAAT | −22 | 3.24 | 1.000 |
| ds20/1 | GCCTATGAAAGGTCTCGACCCGGATACTTTCCAGAGCTGG | −36 | 3.24 | 0.953 |
| ds20/0 | GCCTATGAAAGGTCTCGACCCGGATACTTTCCAGAGCTGG | −38 | 3.40 | 1.000 |
| ds23/1 | TTGGCCTATGAAAGGTCTCTCTAATAACCGGATACTTTCCAGAGAGATTA | −42 | 3.28 | 0.962 |
| ds23/0 | TTGGCCTATGAAAGGTCTCTCTAATAACCGGATACTTTCCAGAGAGATTA | −44 | 3.41 | 1.000 |
The acronyms indicate whether the oligomer is single- or double-stranded, the number of nucleotides or base pairs, followed by a slash and the number of positively charged internucleoside DEED linkers. Controls without DEED linkers have a zero following the slash.
The nucleotides in bold precede a cationic DEED linker.
Measured in 20 mM HEPES buffer or 20 mM KDM buffer; the mobilities in these two buffers were equal within experimental error. The average standard deviation of the mobilities was ±0.03 m.u.
Mobility ratio = mobility (charge variant) / mobility (parent DNA with the same number of nucleotides or base pairs).
Average mobility of a closely spaced doublet in the electropherogram.
2.3 Buffers
The background electrolytes (BGE) used to measure the mobilities of the thymine-modified DNAs contained 67 mM ammonium diethylmalonate (DM), prepared by titrating a stock solution of diethylmalonic acid ((CH3CH2)2C(COOH)2, Sigma-Aldrich, St. Louis, MO) to pH 7.3, the midpoint of the second carboxyl group, with a concentrated solution of NH4OH. The BGE used to characterize the DEED linker-modified oligomers was either 20 mM HEPES (CalBiochem, La Jolla, CA) buffer, pH 7.5, or 20 mM potassium DM buffer, pH 7.5. The mobilities observed in these two buffers were equal within experimental error.
2.4 Capillary Electrophoresis
The free solution mobilities of the charge-modified DNAs were measured with a Beckman Coulter P/ACE MDQ Capillary Electrophoresis System (Fullerton, CA), using methods described previously [17, 24]. All measurements were made in the reverse polarity mode (anode on the detector side) with UV detection at 254 nm. The capillaries were coated internally with linear polyacrylamide (Polymicro Technologies, Phoenix, AZ) to minimize the electroosmotic flow (EOF) of the solvent. Previous studies have shown that this coating does not affect the observed mobilities [25]. The capillaries were 31.1 ± 0.2 cm in length (20.9 ± 0.1 cm to the detector) and 75 μm in internal diameter and were mounted in a liquid-cooled cassette thermostated at 20°C. The applied electric field was 120 V/cm for the thymine-modified DNAs and 266 V/cm for the DEED linker-modified oligomers. The DNA samples were injected hydrodynamically at 0.5 psi (0.0035 MPa) for 3s; the sample plug occupied ~2.6% of the capillary length. The mobilities measured under such conditions are independent of DNA concentration and the electric field strength [25]
2.5 Mobility Calculations
The observed mobilities, μobs, of the DNA samples were calculated from Eq. 1:
| (1) |
where Ld is the distance from the inlet of the capillary to the detector in cm, t is the migration time in seconds, and E is the electric field strength in volts per cm. As described previously [26, 27] the observed mobility corresponds to the algebraic sum of the actual mobility of the DNA, μ, and the mobility caused by the electroosmotic flow (EOF) of the solvent toward the cathode, μEOF. Although the internally coated capillaries used in the present experiments had very low EOF, the observed mobility varies somewhat from run to run because of transient changes in the capillary coating. Changes in μEOF were monitored for the thymine-modified samples by including a small single-stranded DNA oligomer with the sequence ACCTGAT or ACCTGATCAG in each sample as a marker. Except as indicated below, the mobilities of all samples were independent of the presence or absence of the marker. The average mobility of the marker in a given series of experiments was determined and the observed mobilities of the thymine-modified sequence variants were normalized to the average mobility of the marker, as described previously [26, 27]. All mobilities are reported in mobility units, m.u. (1 m.u. = 1 × 10−4 cm2 V−1 s−1). The average standard deviation of the normalized mobilities of the thymine-modified DNAs, based on replicate measurements, was ±0.003 m.u. The mobilities of the linker-modified DNA oligomers were determined without a marker in the solution; the average standard deviation of the mobilities of these samples was ±0.03 m.u.
In order to compare the mobilities of the thymine-modified and linker-modified DNAs with each other and with previous studies of charge-modified DNAs in the literature [16,17], mobility ratios were calculated for each of the data sets by dividing the mobilities of the charge variants in each data set by the mobility of the parent unmodified DNA, measured under the same conditions. Mobility ratios are useful because they minimize the dependence of the mobility on the physical properties of the BGE, allowing the mobility ratios of different samples measured in different BGEs to be compared [15, 27]. In all cases, the mobility ratios in each data set were proportional to the observed mobilities. The mobility ratios observed for the charge variants in each data set were plotted as a function of the fractional negative charge of the variant, calculated by dividing the net number of negative charges in each charge variant by the number of negative charges in the unmodified parent DNA. Since the parent DNA and the charge variants in each data set contain the same number of nucleotides or base pairs, the frictional coefficients of the DNAs in each data set are essentially constant.
3 Results and Discussion
3.1 Electropherograms of Charge-Modified DNAs
Typical electropherograms observed for sample T and its charge variants amm, carb, and phos in 100 mM NH41+ are illustrated in Figure 2A. The peaks on the left in each electropherogram correspond to the DNA; the marker peaks are on the right. The leading edges of the DNA peaks were broadened near the base, most likely because of failure sequences that were carried along in the preparative PCR reactions and migrated with mobilities close to the mobility of the desired product [28]. The trailing edges of the T, amm and carb peaks were reasonably sharp and Gaussian in shape, as were the trailing edges of the peaks observed for DNAs containing neutral thymine substituents (not shown). However, the trailing edge of the phos peak was broad and returned to the baseline very slowly, suggesting that this DNA and the marker were interacting during electrophoresis. Similar results were observed for phos/2 (not shown). When two DNAs are interacting during electrophoresis, the migration time observed for each peak corresponds to the weighted average of the mobilities of the DNAs in each peak [13, 14, 26, 29]. Therefore, to obtain the true mobilities of phos and phos/2, the mobilities of these samples were measured without a marker in the solution.
Figure 2.
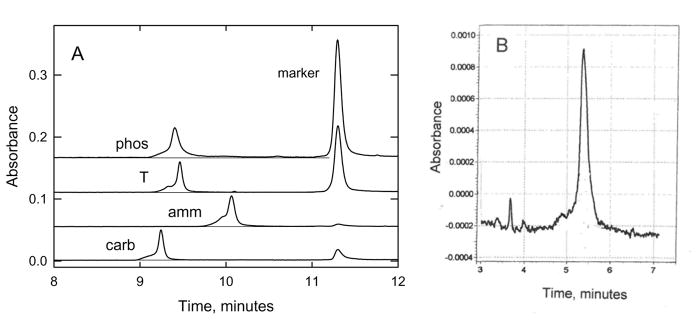
Typical CE electropherograms observed for charge-modified DNAs. The absorbance at 254 nm (in arbitrary units) is plotted as a function of the time after injection of the sample into the capillary. (A), thymine-modified charge variants in 100 mM ammonium DM. The peaks on the left correspond to phos, T, amm and carb (top to bottom); the peaks on the right correspond to the marker ACCTGATCAG. Note the long tail following the peak observed for phos. The slow return of the absorbance to the baseline indicates that phos and the marker were interacting during electrophoresis (see text). (B), Linker-modified DNA, ss20/6, in 20 mM HEPES buffer.
The effect of DNA-marker interactions on the mobilities observed for phos and phos/2 in 100 mM NH41+ is quantified in Table 3, which compares the difference in mobility between the various charge-modified DNAs and sample T, with and without a marker in the solution. The average mobility difference observed for amm and carb with respect to sample T, Δμ, was 0.008 ± 0.003 m.u. with and without the marker, close to the experimental uncertainty in the measurements. However, the Δμvalues observed for phos and phos/2 with and without the marker were 0.072 and 0.022 m.u., respectively, approximately nine and three times larger than the average mobility difference observed for amm and carb. Hence, the mobilities observed for phos and phos/2 without a marker in the solution are used in the subsequent text.
Table 3.
Difference between the mobilities of thymine-modified DNAs and sample T, with and without a marker in the solution.
| DNA sample | Mobility Difference With Marker, m.u. | Mobility Difference Without Marker, m.u. | ΔΔμ1, m.u. |
|---|---|---|---|
| amm | −0.181 | −0.192 | 0.011 |
| carb | +0.064 | +0.058 | +0.006 |
| phos | +0.011 | +0.083 | −0.072 |
| phos/2 | +0.011 | +0.033 | −0.022 |
ΔΔμ = mobility difference with and witout marker.
The electropherogram observed for a linker-modified DNA oligomer, ss20/6, in 20 mM HEPES buffer is illustrated in Figure 2B. The peak is reasonably sharp and nearly Gaussian in shape. The relatively poor signal/noise ratio is due to the low DNA concentration in the sample. Similar electropherograms were observed for the other linker-modified DNAs (not shown).
3.2 Mobility Ratios
Mobility ratios were calculated for each of the charge variant DNAs using the mobility of the unmodified parent DNA, measured under the same conditions, as the reference. Mobility ratios minimize variations due to the physical properties of the solvent, such as viscosity and dielectric constant, making it possible to compare the results obtained in different BGEs. Mobility ratios for the thymine-modified DNAs were calculated by dividing the mobility of the modified DNA by the mobility of sample T (thymine). Mobility ratios of the linker-modified DNAs were calculated with respect to the mobility of an unmodified DNA containing the same number of nucleotides or base pairs. For convenience, the mobilities and mobility ratios calculated for each of the charge variant DNAs are given in Tables 1 and 2.
The mobility ratios observed for the thymine-modified DNAs are illustrated in Figure 3. The average mobility ratio observed for the uncharged DNAs, T, U, meth, hyd, and ester, was 0.998 ± 0.002, slightly smaller than observed for the unmodified sample T. Hence, the bulkiness of an uncharged substituent at the C5 position of thymine has little effect on the observed mobility. The positively charged derivative, amm, with a mobility ratio of 0.940, migrated significantly more slowly than sample T. The negatively charged derivatives, phos, phos/2, carb and carb/2, with mobility ratios ranging from 1.011 to 1.028, migrated faster than sample T.
Figure 3.
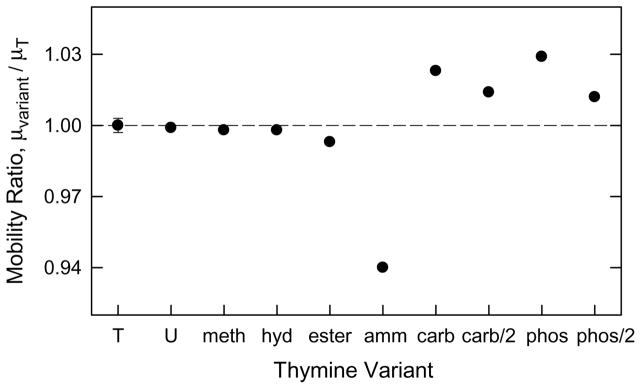
Comparison of mobility ratios observed for the thymine-modified DNAs in 100 mM NH4+. Mobility ratio = mobility (variant) / mobility (sample T). The average standard deviation of the mobility ratios is indicated by an error bar appended to sample T.
3.3 Fractional negative charge of modified DNAs
3.3.1 Thymine-modified DNAs
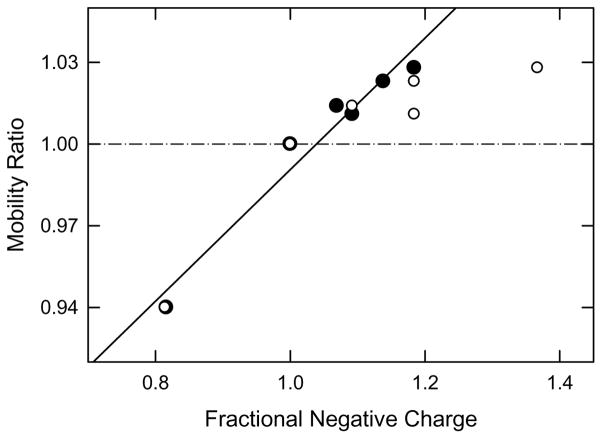
The fractional negative charge of each of the thymine-modified DNAs was calculated by dividing the net number of negative charges in amm, phos, phos/2, carb and carb/2 by the number of negative charges in the unmodified parent DNA, sample T. This procedure is based on the assumption that the charge variants and their parent DNAs have essentially the same frictional coefficients because they contain the same number of base pairs, allowing the mobility ratios and fractional net charges to be compared. The mobility ratios of the thymine-modified DNAs are plotted as the open circles in Figure 4. As expected, the mobility ratios increase with the increasing fractional negative charge of the DNA, due to the increase in the net number of negative charges in the molecule. However, the curvature of the mobility ratios at high fractional negative charge suggests that the mobility ratios are anomalously low, most likely because the charged residues are localized in the DNA major groove (see Figure 1). Previous experimental and theoretical studies have shown that the pH in DNA grooves is approximately two orders of magnitude lower than observed in the bulk solution [30, 31]. Hence, the pH in the grooves of the thymine-modified DNAs would be ~5.3, significantly lower than the pH of 7.3 in the bulk. As a result, phos and phos/2 probably exhibit only a single negative charge in DM buffers if the pK values of the phosphate groups are similar to those of n-propylphosphate (pK = 1.88 and 6.67 at 25°C [32]). The net charge of phos and phos/2 would then be 196 (because of 196 negatively charged phosphate linkers between each nucleotide), plus 36 (negatively charged phosphate groups in phos) or 18 (phosphate groups in phos/2), for a total of 232 negative charges for phos and 214 for phos/2.
Figure 4.
Dependence of the mobility ratios observed for thymine-modified DNAs on the fractional negative charge, normalized to a value of 1.00 for sample T. The fractional charges were calculated from: (○), the expected charge of the functional group added to thymine; and (●), the pH-corrected charge (see text). The line correlating the pH-corrected values was drawn by linear regression; r2 = 0.957.
The carboxylate derivatives, carb and carb/2, probably also exhibit a reduced negative charge in DM buffers, since acetic and propionic acids, for example, have pK values of 4.8 [32]. Taking this pK as characteristic of the carboxylate group in carb and carb/2, the carboxylate derivatives would be 75% ionized at a pH of ~5.3. Hence, carb and carb/2 would contain 223 and 210 negative charges, respectively. The ionization of the ammonium group in amm would be unaffected by the lower pH in the DNA grooves, since the pK value of alkyl amines is greater than 10 [32]. For convenience, the pH-corrected net charges of the thymine-modified DNAs are given in the third column of Table 1.
The dependence of the mobility ratios of the thymine-modified DNAs on the pH-corrected fractional negative charge is illustrated by the solid symbols in Figure 4. The mobility ratios increase approximately linearly with the pH-corrected fractional negative charge, with a correlation coefficient of 0.957. The results indicate that the pH in the DNA grooves is significantly lower than in the bulk solution, in agreement with previous studies [30.31].
3.3.2 Linker-modified DNAs
The net negative charges calculated for the linker-modified DNA oligomers are given in the third column of Table 2, along with the negative charges of unmodified controls containing the same number of nucleotides or base pairs. Note that the number of charged residues in the controls is one less than the number of nucleotides in each chain, because the oligomers are unphosphorylated on their 5′ ends. The fractional charge of each linker-modified oligomer was calculated by dividing the net number of negative charges in the oligomer by the number of negative charges in the appropriate control. The mobilities and mobility ratios observed for the linker-modified DNAs are given in the last two columns of Table 2.
3.4 Dependence of mobility ratios on fractional negative charge
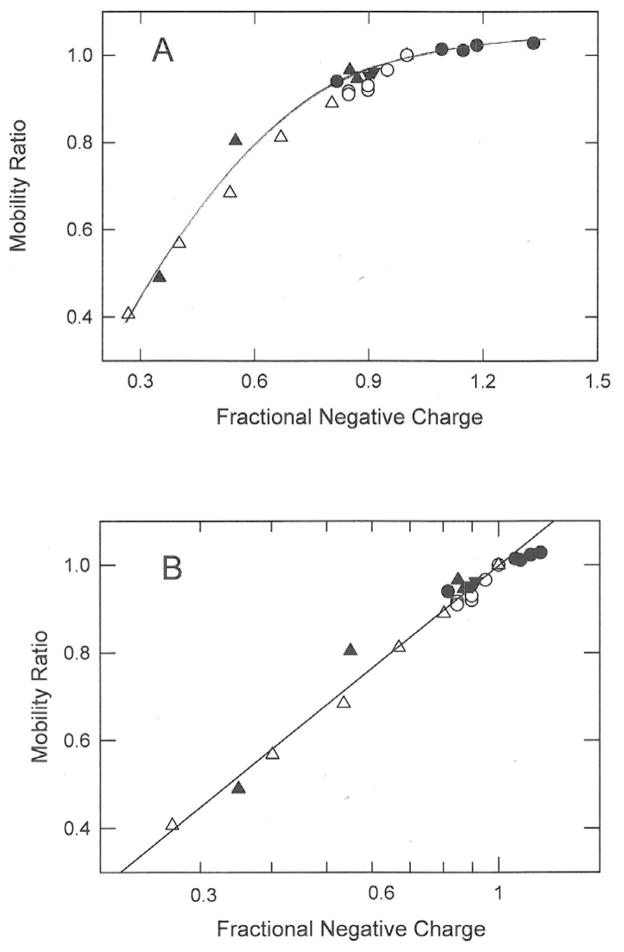
The mobility ratios observed for the thymine-modified and linker-modified DNAs in this study are plotted as the solid symbols in Figure 5. In panel A, the mobility ratios are displayed as a linear function of the fractional negative charge of each DNA; in Figure 5B the mobility ratios are plotted as a function of the logarithm of the fractional negative charge. The mobility ratios in Figure 5 are also compared with mobility ratios calculated from two previous studies of linker-modified DNAs (open symbols). In one study, three to nine negatively charged phosphate linkers at one or both ends of a 118-bp restriction fragment were replaced by cationic DEED linkers [16]; in the second study, three to ten phosphate linkers in a 16-nucleotide oligomer replaced by neutral methoxyethyleneamine phosphoramidate (MEA) linkers [17]). The MEA linker has the sequence O=P(O-ribose)2NHCH2CH2OCH3. For convenience, the DEED and MEA charge modified DNAs are listed in Table 4, along with their properties.
Figure 5.
Dependence of mobility ratios on the fractional negative charge of the analyte. (A), Mobility ratios plotted as the first power of the fractional negative charge of the charge variant; (B), mobility ratios plotted as the logarithm of the fractional negative charge. In both (A) and (B) the symbols correspond to: (●), thymine-modified 98-bp dsDNAs, using pH-corrected fractional charges; (▼), cationic DEED linker-modified 20- or 23-bp dsDNAs; (▲), cationic DEED linker modified 20- or 23-nt ssDNAs; (○), cationic DEED linker-modified 118-bp dsDNAs, calculated from the data in Ref. [16]; and (△), neutral MEA linker-modified 16 nt ssDNAs, calculated from the data in Ref. [17]. The line was drawn by linear regression; r2 = 0.979.
Table 4.
Acronyms, DNA net charge, mobilities and mobility ratios of linker modified DNAs taken from the literature.
| Acronym1 | Net Charge | Mobility, m.u.2 | Mobility Ratio 3 | Reference |
|---|---|---|---|---|
| ds118/0 | −236 | 3.680 | 1.000 | [16] |
| ds118/3 | −224 | 3.551 | 0.965 | |
| ds118/3 | −224 | 3.554 | 0.966 | |
| ds118/6 | −212 | 3.421 | 0.930 | |
| ds118/6 | −212 | 3.387 | 0.920 | |
| ds118/9 | −200 | 3.375 | 0.917 | |
| ds118/9 | −200 | 3.350 | 0.910 | |
|
| ||||
| ss16/0 | −15 | 3.10 | 1.000 | [17] |
| ss16/3 | −12 | 2.76 | 0.890 | |
| ss16/5 | −10 | 2.52 | 0.813 | |
| ss16/7 | −8 | 2.12 | 0.684 | |
| ss16/9 | −6 | 1.76 | 0.568 | |
| ss16/11 | −4 | 1.26 | 0.406 | |
The acronyms indicate whether the oligomer is single- or double-stranded and the number of nucleotides or base pairs, followed by a slash and the number of cationic DEED linkers in the 118-bp samples or the number of neutral MEA linkers in the 16-nt samples. Controls without modified linkers have a zero following the slash.
The mobilities of the 118-bp samples were measured with a marker in the solution [16]; the mobilities of the 16-nt samples were measured without a marker [17]. The average standard deviations of the mobilities were ±0.05 m.u. for the 16-nt samples [16] and ±0.03 m.u. for the 118-bp samples [17].
Mobility ratio = mobility (charge variant) / mobility (parent unmodified DNA).
A comparison of Figures 5A and 5B shows clearly that the mobility ratios observed for all charge-modified DNAs, those described in this work and those measured previously, increase linearly with the logarithm of the fractional negative charge (r2 = 0.979), not the first power of the charge. Hence, if the frictional coefficient is kept approximately constant by comparing sets of DNAs containing the same number of nucleotides or base pairs, measured under a given set of experimental conditions, the electrophoretic mobilities (and the mobility ratios) are determined by the logarithm of the fractional negative charge, regardless of DNA molecular weight (over the range tested) and independent of whether the DNA is single- or double-stranded, whether the linkers or the thymine residues are modified, whether the charge modifications are positive, negative or neutral in sign, or whether the charge modifications are concentrated at one end of the DNA chain or distributed throughout the sequence.
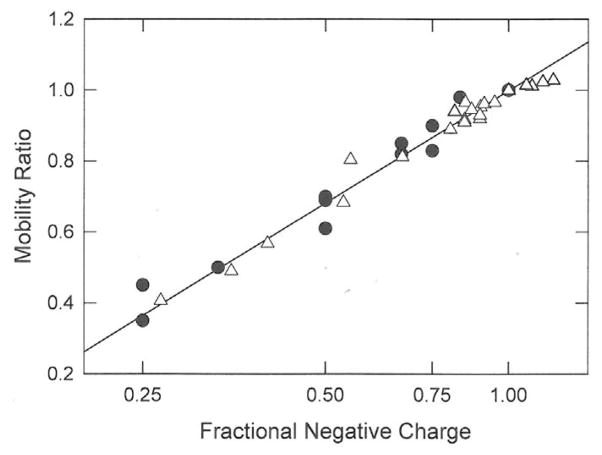
3.5 Comparison with charge variants of small analytes
Several studies of the mobilities of small organic molecules containing variable numbers of carboxylate [18], sulfate [19] or phosphate [33] residues have been reported in the literature. The mobility ratios of these analytes have been calculated by dividing the mobilities of each of the charge variants by the mobility of the molecule in each data set containing the largest number of charged residues. The various organic molecules and their properties are summarized in Table 5. The mobility ratios are plotted in Figure 6 as a function of the logarithm of the fractional negative charge of each charge variant (solid symbols). The open symbols and the straight line in this figure correspond to the DNA data illustrated Figure 5B. In all cases, the mobility ratios of the small multi-charged organic molecules, as well as the DNA charge variants, increase as the logarithm of the fractional negative charge. Hence, the mobilities of small organic molecules with variable charge densities are related to the logarithm of the fractional charge, not the first power of charge as assumed by the Debye-Hückel-Onsager theory [1, 2, 20, 21], or the square root of the number of charged residues as postulated by some authors [18, 19, 33].
Table 5.
Properties of small multi-charged organic molecules taken from the literature.
| Sample | Number of Charged Residues | Mobility, m.u. | Mobility Ratio1 | Reference |
|---|---|---|---|---|
| 2-Naphthalenesulfonic acid | −1 | 3.37 | 0.50 | [19] |
| Naphthalene-2,6-disulfonic acid | −2 | 5.49 | 0.82 | |
| Naphthalene-1,3,6-trisulfonic acid | −3 | 6.75 | 1.00 | |
| AZO-3,6′,6′-trisulfonic acid | −3 | 5.52 | 0.70 | |
| AZO-3,6,3′,6′-tetrasulfonic acid | −4 | 6.71 | 0.85 | |
| AZO-3,6,3′,6′,8′-pentasulfonic acid | −5 | 7.73 | 0.98 | |
| AZO-3,6,8,3′,6′,8′-hexasulfonic acid | −6 | 7.89 | 1.00 | |
|
| ||||
| Benzoic acid | −1 | 3.37 | 0.35 | [18] |
| o-Phthalic acid | −2 | 5.35 | 0.61 | |
| Trimesic acid | −3 | 6.47 | 0.83 | |
| Pyromellitic acid | −4 | 7.30 | 1.00 | |
| Dichondroitin ΔDi-0S | −1 | 1.66 | 0.35 | |
| Dichondroitinesulfate ΔDi-4S | −2 | 2.87 | 0.61 | |
| Dichondroitinesulfate ΔDi-diSB | −3 | 3.94 | 0.83 | |
| Dichondroitinesulfate ΔDi-triS | −4 | 4.73 | 1.00 | |
|
| ||||
| cAMP | −1 | 1.86 | 0.45 | [33] |
| AMP | −2 | 2.77 | 0.69 | |
| ADP | −3 | 3.67 | 0.90 | |
| ATP | −4 | 4.09 | 1.00 | |
Mobility ratio = mobility (charge variant) / mobility (most highly charged derivative).
Figure 6.
Comparison of the mobility ratios observed for the DNA charge variants (△) with those observed for small organic analytes (●), calculated from data in Refs. [18], [19], and [33]. The open symbols and straight line correspond to data in Figure 5B.
4 Concluding remarks
This study has shown that DNA charge variants containing the same number of nucleotides or base pairs, electrophoresed under a given set of experimental conditions, can be compared by calculating the ratio of the mobility of the charge variant to that of the unmodified parent DNA and comparing the mobility ratios to the fractional net charge of the variant. The mobilities of small organic molecules containing different numbers of charged residues can be compared in a similar manner by calculating the ratio of the mobility of the charge variant to that of the most highly charged molecule in the data set. Mobility ratios eliminate most of the contribution of the solvent to the observed mobility, making it possible to compare analytes measured in different BGEs. Mobility ratios are independent of whether the mobilities in a given data set have been extrapolated to zero analyte concentration and/or zero buffer concentration (data not shown). Finally, comparing the mobility ratios with the fractional negative charge of the charge variants in each data set minimizes the effect of differences in analyte size on the measurements, making it possible to compare the mobilities of molecules ranging in size from benzoic acid with a molecular mass of 122 g to 118-bp double-stranded DNA with a molecular mass of ~78 000 g.
The mobility ratios observed for the charge variants of large and small molecules increase linearly with the logarithm of the fractional negative charge, as shown in Figures 5B and 6. Previous studies of mobility as a function of the number of charged residues in a given set of molecules have covered too small a range of charge densities to observe the logarithmic dependence of the mobility on fractional charge. The results observed here are in contrast to most theories of electrophoresis, including the Debye-Hückel-Onsager [1, 2] and Pitts [20, 21] theories, which predict that the mobility of an analyte should increase linearly with the first power of the effective charge, not the logarithm of the effective charge, when the frictional coefficient is held constant. To our knowledge, the only theory that includes a logarithmic dependence of the mobility on the charge of the analyte is the Manning theory of electrophoresis [34], which posits that the mobility of DNA is approximately proportional to the logarithm of the linear charge density. A detailed comparison of the results obtained here with the mobilities predicted by the Manning equation is underway
Acknowledgments
The authors acknowledge S. Yelgaonkar, S. Srivatsan and Y. Tor for the synthesis and characterization of the thymine analogs and J. Dagle for the gift of the DEED-modified oligomers. Partial financial support from Grant CHE0748271 from the Analytical and Surface Chemistry Program of the National Science Foundation (to N.C.S.) and by the Mayo Graduate School, the Mayo Foundation, and National Institutes of Health Grant GM075965 (to L.J.M.) is also acknowledged.
Abbreviations
- BGE
background electrolyte
- bp
base pair
- CE
capillary electrophoresis
- DEED
N,N′-diethylethylenediamine phosphoramidate
- DM
diethylmalonate
- MEA
methoxyethyleneamine phosphoramidate
- m.u.
mobility unit, 1 m.u. = 1 × 10−4 cm2V−1s−1
- nt
nucleotide
- PCR
polymerase chain reaction
Footnotes
The authors have declared no conflict of interest.
References
- 1.Viovy J-L. Rev Mod Phys. 2000;72:813–872. [Google Scholar]
- 2.Bockris JO’M, Reddy AKN. Modern Electrochemistry. Plenum Press; New York: 1998. [Google Scholar]
- 3.Manning GS. Q Rev Biophys. 1978;11:79–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- 4.Stellwagen E, Stellwagen NC. Biophys J. 2003;84:1855–1866. doi: 10.1016/S0006-3495(03)74993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C, Bloomfield VA. Biopolymers. 1994;35:211–216. doi: 10.1002/bip.360350209. [DOI] [PubMed] [Google Scholar]
- 6.Li AZ, Qi LJ, Shih HH, Marx KA. Biopolymers. 1995;38:367–376. doi: 10.1002/(sici)1097-0282(199603)38:3<367::aid-bip9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Li AZ, Huang H, Re X, Qi LJ, Marx KA. Biophys J. 1998;74:964–973. doi: 10.1016/S0006-3495(98)74019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grass K, Holm C. Faraday Disc. 2010;144:57–70. doi: 10.1039/b902011j. [DOI] [PubMed] [Google Scholar]
- 9.Frank S, Winkler RG. J Chem Phys. 2009;131:234905. doi: 10.1063/1.3274681. [DOI] [PubMed] [Google Scholar]
- 10.Wernersson E, Heyda J, Kubíčková A, Křížek T, Coufal P, Jungwirth P. Electrophoresis. 2012;33:981–989. doi: 10.1002/elps.201100602. [DOI] [PubMed] [Google Scholar]
- 11.Ohshima H. Colloids Surfaces A: Physicochem Eng Aspects. 2003;222:207–211. [Google Scholar]
- 12.Penafiel LM, Litovitz TA. J Chem Phys. 1992;96:3033–3038. [Google Scholar]
- 13.Stellwagen NC, Gelfi C, Righetti PG. J Chromatogr A. 1999;838:179–189. doi: 10.1016/s0021-9673(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 14.Stellwagen NC, Bossi A, Gelfi C, Righetti PG. Anal Biochem. 2000;287:167–175. doi: 10.1006/abio.2000.4848. [DOI] [PubMed] [Google Scholar]
- 15.Lu YJ, Stellwagen E, Stellwagen NC. Electrophoresis. 2006;27:1462–1470. doi: 10.1002/elps.200500941. [DOI] [PubMed] [Google Scholar]
- 16.Stellwagen NC, Magnusdottir S, Dagle JM, Gelfi C, Righetti PG. J Chromatogr A. 2000;883:267–275. doi: 10.1016/s0021-9673(00)00415-5. [DOI] [PubMed] [Google Scholar]
- 17.Dong Q, Stellwagen E, Dagle JM, Stellwagen NC. Electrophoresis. 2003;24:3323–3329. doi: 10.1002/elps.200305589. [DOI] [PubMed] [Google Scholar]
- 18.Cottet H, Gareil P. Electrophoresis. 2000;21:1493–1504. doi: 10.1002/(SICI)1522-2683(20000501)21:8<1493::AID-ELPS1493>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Friedl W, Reijenga JC, Kenndler E. J Chromatogr A. 1995;709:163–170. [Google Scholar]
- 20.Pitts E, Tabor BE, Daly J. Trans Faraday Soc. 1970;66:693–707. [Google Scholar]
- 21.Li D, Fu S, Lucy CA. Anal Chem. 1999;71:687–699. doi: 10.1021/ac980843x. [DOI] [PubMed] [Google Scholar]
- 22.Peters JP, Yelgaonkar SP, Srivatsan SG, Tor Y, Maher LJ., III Nucleic Acids Res. 2013;41:10593–10604. doi: 10.1093/nar/gkt808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagle JM, Weeks DL. Nucleic Acids Res. 1996;24:2143–2149. doi: 10.1093/nar/24.11.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Q, Stellwagen E, Stellwagen NC. Biochemistry. 2009;48:1047–1055. doi: 10.1021/bi8020718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stellwagen NC, Gelfi C, Righetti PG. Biopolymers. 1997;42:687–703. doi: 10.1002/(SICI)1097-0282(199711)42:6<687::AID-BIP7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Stellwagen E, Abdulla A, Dong Q, Stellwagen NC. Biochemistry. 2007;46:10931–10941. doi: 10.1021/bi701058f. [DOI] [PubMed] [Google Scholar]
- 27.Chang CY, Stellwagen NC. Biochemistry. 2011;50:9148–9157. doi: 10.1021/bi201263n. [DOI] [PubMed] [Google Scholar]
- 28.Lu YJ, Weers BD, Stellwagen NC. Biophys J. 2005;88:1191–1206. doi: 10.1529/biophysj.104.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musheev MU, Kanoatov M, Krylov SN. J Am Chem Soc. 2013;135:8041–8046. doi: 10.1021/ja402257x. [DOI] [PubMed] [Google Scholar]
- 30.Lamm G, Pack GR. Proc Natl Acad Sci USA. 1990;87:9033–9036. doi: 10.1073/pnas.87.22.9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanlon S, Wong L, Pack GR. Biophys J. 1996;72:291–300. doi: 10.1016/S0006-3495(97)78668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sober HA, editor. CRC Handbook of Biochemistry. The Chemical Rubber Co; Cleveland, Ohio: 1968. pp. J-152–J-154. [Google Scholar]
- 33.Stellwagen E, Stellwagen NC. Electrophoresis. 2007;28:1053–1062. doi: 10.1002/elps.200600487. [DOI] [PubMed] [Google Scholar]
- 34.Manning GS. J Phys Chem. 1981;85:1506–1515. [Google Scholar]