Abstract
Background
Current endoscopic pancreatic function test (ePFT) methods use either secretin or cholecystokinin (CCK) to measure pancreatic function.
Objective
Evaluate a novel ePFT protocol that includes both secretin and CCK stimulation, and assess which fluid parameters best discriminate patients with chronic pancreatitis (CP).
Design
Prospective, cross-sectional diagnostic study.
Setting
Single, tertiary-care institution.
Patients and interventions
Healthy volunteers and patients evaluated for CP were included. All patients underwent a combined secretin-CCK ePFT. Patients evaluated for CP also underwent EUS during the same endoscopic session.
Main outcome measurements
Duodenal fluid bicarbonate, lipase, and amylase concentrations were measured after CCK and secretin stimulation. Results were compared based on the presence of CP detected by EUS (≥5 features)
Results
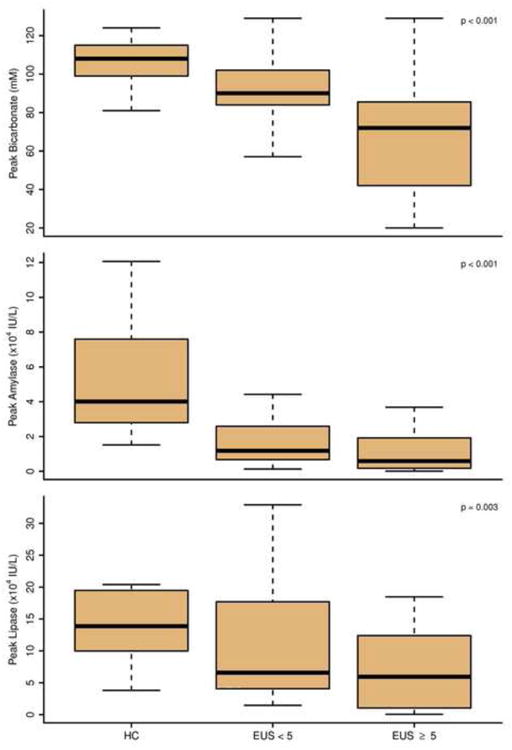
Twenty healthy volunteers and 69 patients evaluated for CP completed the SC ePFT. Patients with EUS score ≥5 had significantly decreased peak bicarbonate concentrations (72 mM) compared with patients with EUS score <5 (90 mM) and healthy subjects (108 mM) (p<0.001). Peak concentrations of amylase and lipase and total fluid volume were not significantly different between patients with CP and controls. ROC analysis revealed that peak bicarbonate concentration had superior discrimination for CP (AUC=0.738) compared with peak amylase (AUC 0.677) and peak lipase (AUC 0.627). The addition of enzyme concentration measurement did not improve discrimination compared with peak bicarbonate alone.
Limitations
SC ePFT results were not compared with single hormone ePFTs.
Conclusions
The addition of CCK infusion and enzyme concentration measurement to a standard secretin ePFT does not enhance the diagnosis of CP.
Keywords: hormone, exocrine insufficiency, CCK, enzyme, bicarbonate, endoscopic ultrasound
Introduction
Hormone-stimulated pancreatic function tests (PFTs) detect mild exocrine insufficiency which may be associated with early chronic pancreatitis (CP). Traditional “tube-based” PFTs have practical limitations which limit their performance to specialized centers. Endoscopic PFTs (ePFTs) have been developed which simplify the performance of PFTs. The ePFT involves timed collection of duodenal fluid samples through the suction channel of the endoscope, and can be performed by any gastroenterologist.
Secretin ePFTs measure bicarbonate concentration as an expression of duct-cell secretion.1,2 , A bicarbonate concentration of ≥80 IU/L after secretin stimulation is considered normal. 1,2 Cholecystokinin (CCK) ePFTs measure enzyme concentrations as an expression of acinar-cell secretion. A previous study of CCK-ePFT has shown that a lipase concentration of ≥810,000 IU/L can distinguish healthy subjects from patients with chronic pancreatitis.3 Either aspect of exocrine function may decline first in early CP.4 As such, a comprehensive ePFT might require both hormones for stimulation.
In this prospective study, we evaluated a novel ePFT protocol with both secretin and CCK. Our aim was to determine which fluid parameters were most accurate in distinguishing patients with EUS structural changes of CP, and whether use of both hormones improves diagnosis of CP.
Methods
Study Design
A single-center prospective cross-sectional diagnostic study was conducted. The study was approved by the Cleveland Clinic Institutional Review Board. All patients underwent ePFT using secretin and CCK (SC ePFT). Patients evaluated for CP also underwent EUS at the time of the SC ePFT as part of their clinical evaluation.
Study Population
Recruitment occurred between September 2008 and December 2010. A focused history and physical exam was conducted using a standardized data collection form. Female subjects underwent a urine pregnancy test before the endoscopic procedure. Inclusion criteria included age ≥18 years, ability to give informed consent, and fitness to undergo an endoscopic procedure. Exclusion criteria included: pregnancy, severe cardiac, pulmonary, or renal disease, recent use of anticholinergic medications or octreotide, recent acute pancreatitis, and a history of gastrointestinal conditions known to impair pancreatic secretion (celiac disease, cirrhosis, gastric or pancreatic surgery, cystic fibrosis). Two groups were enrolled:
Healthy asymptomatic volunteers underwent an SC ePFT in the Cleveland Clinic Clinical Research Unit. Additional exclusion criteria for these subjects included current or former smoking (≥5 pack years), significant past or present alcohol use (≥4 drinks per week), history of drug abuse, recent use of narcotic medications, chronic or recurrent abdominal pain, and history of acute or chronic pancreatitis. Healthy volunteers were given a cash stipend for their participation.
Consecutive patients evaluated for abdominal pain of suspected pancreatic origin underwent combined SC ePFT and EUS. The EUS images were scored based on the presence of 9 parenchymal and ductal criteria (hyperechoic foci, hyperechoic strands, lobularity, cysts, main duct dilation, main duct irregularity, visible side-branches, hyperechoic duct wall, and calcifications). A score of ≥5 was considered positive.5
S-C ePFT Protocol
Endoscopic procedures were performed by a single endoscopist (TS). The procedure lasted approximately 50 minutes [Figure 1]. Patients were placed in leftward supine and reverse Trendelenberg position to maximize pooling of fluid in the dependent portion of the duodenum. Conscious sedation using meperidine and midazolam was administered for healthy volunteers. 6 Propofol-based monitored anesthesia care was used for the majority of the patients evaluated for CP.
Figure 1. Combined EUS and S-C ePFT procedure.
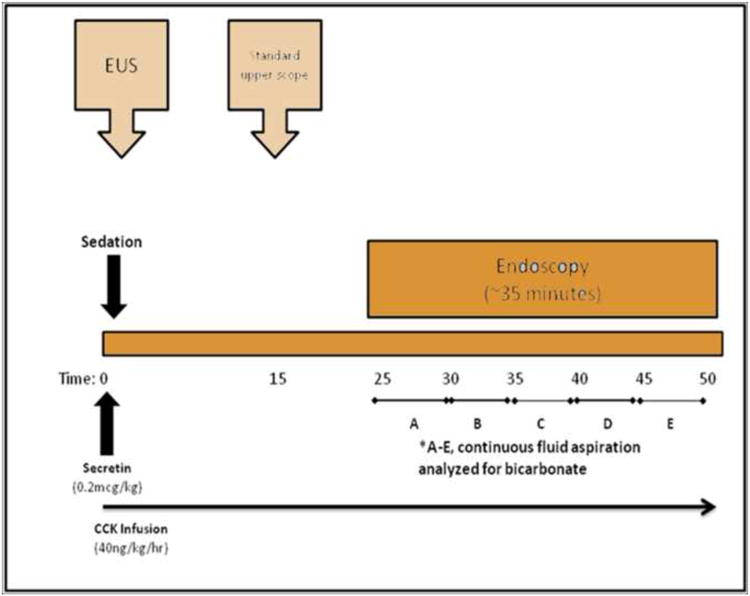
Hormones were administered intravenously starting at time 0. First, an infusion of CCK (Kinevac ®, Bracco Diagnostics Inc., Princeton, NJ) was initiated at 40 ng/kg/hour. Next, a 0.2 microgram test dose of synthetic human secretin was administered (ChiRhoStim®, ChiRhoClin Inc., Burtonsville, MD). After 5 minutes, the standard dose of synthetic human secretin (0.2μg/kg) was administered as an intravenous bolus. A linear-array echoendoscope was passed to examine the head, body, and tail of the pancreas from gastric and duodenal stations (time 0-15). The echoendoscope was withdrawn and a standard upper endoscope was passed into the stomach. All gastric fluid was thoroughly aspirated and discarded. The endoscope was passed through the pylorus and positioned in the second portion of the duodenum. Residual duodenal fluid was aspirated and discarded. Timed duodenal fluid samples were suctioned continuously through the endoscope into a fluid trap at times 25-29, 30-34, 35-39, 40-45, and 46-50 minutes. The time of collection was based on previous studies showing peak concentrations of bicarbonate and pancreatic enzymes at 30-50 minutes after hormonal stimulation.7,8
Each sample was placed on ice and analyzed within 3 hours for lipase, amylase, and bicarbonate concentrations using a hospital autoanalyzer.9 The highest concentrations of lipase, amylase, and bicarbonate were considered the peak concentrations.
Statistical Methods
Descriptive statistics were computed for all variables, including means, standard deviations and percentiles for continuous variables, and frequencies and percentages for categorical factors. Analysis of Variance (ANOVA) or the non-parametric Kruskal-Wallis tests were used for continuous factors and Pearson's chi-square tests for categorical variables. Ad-hoc pairwise comparisons were done using the Steel-Dwass procedure for multiple comparisons and Fisher's Exact tests for categorical factors. Receiver operating characteristics (ROC) analysis was performed to assess the ability of fluid parameters to discriminate patients with positive and negative EUS. After choosing the final prediction model based on the AUC values, internal validation was performed and confidence limits generated using a bootstrapping technique.
A p value < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 software (The SAS Institute, Cary, NC) and R version 2.12.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Twenty healthy subjects completed the SC ePFT. Sixty-nine patients evaluated for CP completed the combined SC ePFT and EUS. Fifty-eight of the 69 patients evaluated for CP had negative or equivocal preceding CT scans. Sixteen of the 69 patients were found to have ≥5 EUS features suggestive of CP. The demographic and clinical characteristics of the groups and the PFT parameters are summarized in Table 1. No procedural complications or pharmacological reactions were noted during the study.
Table 1. Demographic and Clinical Characteristics.
| Factor | HC (N=20) | EUS<5 (N=53) | EUS>=5 (N=16) | p-value |
|---|---|---|---|---|
| Age (yrs) | 34.6±9.6 23 | 43.9±11.6 1 | 47.9±12.0 1 | 0.001 |
| Male | 10(50.0) | 15(28.3) | 9(56.3) | 0.061 |
| White | 12(60.0) 2 | 48(90.6) 1 | 15(93.8) | 0.003 |
| Smoking | <0.001 | |||
| •Never | 20(100.0) 23 | 25(47.2) 1 | 5(31.3) 1 | |
| •Former | 0(0.0) | 11(20.8) | 4(25.0) | |
| •Current | 0(0.0) | 17(32.1) | 7(43.8) | |
| Alcohol | 0(0.0) 3 | 9(17.0) 3 | 8(50.0) 12 | <0.001 |
| Acute Pancreatitits | 0(0.0) 23 | 21(39.6) 13 | 12(75.0) 12 | <0.001 |
| Total Volume (mL)* | 66.0[43.5,92.0] 3 | 50.0[31.0,65.5] | 39.0[32.5,50.0] 1 | 0.005 |
| Peak Lipase concentration (×10 IU/L) | 13.9[10.0,19.5] 23 | 6.6[4.1,17.7] 1 | 5.9[1.04,12.4] 1 | 0.003 |
| Peak Bicarbonate concentration (IU/L) | 108.0[99.0,115.0] 23 | 90.0[84.0,102.0] 13 | 72.0[42.0,85.5] 12 | <0.001 |
| Peak Amylase concentration (×10 IU/L) | 4.0[2.8,7.6] 23 | 1.2[0.67,2.6] 1 | 0.58[0.17,1.9] 1 | <0.001 |
Data not available for all subjects. Missing values: Total Volume (mL) = 1.
Values presented as Mean ± SD with ANOVA; Median [P25, P75] or Median (min, max) with Kruskal-Wallis test, or N (%) with Pearson's chi-square test unless otherwise stated.
Significantly different from HC
Significantly different from EUS<5
Significantly different from EUS>=5
A significance level of 0.017 was used for pairwise ad-hoc comparisons.
Figure 2 demonstrates the distribution of bicarbonate and enzyme concentrations Each of the four parameters (peak bicarbonate, peak amylase, peak lipase, and volume) demonstrated significant overall differences in the 3 groups based on ANOVA. The parameters were then subjected to pairwise comparisons to assess their ability to distinguish patient groups (threshold p-value <0.017). For peak bicarbonate, significant differences were observed for each of the three pairwise comparisons [Table 1]. Peak lipase and peak amylase were significantly different between healthy subjects and those evaluated for CP; however, neither was significantly different between those with ≥5 or <5 EUS features. Total volume was only found to be significantly different between healthy subjects and those with ≥5 EUS features.
Figure 2. Bicarbonate and enzyme results stratified based on patient group.
Logistic regression analysis was done to compare the accuracies of S-C ePFT variables for diagnosing CP based on the EUS gold-standard, and to generate an optimal prediction model [Table 2]. Peak bicarbonate had superior overall accuracy (ROC AUC 0.738) compared with either peak amylase (AUC 0.677) or peak lipase (AUC 0.627). Combining ePFT parameters minimally improved predictive ability. The AUC for the model including peak bicarbonate and peak amylase was 0.746.
Table 2. ROC Analysis.
| ROC Model | AUC (95% CI) |
|---|---|
| Peak Bicarbonate | 0.738 (0.570, 0.906 |
| Peak Amylase | 0.677 (0.508, 0.845) |
| Peak Lipase | 0.627 (0.451, 0.803) |
| Peak Bicarbonate & Peak Amylase | 0.746 (0.589, 0.904) |
AUC: area under ROC curve
Discussion
This is the first report of an ePFT in adults using combined hormone stimulation. We found that the SC ePFT is technically feasible and produced no adverse events. However, the additional enzyme concentration data did not reliably distinguish groups based on EUS structural severity, and did not improve the discrimination compared with bicarbonate alone. We conclude that adding CCK to the secretin ePFT does not augment the test's diagnostic ability.
Sophisticated tube-based SC PFT protocols have included gastric perfusion of inert markers such as mannitol.10-13 Measurement of mannitol concentration in the duodenal fluid adjusts for distal losses and produces a more accurate estimation of total volume. An accurate volume measurement allows calculation of the total enzyme output, the gold standard for acinar secretory capacity. However, perfusion markers add significant complexity based on requirement for a second orogastric tube and specialized laboratory analysis, and are not feasible for ePFT protocols. Therefore, we studied an SC ePFT with measurement of enzyme concentrations, rather than enzyme outputs. We hypothesized that enzyme concentrations would be decreased in patients with CP detected by EUS. Instead, we found that enzyme concentrations do not reliably distinguish patients with and without CP.
Previous studies of the single-hormone CCK ePFT have shown lower enzyme concentrations in patients with CP.3 The relative inaccuracy of enzyme measurement we observed with combined stimulation is most likely due to the dilutional effect of secretin. Secretin causes an increase in water secretion through the pancreatic ductules. This water production is variable and decreases with worsening CP due to loss of duct cell mass, which may paradoxically elevate enzyme concentrations. In a previous study, we found elevated enzyme concentrations in secretin-stimulated fluid of patients with CP compared with healthy controls.14
We confirmed that bicarbonate concentrations are reliable measurements of duct-cell function and negatively correlate with EUS structural features.4,15 In contrast to the above-stated dilutional effect of secretin on CCK-induced enzyme concentrations, the reverse is unlikely because CCK does not generate substantial volume that would dilute bicarbonate concentrations.
Our study has two main limitations that bear mentioning. First, we used EUS as our structural reference standard despite the well documented concerns related to intra-and inter-observer variability. We recognize the imperfections related to this modality, however, most experts would agree that EUS remains the most useful test to evaluate pancreatic parenchymal and ductal changes in the absence of calcifications on cross-sectional imaging. Second, our patient cohort is relatively small, which may impact our ability to definitively establish differences between patient groups.
Based on our current findings, the addition of CCK stimulation and enzyme concentration measurements to the standard secretin ePFT provides no additional diagnostic benefit. At the present time, ePFT protocols using single hormonal stimulation with either secretin or CCK may still provide useful information about acinar- and duct-cell function, respectively.
Acknowledgments
Funding for this investigator-initiated study was provided by Abbott. Portions of this study were performed in The Cleveland Clinic Clinical Research Unit, supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio.
Acronyms
- CCK
cholecystokinin
- CP
chronic pancreatitis
- ePFT
endoscopic pancreatic function test
- EUS
endoscopic ultrasound
- PFT
pancreatic function test
- SC ePFT
secretin and cholecystokinin endoscopic pancreatic function test
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest relevant to this research
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conwell DL, Zuccaro G, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57:37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- 2.Stevens T, Conwell DL, Zuccaro G, et al. A randomized crossover study of secretin-stimulated endoscopic and Dreiling tube pancreatic function test methods in healthy subjects. Am J Gastroenterol. 2006;101:351–5. doi: 10.1111/j.1572-0241.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 3.Conwell DL, Zuccaro G, Vargo JJ, et al. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2003;1:189–94. doi: 10.1053/cgh.2003.50028. [DOI] [PubMed] [Google Scholar]
- 4.Stevens T, Dumot JA, Zuccaro G, et al. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114–9. doi: 10.1016/j.cgh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–64. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 6.Morrow JB, Zuccaro G, Jr, Conwell DL, et al. Sedation for colonoscopy using a single bolus is safe, effective, and efficient: A prospective, randomized, double-blind trial. Am J Gastroenterol. 2000;95:2242–7. doi: 10.1111/j.1572-0241.2000.02308.x. [DOI] [PubMed] [Google Scholar]
- 7.Stevens T, Conwell DL, Zuccaro G, et al. The efficiency of endoscopic pancreatic function testing is optimized using duodenal aspirates at 30 and 45 minutes after intravenous secretin. Am J Gastroenterol. 2007;102:297–301. doi: 10.1111/j.1572-0241.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 8.Conwell DL, Zuccaro G, Morrow JB, et al. Analysis of duodenal drainage fluid after cholecystokinin stimulation in healthy volunteers. Pancreas. 2002;25:350–4. doi: 10.1097/00006676-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Z, Lopez R, Parsi MA, et al. Comparison of autoanalyzer and back titration for measurement of bicarbonate concentration in endoscopically collected pancreatic fluid. Pancreas. 2011;40:237–41. doi: 10.1097/MPA.0b013e3181f82aa3. [DOI] [PubMed] [Google Scholar]
- 10.Sun DC, Shay H. Pancreozymin-secretin test the combined study of serum enzymes and duodenal contents in the diagnosis of pancreatic disease. Gastroenterology. 1960;38:570–81. [PubMed] [Google Scholar]
- 11.Burton P, Evans DG, Harper AA, et al. A test of pancreatic function in man based on the analysis of duodenal contents after administration of secretin and pancreozymin. Gut. 1960;1:111–24. doi: 10.1136/gut.1.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chey WY, Shay H, Nielsen OF. Diagnosis of diseases of the pancreas and biliary tract evaluation of pancreozymin-secretin test. JAMA. 1966;198:257–62. [PubMed] [Google Scholar]
- 13.Nundy S, Baron JH, Beales JS, et al. A simultaneous combined pancreatic test. Gut. 1972;13:844. [PubMed] [Google Scholar]
- 14.Stevens T, Conwell DL, Zuccaro G, et al. Enzyme content in secretin-stimulated duodenal aspirates is paradoxically elevated in patients with chronic pancreatitis. Pancreas. 2005;31:A470. [Google Scholar]
- 15.Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig Dis Sci. 2007;52:1206–10. doi: 10.1007/s10620-006-9469-6. [DOI] [PubMed] [Google Scholar]


