SIRT6 is a member of the Sir2 (Silent Information Regulator-2) family of genes, which regulate fundamental processes in aging and lifespan control in multiple organisms.1-3 SIRT6 deficiency in mice results in genomic instability, metabolic defects, and degenerative phenotypes associated with aging.1 Previously, we showed that SIRT6 deacetylates lysine 9 on the N-terminal tail of histone H3 (H3K9Ac) to modulate telomeric chromatin and gene expression.2,3 Here, we identify a second substrate of SIRT6 at chromatin, lysine 56 on the globular core of histone H3 (H3K56Ac). We show that SIRT6 deacetylates H3K56Ac in vitro and in cells, and identify a physiologic role for this activity in maintaining dynamic changes of H3K56 acetylation levels at telomeric chromatin over the cell cycle. Together, these findings provide the first analysis of how H3K56Ac levels are dynamically regulated at human telomeres in response to a mammalian SIRT.
Acetylation of H3K56 occurs in S. cerevisiae both globally on newly synthesized histones and at specific promoters during S-phase, and proper regulation of this histone mark is important for genomic stability, gene activity and heterochromatin silencing, and histone incorporation into nucleosomal chromatin in DNA replication and repair.4-8 H3K56Ac can be deacetylated in S. cerevisiae by Sir2 and its family members Hst3 and Hst4.9-11 In mammals, regulation of H3K56Ac has recently been linked to stem cell-specific transcriptional networks,chromatin responses to DNA damage, and genomic stability.12-15 Mammalian Sir2 proteins SIRTI and SIRT2 can deacetylate H3K56Ac, but no H3K56Ac deacetylase activity has been detected for the other mammalian SIRTs.12,13,15
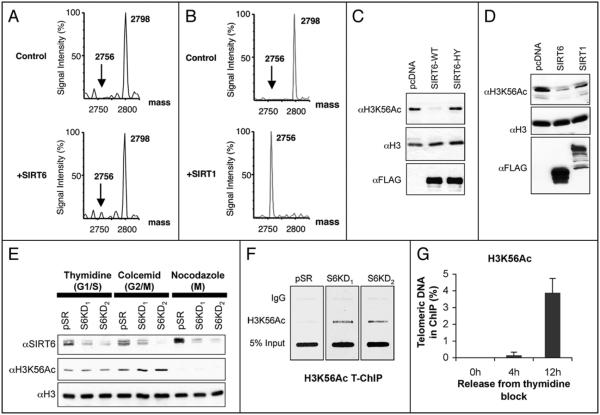
Because SIRT6 has specificity for deacetylating H3K9Ac,2 we compared the sequence context of H3K9 to other acetylated histone residues and found that both H3K56 and H3K9 are present in the sequence K*ST, where K* is the acetylated residue. To ask whether H3K56Ac can serve as a SIRT6 substrate, we performed in vitro deacetylation reactions on an acetylated H3K56 peptide and assessed deacetylation by mass spectrometry. This analysis revealed a modest but reproducible deacetylation of the H3K56Ac peptide by SIRT6 (Fig. 1A). A similar mass spectrometry analysis also revealed virtually complete deacetylation of the H3K56Ac peptide by SIRT1 (Fig. 1B). Thus, using this assay, the H3K56Ac deacetylase activity of SIRT6 is less efficient than that of SIRT1, but is comparable to the H3K9Ac deacetylation activity previously shown for SIRT6.2 We next confirmed the SIRT6 activity in cells; wild-type SIRT6, but not the catalytically inactive SIRT6-H133Y mutant protein, efficiently deacetylated H3K56Ac when expressed in 293T cells (Fig. 1C). Notably, in contrast to the findings from the in vitro peptide assays, H3K56 was deacetylated more efficiently in cells by SIRT6 than by SIRT1 (Fig. 1D). Together, these analyses reveal H3K56Ac as a new substrate for SIRT6, and also provide antibody-independent mass spectrometry validation of the H3K56Ac deacetylase activities of both SIRT6 and SIRT1.
Figure 1.
Evidence for global and telomere-specific regulalion of H3K56Ac by human SIRT6. (A) Mass spedrometry analysis showing in vilro deacelylation by SIRT6 of an acelylaled H3K56 peplide (GTVALREIRRYQK(Ac)STELLIRK), shown by the appearance of a SIRT6-dependent peak (arrow) 42 Da smaller than the acetylaled peptide Molecular weighls (Da) of the acetylated and deacetylated peptide peaks are indicated. NAD-dependent deacelylation reactions were performed as previously described.2 (B) Mass spectrometry as in (A) showing in vitro deacetylation of H3K56Ac peptide by SIRT 1. (C) Western analysis of whole cell extracts from cells expressing Flag-tagged wild-type SIRT6 (SIRT6-WT), calalytically inactive SIRT6 (SIRT6-HY), or empty vector control (pcDNA). (D) Western analysis of whole cell extracts from cells expressing Flag-tagged SIRT6 or SIRT1 compared to pcDNA control. (E) Western analysis of global H3K56Ac levels in SIRT6 knockdown (S6KD1 and S6KD2) or control (pSR) cells following cell cycle arrest due to thymidine, colcemid or nocodazole blocks. The corresponding cell cycle phases enriched in these cultures are indicated. (F) Telomere ChIP analysis showing hyperacetylation of H3K56 at telomeres in S-phase-enriched U2OS cell cultures. IgG ChIP and input DNA are present as controls. (G) Quantification of Telomere ChIP analyses in U2OS cells at 4 and 12 hours following release from a thymidine block, corresponding to S and G2/M phases, respectively. In (C-G), antibodies were αH3K56Ac (Epitomics); αflag (Sigma), αH3 (Abcam); αSIRT6 antibodies were previously described.2
We next asked whether SIRT6 is important for determining the physiologic levels of global H3K56Ac in cells. Western analysis on whole cell extracts of exponentially growing U20S cells revealed no significant difference in global H3K56Ac levels in SIRT6-knockdown cells compared to control cells (data not shown). In budding yeast, H3K56 is acetylated in S-phase and then deacetylated by Hst3 and Hst4 as cells progress through G2/M.9-11 By analogy, it is possible that SIRT6 might be important for deacetylating H3K56Ac in mammalian cells in specific cell cycle phases. Therefore, we compared H3K56Ac levels in SIRT6 knockdown cells (generated by two independent shRNAs2) and control cells, arrested in the cell cycle via thymidine block, colcemid treatment, or nocodazole shake-off–treatments that enriched for G1/S, G2/M and mitotic cells, respectively (data not shown). SIRT6 knockdown had no significant effect on H3K56Ac levels in the thymidine- or nocodazole-synchronized cells, but resulted in a subtle hyperacetylation of H3K56Ac under colcemid conditions, suggesting a potential role for SIRT6 in influencing global H3K56Ac levels in G2 or early mitosis (Fig . 1E). Notably, this experiment also revealed substantial differences in H3K56Ac levels under the different cell cycle arrest treatments, independent of SIRT6 levels: colcemid-treated cells had slightly higher levels of H3K56Ac than thymidine-treated cells, whereas H3K56Ac was strikingly reduced in mitotic cells isolated by nocodazole shakeoff (Fig. 1E). These observations suggest that a dramatic deacetylation of H3K56Ac is triggered by nocodazole treatment, and this process is not affected by depletion of SIRT6 alone. This regulated event could reflect a physiologic mitotic transition, or could be specific to the toxicity of the nocodazole block. It is possible that other mammalian SIRTs contribute to this deactylation event and may be redundant with SIRT6 for this process.
We previously showed that SIRT6 associates dynamically with telomeric chromatin in human U20S cells, with a peak in S-phase of the cell cycle, and that H3K9 is hyperacetylated at telomeres in SIRT6-depleted S-phase cells.2 To study the interplay between SIRT6 and H3K56Ac levels at telomeres, we carried out Telomere Chromatin Immunoprecipitation (T-ChIP) analyses for H3K56Ac. SIRT6 knockdown and control cultures were enriched for S-phase cells via timed release from a thymidine block,and telomeric DNA present in H3K56Ac ChIPs was compared by hybridization to a telomer-repeat probe. H3K56Ac was barely detectable by T-ChIP in control pSR cells, but was dearly present and hyperacetylated in SIRT6 knockdown cells (Fig. 1F) . Similar results were observed with two independent shRNAs, ruling out off-target effects. In contrast, total histone H3 levels at telomeres were not different in the SIRT6 knockdown versus control cells under these conditions (data not shown).
We next asked whether H3K56Ac is present at human telomeric chromatin under physiologic conditions (in the absence of SIRT6 depletion), and if so, whether levels of this mark fluctuate over the cell cycle. Cells were released from a thymidine block as before, and harvesled for T-ChIP at 4 and 12 hours, which correspond to cultures containing predominantly S phase and G2/M phase cells, respectively.2 This analysis revealed a dramatic increase in H3K56Ac levels at the 12-hour time-point (fig. 1G) In contrast to our results at 4 hours, SIRT6 knockdown did not affect H3K56Ac levels at 12 hours (data not shown). The lack of an effect of SIRT6 depletion under these conditions could indicate that SIRT6 does not play a large role in controlling H3K56 levels at telomeres in G2/M, which would be consistent with our previous finding that SIRT6 occupancy at telomeric chromatin peaks in S phase but is substantially decreased by G2/M.2 The dramatic increase in H3K56Ac observed at telomeres in G2/M may be due to regulated activily of other H3K56 deacetylases, such as SIRT1 or SIRT2, or of a yet-to-be identified H3K56 acetyltransferase that could operate at human telomeric chromatin. Together these data provide the first demonstration that H3K56Ac is present at human telomeres and reveal dynamic fluctuation of H3K56Ac levels at telomeric chromatin over the cell cycle, in part dependent on SIRT6.
In conclusion, our study identifies H3K56Ac as a novel substrate for SIRT6, demonstrates an important physiologic role for SIRT6 in modulating this chromatin mark at telomeric chromatin, and provides the first analysis of how H3K56Ac levels in human cells are differentially regulated at telomeres and globally in response to cell cycle arrest. Our findings also suggest new testable hypotheses and models for how SIRT6 may impact on genomic stabilily and DNA damage responses. For example, we recently showed that SIRT6 associates with chromatin flanking induced, site-specific DNA double strand breaks, where it stabilizes the central DNA repair factor DNA-PKcs.16 Our new findings suggest that SIRT6 deacelylation of H3K56Ac might operate at chromatin directly at such sites of damaged DNA, and defects in such a mechanism could underlie aspects of the genomic instability, DNA damage hypersensitivity, and defective DNA repair phenotypes that are observed in SIRT6-deficient cells.1,2,16 It is also possible that in SIRT6 knockdown cells, hyperacetylation of H3K56Ac at telomeres could impact on telomeric chromatin structure and contribute to the telomere dysfunction and genomic instability that result from SIRT6 depletion. Future studies should further elucidate the mechanistic interplay and functional consequences of SIRT6, H3K9Ac and H3K56Ac at telomeric chromatin in human cells. Finally, our discovery of the H3K56Ac deacetylation activity of SIRT6 provides a new link between H3K56Ac control and a central regulator of aging-related molecular pathways.
Acknowledgements
The authors thank Mara Damian and Elisabeth Berber for technical assistance, Ruth Tennen for comments on the manuscript and David Lombard for communicating unpublished results. This work was supported by NIH grants (K.F.C. and O.G.), NIH training grant fellowships (R.A.M. and T.H.) and the Department of Veterans Affairs Merit Review (K.F.C.). E.M. was supported by a Stanford Comprehensive Cancer Center Fellowship and the Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship. K.F.C. is a Paul Beeson Scholar and an Ellison Medical Foundation New Scholar in Aging.
References
- 1.Mostoslavsky R, et al. Cell. 2006;124:315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Michishita E, et al. Nature. 2008;452:492–6. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawahara TLA, et al. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu F, et al. Cell. 2005;121:375–85. doi: 10.1016/j.cell.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, et al. Cell. 2008;134:231–43. doi: 10.1016/j.cell.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masumoto H, et al. Nature. 2005;436:294–8. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 7.Han J, et al. Science. 2007;315:653–5. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll R, et al. Science. 2007;315:649–52. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celic I, et al. Curr Biol. 2006;16:1280–9. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Xu F, et al. Mol Cell. 2007;27:890–900. doi: 10.1016/j.molcel.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maas NL, et al. Mol Cell. 2006;23:109–19. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Das C, et al. Nature. 2009;459:113–7. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, et al. Cell Cycle. 2009;8:1747–53. doi: 10.4161/cc.8.11.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie W, et al. Mol Cell. 2009;33:417–27. doi: 10.1016/j.molcel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjeertes JV, et al. EMBO J. 2009;28:1878–89. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCord R, et al. Aging. 2009;1:109–21. doi: 10.18632/aging.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]