Abstract
Since the identification of matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, as being a driving factor for cancer progression and patient prognosis, MMPs have been studied extensively. Although early programs targeting MMPs were largely unsuccessful in clinical trials, they remain a viable and highly desirable therapeutic target based on preclinical studies and their role in disease progression. As information regarding the structure and function of these proteinases is compiled and biotechnology evolves, tools to develop better inhibitors are within our grasp. Improved methods for high throughput screening and in silico drug design programs have identified compounds which are highly potent, have high binding affinities, and exhibit favorable pharmacokinetic profiles. More recently, advances in drug delivery methods or compounds which bind outside the active site have brought new light to the field. In this review, we highlight the role of MMPs in cancer, clinical trials for MMP inhibitors, and novel approaches to targeting MMPs in cancer.
Keywords: Cancer, Inhibitors, Matrix metalloproteinases, Metastasis, MMPs
Introduction to MMPs in cancer
Matrix metalloproteinases, or MMPs, are responsible for remodeling the extracellular matrix (ECM). Such remodeling processes are necessary for a vast and varied array of physiological events, such as wound repair, organismal growth and development, and mediation of immune responses. However, dysregulation of MMPs has been observed in an equally diverse index of diseases. From pulmonary disorders to autoimmune diseases to cancer (the focus of this review), MMPs have been found to directly contribute to disease progression.1, 2, 3, 4
Cancer research has traditionally focused largely on cancer cell mutations that confer either proliferative or survival advantages. The tumor microenvironment, particularly the extracellular matrix, is now emerging as a key player in influencing cancer progression. MMPs are present in nearly all human cancers; they can be expressed by healthy fibroblasts in the adjacent stroma, cancer-associated fibroblasts, and/or by non-fibroblastic cancer cells. This is of great significance, as MMPs can influence the tumor environment by promoting angiogenesis, tumor growth, and metastasis.5, 6 Accordingly, MMP expression is tied to tumor aggressiveness, stage, and patient prognosis.7, 8
Transcription of MMPs is tightly regulated and expression is generally very low. Further regulation of MMP activity occurs by post-translational modification, production of the enzymes as zymogens requiring activation, and co-expression of tissue inhibitors of metalloproteinases (TIMPs).9, 10 Dysregulation of any of these regulatory mechanisms during pathological conditions may contribute to worsening of disease.9
Increased expression of MMPs is correlated to increased cancer cell proliferation and an increase in tumor size. Overexpression of MMP-3 in the phenotypically normal murine mammary epithelial cell line, SCp2, using a tetracycline inducible system injected into surgically cleared mammary tissue has been shown to be sufficient to induce spontaneous disease progression.11 Similarly, MMP-2 levels detected in cancer tissue are significantly correlated to larger tumor size.12 Several MMPs have been shown to drive cell migration and invasion through the basement membrane. Sequence-specific silencing of MMP-14 alone significantly attenuates both migration and invasion of cancer cells in vitro.13 Similarly, silencing of MMP-9 decreases the ability of glioma cells to migrate or invade.14 Further, the number of metastatic lesions observed in xenografts of MMP-9 knockdown triple negative breast cancer cells was significantly fewer than in xenografts of cells transduced with a non-target lentivirus.15 In a mouse xenograft model of melanoma, increased levels of MMP-2 correlated to a more malignant phenotype.16 Finally, MMPs have been shown to contribute to angiogenesis by degrading basement membranes, allowing for endothelial cell invasion. They also cleave factors that increase or maintain the angiogenic phenotype.17 Nearly every member of the MMP family has been found to be dysregulated in human cancers, with MMP-1,-2,-7,-9,-13, and -14 at the top of this list. Together, these factors all point to MMPs as attractive targets for therapeutics.
MMP substrate specificity
MMPs are calcium-dependent endopeptidases which require coordination of a zinc ion to mediate catalysis. As implied by their name, substrates of MMPs span a vast assortment of extracellular components and the enzyme substrate preferences have been used to classify this family into sub-groups (Table 1). True collagenases are those MMPs which can cleave the major fibrillar collagens (types I, II, and III) at a precise site in the triple helical structure to produce ¾ and ¼ segments.9 Gelatinases proteolyse denatured collagen, also known as gelatin. They also cleave bioactive signaling molecules, including chemokines, as well as activate certain other MMPs. Substrates of stromelysins are among the most diverse, including proteoglycans, fibronectin, laminin, casein, gelatin, types III, IV, IX and X collagen. This subgroup also has the capacity to activate certain other MMPs. While the previous groups are all secreted proteases, matrilysins are an interesting group as they can act either be secreted or act intracellularly. When active within the cytoplasm of a cell, they are involved in maintenance of the innate immune system by activating defensin, a peptide with antibacterial functions.18 Their extracellular functions involve mediation of cell-adhesion (by degrading VE-cadherin) and hydrolysis of certain matrix components, including fibronectin, type IV collagen, and proteoglycans.19
Table 1.
Substrate specificities of matrix metalloproteinases.
| Group | MMP | Major ECM substrates |
|---|---|---|
| Collagenases | MMP-1, MMP-8, MMP-13, MMP-18 | Fibrillar collagens types I, II, III, VII, VIII and X, gelatin, aggrecan |
| Gelatinases | MMP-2, MMP-9 | Gelatin, collagen types IV, V, VII, X, and XIV, gelatin, elastin, galectin-3, aggrecan, fibronectin |
| Stromelysins | MMP-3, MMP-10, MMP-11, MMP-12 | Proteoglycans, fibronectin, laminin, casein, gelatin, collagen types III, IV, IX and X |
| Matrilysins | MMP-7, MMP-26 | Fibronectin, laminin, type IV collagen, proteoglycans, VE-cadherin |
| Membrane-type | MMP-14, MMP-15, MMP-16, MMP-25 | Large tenascin-C, fibronectin, laminin |
The final major group of MMPs encompasses those which are localized at the extracellular side of cell membranes. Substrates of these MMPs include collagen as well as other cell surface molecules and some bioactive molecules.20 Because of their localization to the cell surface, these MMPs are also involved in processes and signaling pathways allowing for angiogenesis, cell migration and invasion, and other cellular functions such as proliferation, apoptosis, and differentiation.21
Owing largely to their vast substrate diversity, MMPs participate in nearly every biological process which involves remodeling of the ECM, from implantation of an embryo into the uterine wall22 to tissue necrosis. It comes as no surprise then that altered expression or activity levels of MMPs is associated with an expansive and extensive array of pathologies.23
Several MMPs have been identified which fall outside of the above described classification system. These “other” MMPs include several isoforms expressed in non-primates of which little is known about substrate preference.
MMP structure
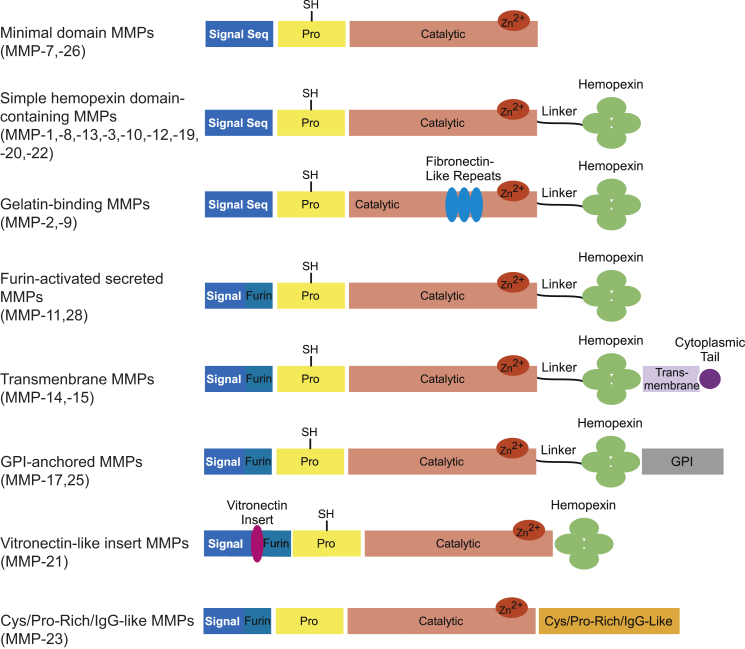
As more MMP family members continue to be discovered and the breadth of substrates is elucidated, a new paradigm for classifying MMPs has emerged which groups the proteases based on structural similarity. In this approach, the known 23 paralogs of vertebrate MMPs are split into eight groups, five of which represent the secreted MMPs and three of which encompass the membrane-type MMPs (Fig. 1).24, 25 Nearly all MMPs share certain structural elements; these include an N-terminal signaling sequence which dictates the localization of each MMP, a propeptide region, a calcium-dependent active site which coordinates a catalytic zinc ion, a linker region of varying length, and a hemopexin-like domain. The exceptions to this generalization are MMP-7 and -23 which both lack the linker and hemopexin domains. The catalytic domains of MMPs are highly similar; they are all roughly spherical with a diameter of approximately 40 Å. Catalytic domains of MMPs are described as shallow, intermediate, or deep. The active-site cleft also harbors a hydrophobic S1′-pocket which is the primary determinant of substrate specificity.9 Aside from the catalytic zinc ion required for proteolysis, a structural zinc and at least one structural calcium ion can be found within the active sites of all MMPs. Sequence analyses indicate that the hemopexin-like domains, which confer substrate specificity or mediate protein-protein interactions, are the least homologous domains.24
Figure 1.
Domain structures of MMPs.
Certain MMPs are distinguished from the others based on the presence of additional domains; these may include a fibronectin-like domain, which contains three repeats of fibronectin type II motifs to which gelatin and type IV collagen substrates bind,26 a cysteine/proline-rich immunoglobulin-like domain,20, 27 or a vitronectin-like domain which has tandem repeats of a motif similar to that of the cell adhesion protein.28 Further diversifying the family is the fact that several MMPs are localized to the extracellular side of a cell's lipid bilayer. MMP-14, -15, -16, (also referred to as membrane type I MMP, or MT-1, MT-2, and MT-3, respectively) and MMP-24 are anchored to the membrane through their transmembrane domain and contain a short carboxy-terminal cytoplasmic domain. MMP-17 and MMP-25 are tethered to the membrane by their glycophosphatidylinositol (GPI) anchor.20, 24, 29
MMPs are usually secreted as zymogens and require activation via proteolysis. The propeptide domain is a critical regulator of MMP activity by simply blocking access to the catalytic domain. The propeptide domain is composed of three alpha chains with flexible connecting loops which interact with each other in order to form a hydrophobic core.20, 30, 31 The prodomain of all MMPs is characterized by inclusion of the common “structural signature” amino acid sequence PRCGXPD which keeps the MMP inactive by a cysteine switch mechanism.32, 33 The sulfyhydryl group of the cysteine in this sequence of the prodomain occupies the catalytic zinc ion and prevents a water molecule necessary for proteolysis from accessing and interacting with the ion.34
The early inhibitor programs
Traditional research on cancer drugs has focused almost solely on compounds which kill cancer cells. However, most forms of aggressive cancers remain resistant to these chemotherapeutics and metastasis remains responsible for 90% of patient deaths.35 In the early 1990's, with the identification of MMPs as contributing to cancer stage and patient prognosis, nearly every pharmaceutical company began heavily investing in a MMP inhibitor (MMPI) program. Optimism was high that these drugs would represent a turning point for cancer therapy.
As most enzyme-targeting drugs do, early inhibitors targeting MMP activity were designed to bind within the catalytic domain of these proteases. The initial therapeutics were peptidomimetics, or compounds derived from the sequences of the amino acids of the MMP's endogenous ligands. Optimization to the backbone of these residues yields compounds which tightly bind the active site but for which the sisisile bond cannot be hydrolyzed. Importantly, these compounds chelate the catalytic zinc ion in order to render the protease inactive.36, 37, 38 The first peptidomimetics designed utilized a hydroxamic acid moiety to chelate the zinc and bound with nanomolar affinity. These compounds were designed around the glycine-leucine cleavage site in collagen that can be cleaved by MMP-1,-8, and -13.39
Batimastat, a broad-spectrum, competitive peptidomimetic is an injectable drug and the first MMPI to be tested clinically (see Table 2 for a summary of clinically tested MMPIs). Endpoints of these trials included investigation of the drug's ability to attenuate tumor invasion, angiogenesis, and migration.40 Although highly potent against MMP-1,-2,-3,-7,-9 and -14 and effective in clinical trials for malignant ascites and malignant pleural effusion, trials were stopped during phase III. Due to its poor solubility and very low oral bioavailability, the program was cancelled in favor of a newer, chemically similar analogue (marimastat) with much improved oral bioavailability.41, 42 During clinical trials, marimastat exhibited modest efficacy in delaying disease progression; however, significance could not be established due to dose-limiting toxicity. Patients taking marimastat experienced significant musculoskeletal pain and inflammation. As results of these trials were not statistically significant in regards to either symptomatic progression or overall survival, development of this drug was stopped.43
Table 2.
Clinically tested MMP inhibitors.
| Inhibitor | Class | Selectivity | Indication | Study phase |
|---|---|---|---|---|
| Batimastat | Peptidomimetic | Broad spectrum | Malignant ascites, malignant effusion | Cancelled in phase III |
| Marimastat | Peptidomimetic | Broad spectrum | Breast cancer, small cell lung cancer, non-small cell lung cancer | Cancelled in phase III |
| CGS 27023A | Small molecule | Broad spectrum | Advanced solid cancer | Cancelled in phase I |
| Prinomastat | Small molecule | Broad spectrum | Non-small cell lung cancer, glioblastoma multiforme, prostate cancer | Cancelled in phase III |
| Tanomastat | Small molecule | MMP-2, MMP-3, MMP-9 | Ovarian cancer, adenocarcinoma of the pancreas, non-small cell lung cancer, rheumatoid arthritis, rejection of organ transplant | Cancelled in phase III |
| Doxycycline | Tetracycline derivative | Broad spectrum | Approved for periodontal diseases, multiple sclerosis (phase IV), type II diabetes (phase IV), aortic aneurysm (phase II), coronary artery disease (phase II), polycystic ovarian cancer (recruiting) | Approved or ongoing |
The hydroxamic acid moiety used in these early compounds to chelate the zinc lacks specificity for zinc over other divalent transition metals. Further, it has the ability to bind metal ions such as Fe3+ which have several oxidation states.44 Thus, efforts were undertaken to diversify the groups chelating the catalytic zinc in order to improve selectivity for this ion. As crystal structures of MMPs became available, computational structure-based drug design was utilized to develop small molecule inhibitors which bound to the active site and exhibited improved pharmacokinetic properties. A review by Whittaker et al established a classification system of small molecule MMPIs based on the group used to bind the catalytic zinc; these groups are hydroxamates, carboxylates, thiols, phosphorous-based zinc binding groups, or others (Table 2). Changing the chelating moiety from a hydroxamate to practically anything else reduces the potency of the drug greatly, which is believed to be due to a change in the binding mode and diminished donor ability. However, studies indicate that the choice in zinc-binding group can influence MMP isoform selectivity. This is attributed to slight differences in hydrophobic interactions, the pKa of the inhibitor, and ring sterics.45
CGS 27023A (Novartis, also known as MMI-270) is a small-molecule inhibitor which targets MMP-2, -8, and -9. An isopropyl substituent α to the Zn2+-chelating hydroxamic acid moiety confers specificity by binding in the S1′ subsite. CGS 27023A also has a bulkier moiety believed to bind within the shallow, solvent-exposed S2′ pocket on the enzyme's surface which is believed to further increase specificity.46 Though no anti-proliferative effect was discernible in preclinical studies, CGS 27023A did significantly attenuate angiogenesis and metastasis while reducing tumor burden in rat models of breast and endometrial cancer.47 Ultimately, in clinical trials for patients with non-small cell lung carcinoma, this drug also was terminated due to poorly tolerated joint and muscle pain.39
Several other small-molecule MMPIs made their way to clinical trials. Prinomastat, an optimized version of CGS 27023A, was tested in clinical trials for use as an anti-angiogenic.48 Once again, phase III clinical trials were cancelled before completion due to a lack of efficacy in patients with late-stage disease. Reportedly however, the typical musculoskeletal adverse effects associated with previously tested MMPIs were not an issue.49 Tanomastat utilizes a carboxylate group to chelate the catalytic zinc ion and was found to be more selective than the prior experimental compounds. The drug was clinically investigated for treatment of solid tumors, rheumatoid arthritis, and in preventing rejection of transplanted organs.50 Although tanomastat was well-tolerated, once again there was no significant evidence of improved survival outlook or decreased rate of progression in cancer patients.48, 50
All of the compounds discussed thus far had an auspicious start pre-clinically but ultimately failed to achieve clinical success. The reasons for this are many. As noted earlier, there are clear structural similarities in the catalytic domain across the family of MMPs. Thus even the most selective of the drugs tested failed to achieve sufficient selectivity to circumvent adverse effects. Compounding the lack of selectivity is the fact that MMPs have normal functions remodeling the ECM system-wide, hence the musculoskeletal effects observed for the majority of the clinically tested drugs. Particularly of interest, preclinical testing of efficacy for all of these compounds focused on models of early-stage cancers. However, patients were enrolled in all of the clinical trials without regard to the stage of their disease. Because MMPs contribute to driving disease progression, MMPIs may be more successful if used as preventative measures or as early-stage therapeutics.4, 25, 51, 52 Indeed, in a prevention study in mice batimastat reduced angiogenic islands in a model of pancreatic cancer by 49%. In an intervention trial using the same mouse model, the compound reduced tumor burden by 83%. However, batimastat had no effect on regression of large tumors or invasive carcinoma.53, 54 In hindsight, we can now appreciate that the clinical studies may not have been optimally designed. Considering the role of MMPs in cancer, it is very likely that MMPIs would be found useful only if trials were designed similarly to the preclinical approach. In other words, these drugs may have had better success had they been tested in patients diagnosed with early stage disease or provided as a post-operative preventative measure for those who have had surgical resection of a primary tumor.
The era of pharma's interest in MMPIs ended quickly and with little clinical success. For some time after the failures of these early campaigns, the industry largely shied away from investing in development of MMPIs. But because MMPs have been implicated in so many diseases, they are too tempting a target to be given up on completely. More recently, design and development of MMPIs has focused on development of compounds with high potency and selectivity for a particular MMP (instead of a broad-spectrum approach) and/or novel delivery mechanisms to target only the diseased tissue(s). Because of the structural and sequence similarity conserved at the catalytic domains, efforts have shifted to investigating compounds which confer allosteric inhibition.
Monoclonal antibodies as catalytic domain inhibitors
To date, at least two monoclonal antibodies have been tested which bind to the catalytic domain, likely blocking substrate access but without interacting with the catalytic zinc. DX-2400 is a MMP-14 specific inhibitor which binds to the catalytic domain with a Ki in the sub-nanomolar range.55 The drug, developed by Dyax before Kadmon Corporation acquired the rights, was identified by screening using antibody phage display technology. MMP-14, which is a membrane-bound MMP, is overexpressed in most human cancers and is capable of cleaving the major fibrillar collagens. MMP-14 is also known to activate pro-MMP-2 and participates in such physiological processes as angiogenesis, cell invasion, and metastasis.21 Thus far, preclinical studies of DX-2400 indicate that the antibody is capable of inhibiting all of these activities but yet has no measurable effect on other MMPs.55 In mouse studies, the drug was observed to significantly decrease tumor burden and decreased number of metastases observed in the lung and liver. Further, DX-2400 was effective against HER2-positive xenografts both when used as a single agent or in combination with paclitaxel. This marks DX-2400 as an attractive candidate for patients diagnosed with the especially hard-to-treat triple-negative breast cancer.55 To date, clinical trials for this therapeutic have not yet been initiated.
A murine monoclonal antibody, termed REGA-3G12, has also been generated by hybridoma technology against the catalytic domain of human MMP-9 secreted by neutrophils.56 This compound is unique because it inhibits MMP-9 without affecting activity of MMP-2, another gelatinase to which it is closely related and with which it shares high homology.57 MMP-9 is secreted by most human cancer cells and can be secreted by infiltrating immune cells (including macrophages and neutrophils) and contributes to tumor progression, angiogenesis, and tumor cell invasion. MMP-9 activation can be induced by inflammatory cytokines, growth factors, and cell/stroma interactions particularly in the most malignant cells whereas MMP-2 is thought to be constitutively expressed by many cell types.58, 59 Therefore, a therapeutic which can differentiate between these two highly similar enzymes may prove very useful.
Hemopexin domain inhibitors
Because the hemopexin domain exhibits significantly less sequence and structural homology compared to the catalytic domain, much research has focused on discovery of inhibitors which can bind to this region. This domain comprises a succession of four structurally similar hemopexin-like repeats. Each of these repeats appears in the shape of a blade, forming a propeller-like structure with the blades oriented in such a way to create a central funnel-like tunnel. Each blade is made up of four β-strands; the first three β-strands bear the highest homology across the MMP family whereas the β4 strands bear the least. The tunnel created by this propeller structure traverses its length and is highly solvated. As many as four structural ions have been found to be coordinated within this tunnel and it has been proposed that these ions confer a stabilizing function for the whole domain.60 The evidence for this is that MMP-9, which has only a sodium ion, displays a flexible architecture and considerable deviation from the structure of hemopexin domains reported for other MMPs.61 The first ion binding position, which is closest to the linker region connecting the hemopexin domain to the catalytic domain, is generally either a sodium or calcium ion. Next typically comes a chloride ion, followed in the third position by another cation, frequently calcium. The fourth position is generally occupied by another anion, which is usually chloride.60
Compounds which bind to the hemopexin domains and prevent dimerization have been shown to significantly decrease tumor size, reduce MMP-mediated cell scattering/invasion, angiogenesis, and tumor metastasis both in vitro and in animal models.62, 63, 64, 65 Small molecule inhibitors which bind at the hemopexin domain of MMP-14 (a membrane-type MMP) and MMP-9 have been described. In silico analysis of the MMP-14 hemopexin domain identified a druggable pocket-like site in the center of the hemopexin structure. Binding of small molecule compounds in this site should, in theory, allosterically block dimerization. Subsequent docking studies of small-molecule compounds led to identification of a compound which is selective for MMP-14 compared to MMP-2 and was not cytotoxic and did not affect catalytic activity (including MMP-14-mediated activation of MMP-2). This compound was effective in attenuating cancer cell migration and in vivo reduced tumor volume and caused a fibrotic tumor phenotype (hypothesized to be a result of a decreased ability for the cancer cells to invade).63 Similar studies have been spearheaded against MMP-9, in which docking programs were utilized to map potential ligand binding sites in the hemopexin domain at the dimerization interface. The compound identified in that study also yielded one with no proteolytic or cytotoxic effects but which significantly decreased cancer cell migration and invasion and, in vivo, significantly decreased tumor size as well as the number of metastases.64
Similar to the small molecule compounds described above, small peptides have been used successfully to block dimer-induced functions of MMPs, including cancer cell migration, invasion, angiogenesis, and in vivo metastasis. MMP-14 homodimerizes via the outer strand of blade IV and also heterodimerizes through the outer strand of blade I with CD44 in order to induce intracellular cytoskeleton rearrangements necessary for processing of migration and invasion machinery, including proteolysis of proMMP-2 by MMP-14.66, 67 On the other hand, MMP-9 homodimerizes via blade IV to induce cell migration.68 Short peptides that mimic the sequence of the residues with which the hemopexin-like domains dimerize were able to reduce tumorigenic effects in vitro and, in the case of the MMP-14 peptides, in vivo.62, 65
Taking another approach, a fusion protein has been designed which links the ten amino acid sequences of a MMP-2 selective inhibitory peptide (APP-IP, a β-amyloid precursor protein) to the N-terminus of TIMP-2. This macromolecular protein, which binds with a Ki in the sub-picomolar range, is designed to interact with both the active site and the hemopexin-like domain of MMP-2. This bimodal approach confers increased selectivity for MMP-2 compared to either subunit individually. The binding of the inhibitor at the catalytic domain blocks catalytic activity while the interaction with the hemopexin-like domain prevents binding of endogenous partners that can promote angiogenesis or cancer cell migration. Selectivity for MMP-2 was confirmed via fluorometric enzyme activity assay and indicates that the concentration of APP-IP-TIMP-2 necessary to inhibit MMP-1, MMP-3, MMP-7, MMP-8, MMP-9, or MMP-14 is over six-fold higher than for MMP-2.69
Targeting structural elements to upset the global architecture
To date, there is a single approved therapeutic which inhibits MMPs, albeit it is approved at this time only for periodontal diseases. A chemically modified tetracycline, doxycycline (Periostat®), works to inhibit MMPs via a mechanism unrelated to its well-characterized antimicrobial activities. Though considered broad-spectrum inhibitors, chemically modified tetracyclines are most useful against the collagenases. In the case of doxycycline, the drug's lower half is rich in oxygen, making it a potent chelator of divalent metals.70 Exactly how doxycycline binds remains enigmatic. However, models indicate that doxycycline is capable of chelating the structural cations coordinated in either the catalytic domain or the hemopexin-like domain but is likely not capable of chelating the catalytic zinc.71, 72 In the case of MMP-7, doxycycline has been demonstrated by deuterium exchange mass spectroscopy to interact with hydrophobic tryptophan residues in the MMP which are proximal to the structural zinc within the catalytic domain, thus affecting global conformation of the enzyme and rendering it inactive.73
As an added bonus, chemically modified tetracyclines have been shown to inhibit TNF-α and IL-8 production, thereby targeting the MMP expression pathway and reducing overall MMP levels.74 The safety, toxicity, and efficacy profiles of tetracyclines and their derivatives, including doxycycline, are well known and have been used as antibiotics safely for years. Further, tetracyclines are attractive for development as they are natural compounds, easily isolated, and their toxicity, efficacy, and pharmacokinetic profiles are well described.75 Thus, unsurprisingly, several clinical trials recruiting or in progress are investigating doxycycline in combination with standard chemotherapeutics for use in cancer therapy.
Designing targeted drug delivery systems
The majority of current chemotherapeutics target any dividing cell, system-wide. These non-specific, systemic effects are what lead to the adverse symptoms typically associated with cancer treatment such as hair loss or loss of appetite. Because the early, unsuccessful MMPIs were non-specific and systemically available, recent research has begun looking at drug delivery systems which will only be effective within the tumor environment.
Liposome systems conjugated to a selectivity marker which encapsulate cytotoxic agents have emerged as an innovative way to deliver therapeutics specifically to tumor cells. Liposomes are spherical vesicles composed of a lamellar phase lipid bilayer with a solvated inner core. Various compounds may be chemically conjugated to these systems to stabilize the system, confer selectivity, or trigger cellular uptake. One recent study demonstrates the use of a monoclonal antibody, mAb 2C5, which recognizes cell surface nucleosomes expressed on cancer cells, along with a MMP-2 cleavable peptide to provide additional selectivity at the tumor site. The MMP-2 cleavable peptide also acts to shield a cell-penetrating peptide that enables entry of the system into the target cell. In this approach, the cytotoxic effects conferred by the encapsulated drug cannot be initiated unless MMP-2 first cleaves the linker to remove the blocker and expose this membrane-penetrating peptide.76
Similar to liposomes, nanoparticles are another system which can be modified in order to selectively deliver a cytotoxic payload to cancer cells. Nanoparticles have been engineered in such a manner that hydrophilic compounds can be conjugated to the particle surface and more hydrophobic compounds, such as cancer drugs like doxorubicin, can be incorporated inside the particle. The MMP substrate peptide PLGVR was conjugated to mesoporous silica nanoparticles to provide selectivity at the MMP-expressing tumor site. Cellular uptake of these nanoparticles is only initiated after cleavage of this peptide. Although nanoparticles on their own can arbitrarily cross any cell membrane, the fact that the tumor microenvironment is very acidic can be leveraged to reduce non-specific effects. The loading of polyanions onto the surface of the particles repels non-cancer cells and allows the particles to accumulate in cancerous tissues. Cancer chemotherapeutics loaded into the core can induce tumor cell death following nanoparticle uptake. In vitro experiments demonstrate these nanoparticles are taken up by cells expressing MMPs and cytotoxic effects are observed following cleavage of the MMP substrate by cancer cells.77 Like liposomes, nanoparticles have potential clinical use for selective delivery of chemotherapeutics and may attenuate the harsh side effects typically associated with traditional approaches.
As discussed earlier and reviewed in detail in reference 35, the biggest contributing factor to the failure of the early MMPIs in the clinic was the system-wide exposure. For skin dysplasias however, application of a topical ointment can easily bypass this issue. In many cancers, natriuretic peptide receptor-A (NPR-A) is overexpressed and is associated with cancer aggressiveness as well as increased inflammation (which drives cancer progression).78 In vivo studies of a mouse model of melanoma in which mice were treated with an ointment containing a NPR-A inhibitor demonstrated significantly decreased activity and expression levels of MMP-2 and MMP-9.79 Similar studies have also been completed successfully using an ointment containing a synthetic retinoid to directly inhibit MMPs but in a diabetic ulcer model. In this case, not only did expression and activity of MMP-1, -2, and -9 decrease but the rate of wound healing increased.80, 81, 82
Conclusions
MMPs remain a viable target for cancer therapeutics. However, for MMPIs to be clinically successful, they will need to be highly selective for a particular MMP and able to accumulate in cancerous tissues without eliciting adverse systemic effects. Although MMPI programs were initiated industry-wide in the era following identification of their role in cancer, the initial trials failed across the board despite preclinical data indicating that MMPIs have great potential for therapeutic use. Since these first trials were initiated, vast amounts of literature have been published regarding the biochemistry of MMP expression, function, and their contribution to pathological progression. Furthermore, post-clinical reviews of how clinical trials were conducted provide a better understanding of the timing for which MMPIs will be beneficial. Taking this into consideration, it is clear that the time is now ripe for the industry to once again begin investing in MMPI programs.
Conflicts of interest
All authors have none to declare.
Acknowledgements
This work is supported in part by Baldwin Breast Cancer Foundation and National Cancer Institute (1R01CA166936 to J.C.).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Roy R., Yang J., Moses M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker M., Floyd C.D., Brown P., Gearing A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- 3.Bloomston M., Zervos E.E., Rosemurgy A.S., 2nd Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668–674. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. http://www.nature.com/nrc/journal/v2/n3/suppinfo/nrc745_S1.html [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick N.A., Neilson E.G., Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Vihinen P., Kähäri V.M. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T., Tanioka M., Yoshida H., Yoshioka T., Nishimoto H., Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 9.Tallant C., Marrero A., Gomis-Rüth F.X. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Cerdà-Costa N., Xavier Gomis-Rüth F. Architecture and function of metallopeptidase catalytic domains. Protein Sci. 2014;23:123–144. doi: 10.1002/pro.2400. doi:10.1002/pro.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternlicht M.D., Lochter A., Sympson C.J. The Stromal proteinase MMP3/Stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/S0092-8674(00)81009%130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S.-C., Yang S.-F., Yeh K.-T. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clin Chim Acta. 2006;371:92–96. doi: 10.1016/j.cca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Ueda J., Kajita M., Suenaga N., Fujii K., Seiki M. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003;22:8716–8722. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- 14.Lakka S.S., Rajan M., Gondi C. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–8019. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 15.Mehner C., Hockla A., Miller E., Ran S., Radisky D.C., Radisky E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. May 2014;5:2736–2749. doi: 10.18632/oncotarget.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann U.B., Westphal J.R., Zendman A.J., Becker J.C., Ruiter D.J., van Muijen G.N. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathology. 2000;191:245–256. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH632>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Stetler-Stevenson W.G. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Investigation. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson C.L., Ouellette A.J., Satchell D.P. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 19.Ii M., Yamamoto H., Adachi Y., Maruyama Y., Shinomura Y. Role of matrix metalloproteinase-7 (Matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med. 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 20.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lemaître V., D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- 22.Alexander C.M., Hansell E.J., Behrendtsen O. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez D., Morrison C.J., Overall C.M. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochimica Biophysica Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Massova I., Kotra L.P., Fridman R., Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 25.Overall C.M., Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 26.Murphy G., Nguyen Q., Cockett M.I. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J Biol Chem. 1994;269:6632–6636. [PubMed] [Google Scholar]
- 27.Ohnishi J., Ohnishi E., Jin M. Cloning and characterization of a rat ortholog of MMP-23 (matrix metalloproteinase-23), a unique type of membrane-anchored matrix metalloproteinase and conditioned switching of its expression during the ovarian follicular development. Mol Endocrinol. 2001;15:747–764. doi: 10.1210/mend.15.5.0638. [DOI] [PubMed] [Google Scholar]
- 28.Yang M., Murray M.T., Kurkinen M. A novel matrix metalloproteinase gene (XMMP) encoding vitronectin-like motifs is transiently expressed in Xenopus laevis early embryo development. J Biological Chem. 1997;272:13527–13533. doi: 10.1074/jbc.272.21.13527. [DOI] [PubMed] [Google Scholar]
- 29.Zucker S., Pei D., Cao J., Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP) Curr Top Dev Biol. 2003;54:1–74. doi: 10.1016/s0070-2153(03)54004-2. [DOI] [PubMed] [Google Scholar]
- 30.Jozic D., Bourenkov G., Lim N.-H. X-ray structure of human proMMP-1: new insights into procollagenase activation and collagen binding. J Biological Chem. 2005;280:9578–9585. doi: 10.1074/jbc.M411084200. [DOI] [PubMed] [Google Scholar]
- 31.Maskos K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie. 2005;87:249–263. doi: 10.1016/j.biochi.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Park A.J., Matrisian L.M., Kells A.F., Pearson R., Yuan Z.Y., Navre M. Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the “cysteine switch”. J Biol Chem. 1991;266:1584–1590. [PubMed] [Google Scholar]
- 33.Vartak D.G., Gemeinhart R.A. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15:1–20. doi: 10.1080/10611860600968967. doi:772726202 [pii]10.1080/10611860600968967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Wart H.E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Dormán G., Cseh S., Hajdú I. Matrix metalloprotease inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010;70:949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo M., Eckhardt S.G. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 38.Hu J., Van den Steen P.E., Sang Q.-X.A., Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. http://www.nature.com/nrd/journal/v6/n6/suppinfo/nrd2308_S1.html [DOI] [PubMed] [Google Scholar]
- 39.Rao B.G. Recent developments in the design of specific matrix metalloproteinase inhibitors aided by structural and computational studies. Curr Pharm Des. 2005;11:295–322. doi: 10.2174/1381612053382115. [DOI] [PubMed] [Google Scholar]
- 40.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen H.S., McCann P.P. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75:69–75. doi: 10.1016/S0163-7258(97)00023%135. [DOI] [PubMed] [Google Scholar]
- 42.Steward W.P. Marimastat (BB2516): current status of development. Cancer Chemother Pharmacol. 1999;43:S56–S60. doi: 10.1007/s002800051099. [DOI] [PubMed] [Google Scholar]
- 43.Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 44.Overall C.M., Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal A., Romero-Perez D., Jacobsen J.A., Villarreal F.J., Cohen S.M. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008;3:812–820. doi: 10.1002/cmdc.200700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supuran C.T., Casini A., Scozzafava A. Protease inhibitors of the sulfonamide type: anticancer, antiinflammatory, and antiviral agents. Med Res Rev. 2003;23:535–558. doi: 10.1002/med.10047. doi:10.1002/med.10047. [DOI] [PubMed] [Google Scholar]
- 47.Wood J., Schnell C., Cozens R. CGS 27023A, a potent and orally active matrix metalloprotease inhibitor with antitumor activity. Proc Am Assoc Cancer Res. 1998 [Google Scholar]
- 48.Fisher J.F., Mobashery S. Recent advances in MMP inhibitor design. Cancer Metastasis Rev. 2006;25:115–136. doi: 10.1007/s10555-006-7894-9. [DOI] [PubMed] [Google Scholar]
- 49.Phase III Clinical Trial of Prinomastat Halted. 2000. [Google Scholar]
- 50.Hirte H., Vergote I.B., Jeffrey J.R. A phase III randomized trial of BAY 12–9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: a national Cancer Institute of Canada clinical trials group study. Gynecol Oncol. 2006;102:300–308. doi: 10.1016/j.ygyno.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Pavlaki M., Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22:177–203. doi: 10.1023/a:1023047431869. [DOI] [PubMed] [Google Scholar]
- 52.Zucker S., Cao J., Chen W.-T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 53.McCawley L.J., Matrisian L.M. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/S1357-4310(00)01686%135. [DOI] [PubMed] [Google Scholar]
- 54.Bergers G., Javaherian K., Lo K.-M., Folkman J., Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 55.Devy L., Huang L., Naa L. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 56.Zhou N., Paemen L., Opdenakker G., Froyen G. Cloning and expression in Escherichia coli of a human gelatinase B-inhibitory single-chain immunoglobulin variable fragment (scFv) FEBS Lett. 1997;414:562–566. doi: 10.1016/S0014-5793(97)01072%137. [DOI] [PubMed] [Google Scholar]
- 57.Martens E., Leyssen A., Van Aelst I. A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochimica Biophysica Acta. 2007;1770:178–186. doi: 10.1016/j.bbagen.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Klein G., Vellenga E., Fraaije M.W., Kamps W.A., de Bont E.S.J.M. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Westermarck J., Kähäri V.-M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 60.Xavier Gomis-Rüth F. Hemopexin domains. Encycl Inorg Bioinorganic Chem. 2004 [Google Scholar]
- 61.Cha H., Kopetzki E., Huber R., Lanzendörfer M., Brandstetter H. Structural basis of the adaptive molecular recognition by MMP9. J Mol Biol. 2002;320:1065–1079. doi: 10.1016/s0022-2836(02)00558-2. [DOI] [PubMed] [Google Scholar]
- 62.Zarrabi K., Dufour A., Li J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biological Chem. 2011;286:33167–33177. doi: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remacle A.G., Golubkov V.S., Shiryaev S.A. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012;72:2339–2349. doi: 10.1158/0008-5472.CAN-11-4149. doi:10.1158/0008-5472.CAN-11-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dufour A., Sampson N.S., Li J. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011;71:4977–4988. doi: 10.1158/0008-5472.CAN-10-4552. doi:10.1158/0008-5472.can-10-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dufour A., Zucker S., Sampson N.S., Kuscu C., Cao J. Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. J Biological Chem. 2010;285:35944–35956. doi: 10.1074/jbc.M109.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mori H., Tomari T., Koshikawa N. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21:3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lichte A., Kolkenbrock H., Tschesche H. The recombinant catalytic domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) induces activation of progelatinase A and progelatinase A complexed with TIMP-2. FEBS Lett. 1996;397:277–282. doi: 10.1016/s0014-5793(96)01206-9. [DOI] [PubMed] [Google Scholar]
- 68.Dufour A., Sampson N.S., Zucker S., Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217:643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higashi S., Hirose T., Takeuchi T., Miyazaki K. Molecular design of a highly selective and strong protein inhibitor against matrix metalloproteinase-2 (MMP-2) J Biological Chem. 2013;288:9066–9076. doi: 10.1074/jbc.M112.441758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golub L.M., Ramamurthy N.S., McNamara T.F., Greenwald R.A., Rifkin B.R. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 71.Griffin M.O., Fricovsky E., Ceballos G., Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Rev Literature. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith G.N., Jr., Mickler E.A., Hasty K.A., Brandt K.D. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum. 1999;42:1140–1146. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 73.García R.A., Pantazatos D.P., Gessner C.R., Go K.V., Woods V.L., Villarreal F.J. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass Spectrometry. Mol Pharmacol. 2005;67:1128–1136. doi: 10.1124/mol.104.006346. [DOI] [PubMed] [Google Scholar]
- 74.Perdigão J., Reis A., Loguercio A.D. Dentin adhesion and MMPs: a comprehensive review. J Esthet Restor Dent. 2013;25:219–241. doi: 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- 75.Jones C.H., Petersen P.J. Tigecycline: a review of preclinical and clinical studies of the first-in-class glycylcycline antibiotic. Drugs Today. 2005;41:637. doi: 10.1358/dot.2005.41.10.937460. [DOI] [PubMed] [Google Scholar]
- 76.Zhu L., Kate P., Torchilin V.P. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Yuan Z.-F., Wang Y. Multifunctional Envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J Am Chem Soc. 2013;135:5068–5073. doi: 10.1021/ja312004m. doi:10.1021/ja312004m. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Raulji P., Mohapatra S.S. Natriuretic peptide receptor a as a novel target for prostate cancer. Mol Cancer. 2011;10:56. doi: 10.1186/1476-4598-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian V., Vellaichamy E. Atrial natriuretic peptide (ANP) inhibits DMBA/croton oil induced skin tumor growth by modulating NF-κB, MMPs, and infiltrating mast cells in Swiss albino mice. Eur J Pharmacol. 2014;740:388–397. doi: 10.1016/j.ejphar.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Warner R.L., Bhagavathula N., Nerusu K. MDI 301, a nonirritating retinoid, improves abrasion wound healing in damaged/atrophic skin. Wound Repair Regen. 2008;16:117–124. doi: 10.1111/j.1524-475X.2007.00338.x. doi:10.1111/j.1524-475X.2007.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varani J., Perone P., Merfert M.G., Moon S.E., Larkin D., Stevens M.J. All-trans retinoic acid improves structure and function of diabetic rat skin in organ culture. Diabetes. 2002;51:3510–3516. doi: 10.2337/diabetes.51.12.3510. doi:10.2337/diabetes.51.12.3510. [DOI] [PubMed] [Google Scholar]
- 82.Zeng W., Tahrani A., Shakher J. Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes. J Diabetes Complications. 2011;25:398–404. doi: 10.1016/j.jdiacomp.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]