Abstract
IMPORTANCE
Voltage-gated potassium channel complex antibody (VGKCc-Ab) encephalitis is an immunotherapy-responsive syndrome usually associated with causative antibodies that target the leucine-rich, glioma inactivated 1 (LGI1) protein. Although it is expressed throughout the brain, LGI1 is not known to be expressed in cardiac tissue. We describe a novel neurocardiac prodrome of VGKCc-Ab/LGI1-encephalitis.
OBSERVATIONS
Among 14 patients with VGKCc/LGI1-Ab encephalitis evaluated in the University of California, San Francisco Autoimmune Encephalitis Clinic and Rapid Dementia Research Program, 3 patients (2 men and 1 woman; aged 53, 55, and 64 years) exhibited episodic bradycardia that preceded the onset of encephalopathy by approximately 2 months and was severe enough to lead to pacemaker implantation. Serum LGI1-Ab results were positive when tested at the time of the subsequent encephalopathy. All 3 patients developed hyponatremia; none had faciobrachial dystonic seizures or malignancy. Brain magnetic resonance imaging was abnormal in 2 cases. None of the patients experienced further symptomatic bradyarrythmias after 1.7 to 7 years of follow-up.
CONCLUSIONS AND RELEVANCE
Episodic bradycardia is a distinctive neurocardiac prodrome of VGKCc/LGI1-Ab encephalitis. The neuroanatomical localization most likely relates to insular and temporal lobe involvement, cortical regions that modulate cardiac autonomic function. Further study is needed to determine if recognition of this neurocardiac prodrome and earlier institution of immunosuppression can prevent the development of encephalopathy.
Voltage-gated potassium channel complex antibody (VGKCc-Ab) encephalitis is characterized by rapid-onset dementia, seizures, and hyponatremia.1,2 The leucine-rich, glioma inactivated 1 (LGI1) protein, part of the VGKC-complex, is the established antigenic target in most cases of this antibody-mediated limbic syndrome.3,4 We describe 3 patients with VGKCc/LGI1-Ab encephalitis who developed episodic bradycardia requiring pacemaker implantation prior to the onset of encephalopathy. As LGI1 is widely expressed throughout brain but not cardiac tissue4 and as cardiac conduction can be modulated by cortical autonomic centers,5 we propose that the antibody-associated encephalitis generates episodic bradycardia early within the disease process.
Methods
We identified 14 patients with VGKCc-Ab encephalitis6,7 evaluated between January 1, 2006, and August 1, 2013, in the University of California, San Francisco Rapid Dementia Research Program and Autoimmune Encephalitis Clinic. Testing for VGKCc-Abs and LGI1-Abs was performed in US clinical laboratories and/or the international research laboratories of Josep Dalmau, MD, PhD, University of Barcelona, University of Pennsylvania, and Angela Vincent, FRS, University of Oxford. All participants provided written informed consent. The University of California, San Francisco Committee on Human Research approved the study protocol.
Report of Cases
Of these 14 patients with VGKCc-Ab encephalitis, 3 had prodromal bradycardia and pacemaker implantation (21%; 2 men and 1 woman; ages 53, 55, and 64 years). None of the patients had a prior cardiac history. All 3 cases had serum VGKCc-Abs and LGI1-Abs at the time of encephalitis, which was characterized by amnesia, seizures, and serum hyponatremia. None of the cases had faciobrachial dystonic seizures, tumors, or contactin-associated protein-like 2 Abs (Table) and none had antibody testing at the time of the neurocardiac prodrome. Cases 2 and 3 were included in our analysis of neuropsychological testing in LGI1 encephalitis6 and case 2 was included in our analysis on the use of rituximab in this disorder.7
Table.
Demographic and Clinical Characteristics
| Characteristic | Case | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age at onset, y | 53 | 64 | 55 |
| Sex | Male | Male | Female |
| Initial VGKCc-Ab titer | 696 pmol/L (normal <450 pmol/L) | 0.11 nmol/L (normal <0.02 nmol/L) | 353 pmol/L (normal <100) |
| LGI1 antibodies | Positive | Positive | Positive |
| CASPR2 antibodies | Negative | Negative | Negative |
| Brain MRI findings | Normal | Abnormal | Abnormal |
| Bradyarrythmia | Bradycardia to 30 bpm, intermittent sinus arrest, slow junctional escape rhythm | Sinus bradycardia to 20 bpm | Bradycardia, 12-s sinus pause |
| Pacemaker implantation | Yes | Yes | Yes |
| Time from cardiac syndrome to encephalopathy onset, mo | Approximately 2 | Approximately 2 | Approximately 2 |
| Neurocognitive impairment | Amnestic, ↓ processing speed | Amnestic, ↓ processing speed | Amnestic, dysexecutive |
| Seizures | Yes, olfactory | Yes, psychic and motor | Yes, psychic and motor |
| Faciobracial dystonic seizures | No | No | No |
| Serum sodium nadir, mEq/L | 128 | 128 | 126 |
| Malignancy | Negative (CT/PET body) | Negative (CT/PET body) | Negative (CT body) |
| Follow-up, y | 2 | 1.7 | 7 |
Abbreviations: CASPR2, contactin-associated protein-like 2; CT, computerized tomography; LGI1, leucine-rich, glioma inactivated 1; MRI, magnetic resonance imaging; PET, positron emission tomography; VGKCc-Ab, voltage-gated potassium channel complex antibody; ↓, decreased.
SI conversion factor: To convert sodium to millimoles per liter, multiply by 1.
Case 1
A 53-year-old man developed 3 unheralded syncopal episodes during 2 months, all lasting seconds to less than 1 minute; 1 episode developed while he was driving. Evaluation revealed intermittent sinus arrest with slow junctional escape. He was diagnosed with sick sinus syndrome. No other accompanying symptoms at that time were reported or noted. A pacemaker was implanted. He subsequently experienced episodic unpleasant olfactory hallucinations lasting 10 to 20 seconds. He also felt fatigued and developed intermittent light-headedness yet pacemaker interrogation at that time revealed no significant abnormalities or pacing episodes. Two months following the pacemaker implantation, he had trouble remembering a recent family trip and was described as speaking gibberish. He was reportedly diagnosed clinically and electrographically with nonconvulsive status epilepticus and his cognition partially improved with levetiracetam. He continued to have mild memory problems and was intermittently anxious and uncharacteristically tearful. Serum VGKCc-Ab and LGI1-Ab levels were elevated (Table).
Five months following his pacemaker implantation, he was treated with intravenous methylprednisolone (1 g/d × 5 d) and an oral prednisone taper. Seven months after the pacemaker implantation, neuropsychological testing revealed mild impairment in verbal memory and processing speed. He subsequently received treatment with intravenous immunoglobulin and azathioprine and had steady cognitive improvement, allowing him to return to full-time work in a cognitively demanding profession. Two years after his pacemaker implantation, he had no detectable VGKCc-Abs or LGI1-Abs.
Case 2
A 64-year-old man developed lightheadedness with associated bradycardia lasting seconds and was diagnosed with sick sinus syndrome. No other accompanying symptoms at that time were reported or noted. A pacemaker was implanted. The next day, he developed episodic anxiety lasting hours and occurring daily. Two months following his pacemaker implantation, he became forgetful, compulsive, and delusional (daily thoughts of people stealing from him and intruders present in the neighborhood). He had a seizure with head version and tonic arm extension (laterality not recalled) and then bilateral clonic limb movements, followed 2 weeks later by a generalized tonic-clonic seizure. Investigations revealed persistent serum hyponatremia (Table). Brain magnetic resonance imaging showed T2/fluid-attenuated inversion recovery hyperintensities (Figure). Neuropsychological testing demonstrated verbal memory impairment and slowed processing speed. He was treated with intravenous methylprednisolone (1 g/d × 5 d) with improvement. About 3 weeks later, his cognition worsened, leading to retreatment with intravenous methylprednisolone, intravenous immunoglobulin, and a prednisone taper over several months. Ten months after his pacemaker implantation, he had no cardiac symptoms (pacemaker interrogation revealed 10%–12% pacing with a baseline setting of 55 bpm). At 12 months, he was retreated with intravenous immunoglobulin and continued oral prednisone, with partial improvement of cognitive deficits but persistent difficulty with verbal recall on cognitive testing. Levels of VGKCc-Abs and LGI1-Abs remained elevated. At 15 months, he was treated with 2 doses of intravenous rituximab, 1000 mg, and continued taking prednisone, 20 mg/d. At 18 months, LGI1-Abs and VGKC-Abs were not detectable. He exhibited additional improvement in episodic memory and emotional lability but continued to have moderate deficits on verbal learning and memory testing.
Figure. Brain Magnetic Resonance Imaging of Cases 2 and 3 at Initial Diagnosis With Voltage-Gated Potassium Channel Complex/Leucine-Rich, Glioma Inactivated 1 Antibody Encephalitis.
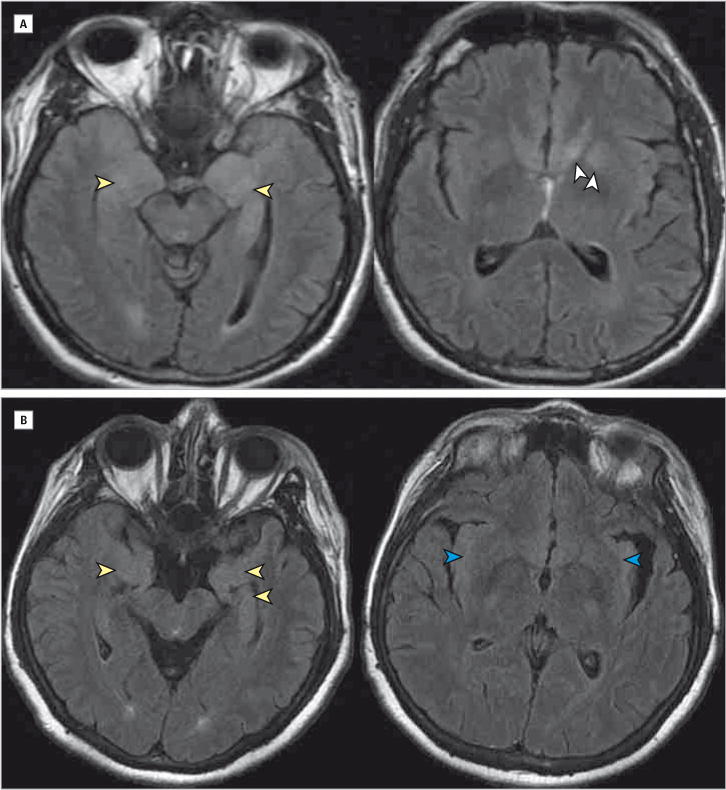
A, Case 2. Fluid-attenuated inversion recovery reveals hyperintensities in the left greater than right medial temporal lobes (yellow arrowheads) and amygdala and striatum (white arrowheads). B, Case 3. Fluid-attenuated inversion recovery sequences showing hyperintensities in the bilateral medial temporal lobes (yellow arrowheads) and bilateral insula (blue arrowheads). Orientation is radiologic. There was no abnormal contrast enhancement or restricted diffusion in either case (not shown).
Case 3
A 55-year-old woman developed discrete episodes of fear lasting several minutes that occurred 1 to 2 times daily for 1 to 2 months. Lorazepam was prescribed for panic attacks. One episode culminated in a 10-second loss of consciousness without postictal confusion. Two weeks later, panic episodes with loss of consciousness recurred with documented bradycardia and a sinus pause of 12 seconds. She was diagnosed with cardiogenic syncope secondary to bradyarrythmias and underwent pacemaker implantation (Table). During the next 3 months, she appeared uncharacteristically apathetic, her short-term memory declined, and she experienced hallucinations. This was followed by a seizure with head deviation and subsequent generalized tonic-clonic activity. Her husband described episodes during which she lost awareness and reached for objects with her right hand, occurring about 6 times a day, each lasting a few seconds. Magnetic resonance imaging results showed T2/fluid-attenuated inversion recovery hyperintensities (Figure). Five months after her pacemaker implantation, she was treated with intravenous methylprednisolone (1 g/d × 5 d), followed by maintenance prednisone with a marked improvement in memory and panic episodes. After tapering off prednisone 2 years following the pacemaker implantation, her panic attacks insidiously recurred and she developed emotional lability. These symptoms resolved following another course of intravenous methylprednisolone and oral prednisone maintenance. Two years after the pacemaker implantation, no significant abnormalities were noted on her cardiac electrophysiology follow-up. Contacted by telephone 7 years after onset, her husband reported that she still had mild short-term memory difficulties such as forgetting details of conversations and had had no further syncopal episodes or cardiac problems.
Discussion
Episodic bradycardia is a distinctive prodrome of VGKCc/LGI1-Ab encephalitis that led to pacemaker implantation in all of our cases and preceded the onset of encephalopathy by about 2 months.
The neuroanatomical localization of this neurocardiac prodrome most likely relates to temporal lobe and insular involvement. In patients undergoing epilepsy surgery, stimulation of the left insular cortex can provoke bradyarrythmias.8 Temporal lobe seizures involving either hemisphere can trigger ictal bradycardia.9 The episodic course of the neurocardiac prodrome in our patients favors an epileptic cause secondary to focal encephalitis of these brain regions. Further supporting this proposed cause and localization, our patients also exhibited episodes of anxiety, emotional lability, and/or olfactory hallucinations prior to the encephalopathy, focal motor, and generalized tonic-clonic seizures in the context of evolving encephalitis. It is also possible that the bradycardia was accompanied by other symptoms that were not reported because they were overshadowed by syncopal symptoms. As LGI1 is found throughout the neocortex10,11 and emerging evidence suggests direct pathogenicity of LGI1-Abs,12,13 it is likely that LGI1-Abs were involved in triggering the autonomic dysfunction that caused bradycardia in our patients.
This finding adds to the spectrum of autonomic symptoms associated with VGKCc encephalitis. Early case reports of VGKCc encephalitis noted some patients with hypersalivation.1,2,14 Episodic hypothermia was also described in a subset of patients with VGKCc-Ab–associated limbic encephalitis.15 Autonomic symptoms are also a prominent feature of the Morvan syndrome, which in addition to neuromyotonia can include hyperhidrosis, lacrimation, constipation, diarrhea, impotence, tachycardia, and alterations in blood pressure. Morvan syndrome is a phenotype more commonly associated with contactin-associated proteinlike 2 Abs than LGI1-Abs, although both antibodies may be present in patients with Morvan syndrome.16,17 Furthermore, seizures with prominent autonomic features have been described in patients with VGKCc-Abs and LGI1-Abs.11,18 Interestingly, ictal asystole has been rarely reported in another cell-surface antibody-associated neurological disorder, N-methyl-D-aspartate receptor antibody encephalitis.19,20
Conclusions
Recognition of episodic bradycardia in combination with other limbic features, particularly amnesia or seizures, might prompt early consideration of VGKCc/LGI1-Ab testing. Furthermore, similar to the observation that early immunosuppressive treatment of prodromal faciobrachial dystonic seizures11 may prevent progression to encephalopathy,21 we hypothesize that the same might be true for treatment of this neurocardiac prodrome. Further research is needed to address these possibilities.
Acknowledgments
Funding/Support: Research was supported by grant KL2TR000143 from the National Center for Advancing Translational Sciences of the National Institutes of Health, grants R01-AG031189 and R01-AG021601 from the National Institute on Aging of the National Institutes of Health, a commission from the UK-US Fulbright Program, the Multiple Sclerosis Society, and Epilepsy Research United Kingdom.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Gelfand and Naasan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Naasan, Irani, Geschwind, Gelfand.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Naasan, Irani, Geschwind, Gelfand.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bettcher, Gelfand.
Obtained funding: Geschwind, Gelfand. Administrative, technical, or material support: Naasan, Geschwind.
Study supervision: Irani, Geschwind, Gelfand.
Conflict of Interest Disclosures: Dr Irani is a coapplicant on a patent for the discovery of VGKC-complex antigenic targets (including LGI1) and receives royalties. Dr Geschwind is a consultant for Lundbeck Inc, MedaCorp, and the Council of Advisors and Neurophage and has received research funding from the Tau Consortium and CurePSP. Dr Gelfand has received compensation for medical legal consulting work related to central nervous system inflammatory demyelinating disease. No other disclosures are reported.
Disclaimer: The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Additional Contributions: We thank Kendra Bechtel, BA, University of California, San Francisco, Sven Forner, BA, University of California, San Francisco, and Aya Abounasar, BS, University of California, San Francisco, for research coordination and our patients and their families. Ms Bechtel and Mr Forner are employees at University of California, San Francisco, paid by grants received from the National Institutes of Health and Ms Abounasar is an employee at University of California, San Francisco.
References
- 1.Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62(7):1177–1182. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(Pt 3):701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 3.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 6.Bettcher BM, Gelfand JM, Irani SR, et al. More than memory impairment in voltage-gated potassium channel complex encephalopathy [published online July 1, 2014] Eur J Neurol. doi: 10.1111/ene.1248224981998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani SR, Gelfand JM, Bettcher BM, et al. Effect of rituximab in patients with leucine-rich, glioma-inactivated 1 antibody-associated encephalopathy. JAMA Neurol. 2014;71(7):896–900. doi: 10.1001/jamaneurol.2014.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 9.Britton JW, Ghearing GR, Benarroch EE, Cascino GD. The ictal bradycardia syndrome: localization and lateralization. Epilepsia. 2006;47(4):737–744. doi: 10.1111/j.1528-1167.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 10.Schulte U, Thumfart JO, Klöcker N, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49(5):697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 12.Lalic T, Pettingill P, Vincent A, Capogna M. Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia. 2011;52(1):121–131. doi: 10.1111/j.1528-1167.2010.02756.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa T, Fukata Y, Yamasaki M, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci. 2013;33(46):18161–18174. doi: 10.1523/JNEUROSCI.3506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50(1):73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, Irani SR, Rajabally YA, et al. Hypothermia in VGKC antibody-associated limbic encephalitis. J Neurol Neurosurg Psychiatry. 2008;79(2):202–204. doi: 10.1136/jnnp.2007.130039. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69(2):303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72(2):241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 18.Wieser S, Kelemen A, Barsi P, et al. Pilomotor seizures and status in non-paraneoplastic limbic encephalitis. Epileptic Disord. 2005;7(3):205–211. [PubMed] [Google Scholar]
- 19.Lee M, Lawn N, Prentice D, Chan J. Anti-NMDA receptor encephalitis associated with ictal asystole. J Clin Neurosci. 2011;18(12):1716–1718. doi: 10.1016/j.jocn.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Millichap JJ, Goldstein JL, Laux LC, Nordli DR, Jr, Stack CV, Wainwright MS. Ictal asystole and anti-N-methyl-D-aspartate receptor antibody encephalitis. Pediatrics. 2011;127(3):e781–e786. doi: 10.1542/peds.2010-2080. [DOI] [PubMed] [Google Scholar]
- 21.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136(pt 10):3151–3162. doi: 10.1093/brain/awt212. [DOI] [PubMed] [Google Scholar]


