Abstract
Ox40 ligand (Ox40L)–Ox40 pathway has been shown to enhance Th2 responses and play a role in pathogenesis of cutaneous leishmaniasis (CL) caused by Leishmania major. Using Ox40l−/− BALB/c mice we analyzed the role of this pathway in determining the outcome to CL caused by L. mexicana and compared to L. major. Contrary to our expectations, Ox40l−/− mice were highly susceptible to both L. major (LV39) and L. mexicana (M379) and developed large non-healing lesions containing parasites comparable to Ox40l+/+ BALB/c mice. Interestingly, upon in vitro stimulation with Leishmania antigen (LmAg), the lymph node cells from L. major infected Ox40l−/− mice produced significantly less IL-4 and IL-10 compared to Ox40l+/+ mice. L. mexicana infected Ox40l−/− and Ox40l+/+ mice did not show any difference in the production of IL-4 and IL-10. No difference was noted in the amount of Th1 cytokines IFN-γ and IL-12 produced by Ox40l−/− and Ox40l+/+ mice infected with either parasite. These results indicate that the Ox40L–Ox40 pathway promotes Th2 bias only in L. major infection but not L. mexicana infection and this pathway is not critical for susceptibility to CL.
Keywords: Ox40L–Ox40, L. major, L. mexicana, Cutaneous leishmaniasis
Graphical abstract
1. Introduction
1.1. Cutaneous leishmaniasis (CL)
CL manifests as localized cutaneous lesions at the site of infection with the parasite Leishmania (Herwaldt, 1999). Nearly 1.5 million new cases of CL are detected annually (Alvar et al., 2012). L. major and L. mexicana are common agents of CL in the Old World and the New World respectively (Herwaldt, 1999). In the mammalian host, Leishmania primarily survives and replicates within host macrophages, and clinical outcomes depend on whether macrophages are fully activated to clear the parasite. CD4+ Th1 cells produce IFN-γ which activates inducible nitric oxide synthase (iNOS) in macrophages, leading to the production of leishmanicidal nitric oxide (NO) (Scott, 1991). IL-12, another Th1 promoting cytokine produced by macrophages and dendritic cells (DCs), indirectly contributes to host immunity by inducing IFN-γ production in NK cells (Stamm et al., 1999). On the other hand, IL-4 produced by Th2 cells inhibits IL-12 mediated Th1 activation. IL-10, an anti-inflammatory cytokine, indirectly enhances Th2 responses by suppressing IL-12, IFN-γ and NO production by Leishmania infected cells. IL-4 and IL-10 thus act to favor parasite persistence and establishment of chronic CL (Chatelain et al., 1999a, 1999b).
1.2. Ox40 ligand (Ox40L)–Ox40 co-stimulation
During antigen presentation, co-stimulatory molecules on antigen presenting cells (APCs) also activate naive CD4+T cells via specific receptors which are critical in influencing differentiation of T cells into Th1 or Th2 lineages (Sharpe and Freeman, 2002). The co-stimulatory molecule, Ox40L is expressed by DCs, macrophages and B cells and signals via its receptor Ox40 which is a protein of the tumor necrosis factor (TNF) receptor super family expressed on activated T cells. Ox40L–Ox40 co-stimulation leads to activation of TNF receptor associated factor (TRAF) 2, 3 and 5. This pathway has been shown to prolong the survival of effector CD4+Th cells via expression of anti-apoptotic factors Bcl-2 and Bcl-XL as well as contributes to generation of memory T cells (Croft, 2010).
Earlier studies using in vitro models indicated that Ox40L–Ox40 interactions led to generation of Th2 responses during antigen presentation. While a number of disease models supported a Th2 response enhancing role (Jember et al., 2001; Tsukada et al., 2000; Yoshioka et al., 2000), other models have contradicted this role (Ishii et al., 2003; Zubairi et al., 2004). Activation of the Ox40 pathway has been shown to promote Th1 responses and contribute to parasite killing during L. donovani infection of mice (Zubairi et al., 2004). On the other hand, studies of L. major infection in Ox40l transgenic BALB/c mice, which overexpress OX40L mainly on T cells and display constitutive Ox40L–Ox40 interaction, showed increased parasite burdens and elevated Th2 responses (Ishii et al., 2003). Further Ox40l gene deficient BALB/c mice were shown to be more resistant to L. major infection than WT BALB/c mice and this was associated with a significant reduction in the production of Th2 cytokines (Ishii et al., 2003). However, the role of Ox40L–Ox40 interactions during L. mexicana infection has not been examined. Murine models have shown that immunological mechanisms governing resistance or susceptibility to CL are different between L. major and L. mexicana (Alexander and Kaye, 1985; McMahon-Pratt and Alexander, 2004).
To further examine the role of Ox40L–Ox40 interactions in CL, we examined host immune responses of wild type (Ox40l+/+) and Ox40l gene deficient (Ox40l−/−) mice to infection by L. mexicana and compared this with similar infection using L. major (LV39). Our results suggest that pathogen derived virulence factors could affect the role of the Ox40L–Ox40 pathway in determining disease outcomes of CL.
2. Materials and methods
2.1. Mice
Female Ox40l+/+ BALB/c mice were purchased from Harlan laboratory. Ox40l−/− BALB/c mice were provided by Dr. Arlene Sharpe (Brigham and Women’s Hospital) which were then bred and maintained at Ohio State University’s Animal facility in accordance with University Laboratory Animal Resource (ULAR) guidelines. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Ohio State University.
2.2. Parasites and infections
Two million L. mexicana (M379) and L. major (LV39) metacyclic promastigotes recovered from animal stocks were injected s.c. into hind left footpad of age and sex matched Ox40l+/+ and Ox40l−/− mice in 50 μl volume. Five mice per group were used in the study and 3 independent sets of such experiments were performed. Mice were monitored weekly for thickness of infected and uninfected contra-lateral footpads using a dial gauge micrometer (Mitutoyo). Difference in thickness of infected hind left footpad compared with the uninfected contralateral footpad is expressed as mean footpad swelling.
2.3. Quantification of parasite load
Infected footpads from mice sacrificed 7 weeks p.i. were cut and ground in 5 ml Schneider’s media (Gibco) supplemented with 10% fetal calf serum (FCS) (Gibco), 100 U/ml penicillin (Gibco) and 100 μg/ ml streptomycin (Gibco) over 70 μm nylon cell strainer (BD Falcon). The suspension was then centrifuged at 3000 rpm and resuspended in 400 μl of Schneider’s media. Limiting dilution assay was performed in duplicate as described previously (Rosas et al., 2005). Parasite viability was recorded after 4–7 days of incubation at 25 °C. The highest log dilution with viable parasites was reported.
2.4. Histopathology
Infected footpads from one representative mouse in each group were fixed in 10% formalin for at least 4 days. Fixed tissues were sent to Ohio State University’s Department of Comparative Pathology for routine H&E staining.
2.5. T cell proliferation assay and cytokine estimation
Popliteal lymph nodes were collected from infected mice, 7 weeks p.i. and single cell suspension was prepared in 5 ml RPMI 1640 (Gibco) supplemented with 10% FCS (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 10 μM HEPES (Gibco) and 500 μl beta-mercaptoethanol (Gibco). T cell proliferation was performed as previously described (Rosas et al., 2005). Supernatants were collected 72 hours after incubation and cytokine ELISA was performed according to manufacturer instructions. Cytokine clones used were IL-4: 11B11-capture, BVD6-24G2-detection (BD Biosciences), IL-10: JES5-16E3-capture, JES5-2A5-detection (Biolegend), IL-12: C18.2-capture, C17.8-detection (Biolegend), IFN-γ: R4-6A2-capture, XMG1.2-detection (Biolegend).
2.6. Antibody ELISA
Tail vein bleeding was done at weeks 2, 4 and 6 p.i., according to ULAR’s guidelines. Serum was obtained by centrifuging blood at 5000 rpm for 10 minutes and stored at −20 °C. L. mexicana and L. major specific IgG1 and IgG2a were detected by ELISA as previously described (Rosas et al., 2005) using HRP conjugated anti IgG1 and IgG2a antibodies and Streptavidin AKP (BD Pharmingen).
2.7. Statistical analysis
All data presented were obtained from 3 independent experiments. Unpaired Student’s t test was performed to compare statistical significance in footpad swelling, parasite load and cytokine concentration results. p value below 0.05 was considered significant. Mann–Whitney U prime test was used to compare antibody titers.
3. Results and discussion
3.1. Ox40l−/− mice are susceptible to L. mexicana infection
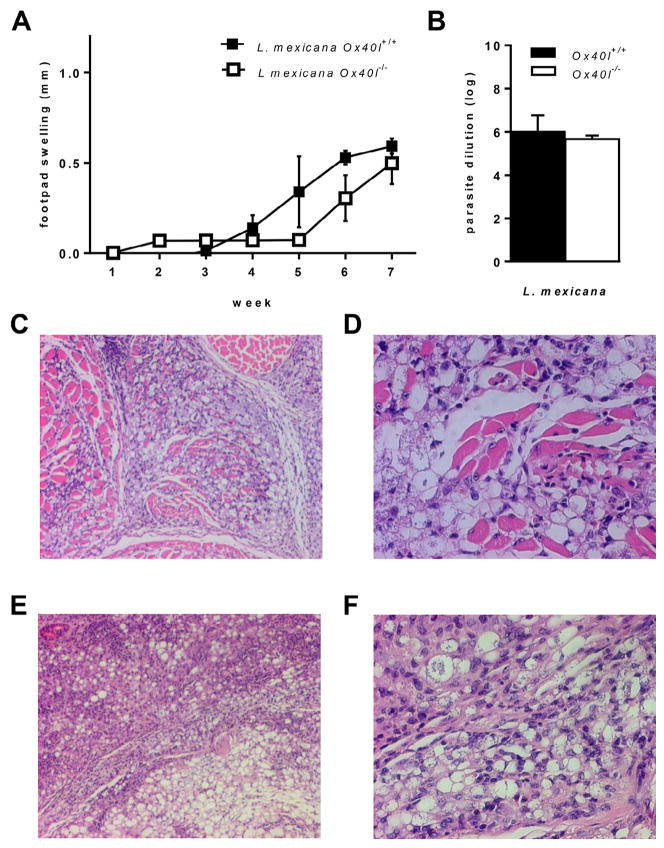
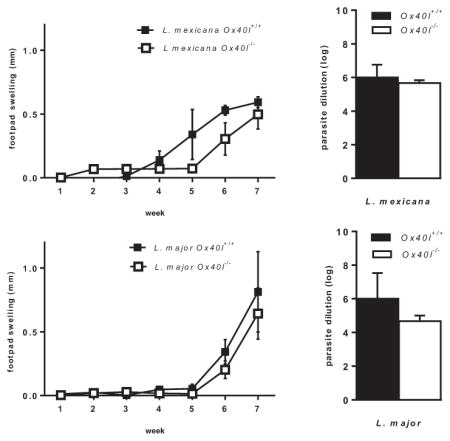
Ox40L–Ox40 co-stimulation has been implicated in inducing susceptibility to CL caused by L. major (Akiba et al., 2000; Ishii et al., 2003) but mediates resistance to visceral leishmaniasis (Zubairi et al., 2004). Although both L. major and L. mexicana cause CL, these parasites are significantly different in their phylogenetic, virulence and immunogenic characteristics (McMahon-Pratt and Alexander, 2004). We therefore analyzed the role of Ox40L–Ox40 co-stimulatory pathway in L. mexicana (M379) infection using Ox40l+/+ and Ox40l−/− BALB/c mice. We observed similar progressive footpad swellings in L. mexicana infected Ox40l−/− and Ox40l+/+ mice (Fig. 1A). Seven weeks p.i., infected footpads in Ox40l−/− and Ox40l+/+ mice further had similar parasite burdens (Fig. 1B). H&E staining of infected footpads also revealed similar inflammatory infiltrate comprising of lymphocytes and parasitized macrophages in both groups of mice (Fig. 1C–F). Our observations indicate that unlike previous studies with L. major, susceptibility to L. mexicana in BALB/c mice is independent of Ox40L pathway.
Fig. 1.
Footpad infection of Ox40l−/− and Ox40l+/+ BALB/c mice with L. mexicana. (Results are representative of three independent experiments.) (A) Lesion size is expressed as mean footpad swelling (millimeters) ± SE (n = 15 mice per group). (B) Parasite load is expressed as the largest log dilution that yields viable parasite upon culture for 4–7 days and is expressed as mean log dilution ± SE (n = 12 mice per group). (C–F) Histopathological examination of infected footpads after H&E staining (n = 3 mice per group). L. mexicana infected Ox40l+/+ mice, 10× (C) and 40× (D) and L. mexicana infected Ox40l−/− mice, 10× (E) and 40× (F).
Immunological mechanisms governing CL are different between L. major and L. mexicana. C57BL/6 mice infected with L. major develop a robust Th1 response and are resistant to infection but develop chronic CL upon infection with L. mexicana. Also, virulence factors which have the ability to modulate host immune responses are markedly different between these two Leishmania species. For example, surface lipophosphoglycan is an important virulence factor for L. major, but not L. mexicana (McMahon-Pratt and Alexander, 2004; Mottram et al., 2004). Cathepsin L-like cysteine protease B (CPB) enzymes have been identified as an important virulence factor in L. mexicana but not L. major (Buxbaum et al., 2003; Mottram et al., 2004). Moreover, genes involved in immune responses modulating susceptibility/ resistance to Leishmania infection are distinct for L. major and L. mexicana (Alexander and Kaye, 1985). It is therefore not surprising that the roles for co-stimulatory molecules in regulating immunity to Leishmania may differ between the two species.
3.2. Ox40L–Ox40 interaction does not affect Th1 or Th2 responses in L. mexicana infected BALB/c mice
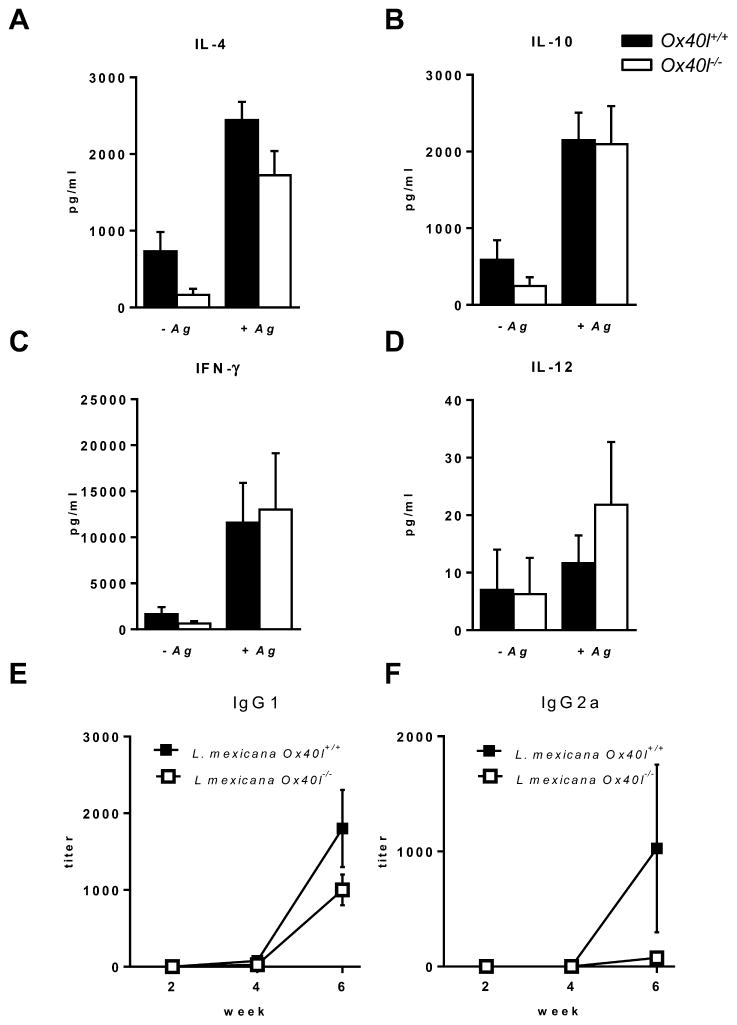
In L. major infected mice, Ox40 co-stimulation is associated with the generation of Th2 responses (Ishii et al., 2003), while in L. donovani infection Ox40 stimulation results in Th1 immune response generation (Zubairi et al., 2004). We therefore examined whether Ox40L–Ox40 interaction has any role in enhancing or suppressing either Th1 or Th2 immune responses during L. mexicana infection. Cytokine analysis of lymph node cells from L. mexicana infected Ox40l−/− and Ox40l+/+ mice re-stimulated with Leishmania antigen (LmAg) showed no differences in production of IL-4, IL-10, IFN-γ and IL-12 (Fig. 2A–D). Further, L. mexicana specific serum IgG1 and IgG2a antibodies, indicative of a Th2 and Th1 response respectively (Rosas et al., 2005), were comparable in both Ox40l−/−and Ox40l+/+ mice infected with L. mexicana (Fig. 2E,F). Our results indicate that Ox40L–Ox40 interaction does not contribute to the development of Th1 nor Th2 immune responses during L. mexicana infection of BALB/c mice. This contrasts with the Th2 promoting role of Ox40 co-stimulatory pathway in response to L. major infection (Ishii et al., 2003), as well as the enhanced Th1 response and subsequent parasite clearance in L. donovani infected mice following OX40 co-stimulation (Zubairi et al., 2004). Although mechanisms behind these observed differences are yet to be fully understood, these results seem to suggest that the immunological consequence of Ox40L–Ox40 interactions depends on prevailing immune conditions elicited by the infecting pathogen (Gupta et al., 2013; Oghumu et al., 2010; Tuladhar et al., 2011).
Fig. 2.
Cytokines and antibodies in Ox40l+/+ and Ox40l−/− mice infected with L. mexicana. IL-4 (A), IL-10 (B), IFN-γ (C) and IL-12 (D) concentrations measured by ELISA are expressed as mean concentration (picogram) ± SE (n = 15 mice per group). Serum antibody was tested for IgG1 (E) and IgG2a (F) specific for L. mexicana by ELISA. Results are represented as mean antibody titer ± SE (n = 15 mice per group).
Ox40L–Ox40 pathway is implicated in prolonging the survival and proliferation of effector T cells (Pippig et al., 1999), and it may be that Ox40 stimulation functions primarily to sustain ongoing T cell activation processes rather than direct T helper differentiation in Leishmania infections (Gramaglia et al., 1998). This would explain why different T helper states are enhanced by the Ox40L–Ox40 pathway after infection with different species of Leishmania. Further, other pathways including CD40L-CD40, B7-CD28/CTLA4 also play a role in skewing Th1/Th2 responses (Tuladhar et al., 2011), and Ox40L–Ox40 interactions have been shown to exert their effects much later after initial T cell activation (Curry et al., 2004; Gramaglia et al., 1998). It is therefore possible that the effects of Ox40 stimulation during L. mexicana infections are minimal, unlike in L. major infection. Another factor that may contribute to this difference is the role of parasite virulence factors. While L. mexicana virulence is mediated primarily by CPB, this factor has a much less profound role in L. major virulence (Mottram et al., 2004). CPB has been shown to be capable of inducing a Th2 response in L. mexicana infected BALB/c mice (Buxbaum et al., 2003). The immunomodulatory ability of L. mexicana specific virulence factors could potentially override any immune response exerted by Ox40 co-stimulation in mice infected with L. mexicana (Buxbaum et al., 2003; McMahon-Pratt and Alexander, 2004; Mottram et al., 2004).
3.3. Ox40l−/− mice display decreased Th2 responses than Ox40l+/+ mice but are equally susceptible to a virulent strain of L. major
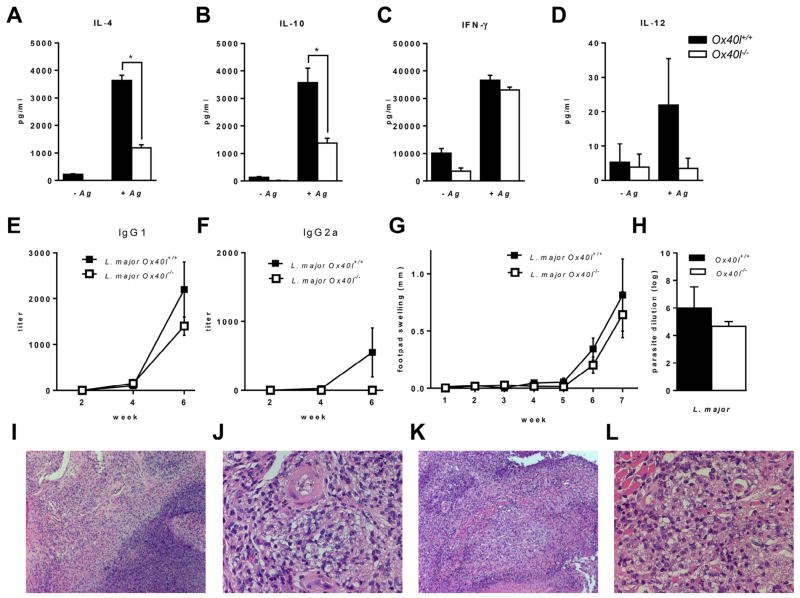
Previous studies indicated that blockade of Ox40L–Ox40 co-stimulation in L. major infection results in decreased IL-4 and IL-10 levels and led to abrogation of CL (Akiba et al., 2000; Ishii et al., 2003). Similar to these reports, we observed significantly reduced IL-4 and IL-10 production by L. major infected Ox40l−/− mice compared to Ox40l+/+ mice upon stimulation of lymph node cells with LmAg (Fig 3A,B). Ox40l−/− and Ox40l+/+ mice did not exhibit any differences in IFN-γ and IL-12 production (Fig. 3C,D). Further, production of serum IgG1 (Fig. 3E) and IgG2a (Fig. 3F) were similar between Ox40l−/− and Ox40l+/+ mice infected with L. major.
Fig. 3.
Footpad infection of Ox40l−/− and Ox40l+/+ BALB/c mice with L. major. (Results are representative of three independent experiments.) IL-4 (A), IL-10 (B), IFN-γ (C) and IL-12 (D) concentrations measured by ELISA are expressed as mean concentration (picogram) ± SE (n = 15 mice per group). * denotes p < 0.05 calculated by unpaired Student’s t test. Serum antibody was tested for IgG1 (E) and IgG2a (F) specific for L. major by ELISA. Results are represented as mean antibody titer ± SE (n = 15 mice per group). (G) Lesion size is expressed as mean footpad swelling (millimeters) ± SE (n = 15 mice per group). (H) Parasite burden in infected footpad is expressed as mean log dilution ± SE (n = 12 mice per group). (I–L) Histopathological examination of infected footpads by H&E staining (n = 3 mice per group). L. major infected Ox40l+/+ mice, 10× (I) and 40× (J) and L. major infected Ox40l−/− mice, 10× (K) and 40× (L).
However, contrary to previously published results, we observed comparable footpad swelling (Fig. 3G) and parasite loads (Fig. 3H) in Ox40l−/− and Ox40l+/+ mice infected with L. major (LV39). H&E staining of infected footpads also revealed similar degree of inflammation in Ox40l−/− and Ox40l+/+ mice (Fig. 3I–L). The difference in the outcome of L. major infection observed between our study and previous studies may possibly be a result of the different strains of L. major used. Previous studies used the L. major strain (MHOM/SU/73/5ASKH) while our current study was performed using L. major strain LV39 (MRHO/SU/59/P). A number of studies have demonstrated that different strains of L. major parasites vary in their pathogenicity and in the induction of immune responses which can alter the outcome of infection (Alimohammadian et al., 2010; Asadpour et al., 2013). Taken together, these results suggest that pathogen derived factors could affect the role of the OX40L–OX40 pathway in determining disease outcomes of CL.
It is surprising that although Th2 cytokine responses were reduced in L. major infected Ox40L−/− mice compared to Ox40L+/+ mice, lesion sizes and antigen specific IgG1 and IgG2a antibodies were comparable between both groups of mice. Although it is generally accepted that the Th2 cytokine IL-4 contributes to the generation of antigen specific IgG1 antibodies, other cytokines (Bryson et al., 2008) and parasite derived factors (Buxbaum, 2013; Mohammadi et al., 2006) have been shown to affect IgG subclass distribution. Moreover, levels of IgG1 and IgG2a antibodies do not always correlate with disease outcome (Fossati-Jimack et al., 2000). For example increased levels of IgG2a antibodies have been shown to contribute to disease exacerbation of CL in PD-L2−/− mice due to increased binding of opsonized parasites to FcγR and suppression of protective immune responses (Liang et al., 2006), which contrasts with the protective immune responses associated with elevated IgG2a levels in WT mice. Taken together our results suggest that IL-4 independent mechanisms affect the generation of antigen specific IgG isotypes in OX40L deficient mice during CL caused by L. major.
4. Conclusions
This is the first report that examines the effect of Ox40L deficiency in CL caused by L. mexicana. Our study demonstrates that the Ox40L–Ox40 pathway does not contribute to Th1 nor Th2 bias and subsequently disease outcome of L. mexicana infection. Our study also shows that this co-stimulatory pathway promotes Th2 responses, but is not required for susceptibility to CL caused by L. major strain LV39.
HIGHLIGHTS.
First study to examine the role of Ox40L–Ox40 in L. mexicana infection.
Ox40L pathway does not induce Th2 responses in experimental L. mexicana infection.
Ox40L–Ox40 co-stimulation is not critical for susceptibility to L. mexicana.
Ox40L–Ox40 promotes Th2 response but does not affect outcome of L. major infection.
Acknowledgments
This work was supported by National Institutes of Health grants R03AI090231, RC4AI092624, R34AI100789, R21AT004160 and R03CA164399 awarded to A.R.S and National Institute of Dental and Craniofacial Research Training Grant T32DE014320 awarded to S.O.
Abbreviations
- CL
cutaneous leishmaniasis
- CPB
cysteine protease B
- FCS
fetal calf serum
- LmAg
Leishmania antigen
- Ox40L
Ox40 ligand
- p.i
post infection
- TNFR
tumor necrosis factor receptor
References
- Akiba H, Miyahira Y, Atsuta M, Takeda K, Nohara C, Futagawa T, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Kaye PM. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin Exp Immunol. 1985;61:674–682. [PMC free article] [PubMed] [Google Scholar]
- Alimohammadian MH, Darabi H, Ajdary S, Khaze V, Torkabadi E. Genotypically distinct strains of Leishmania major display diverse clinical and immunological patterns in BALB/c mice. Infect Genet Evol. 2010;10:969–975. doi: 10.1016/j.meegid.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadpour A, Riazi-Rad F, Khaze V, Ajdary S, Alimohammadian MH. Distinct strains of Leishmania major induce different cytokine mRNA expression in draining lymph node of BALB/c mice. Parasite Immunol. 2013;35:42–50. doi: 10.1111/pim.12018. [DOI] [PubMed] [Google Scholar]
- Bryson KJ, Wei XQ, Alexander J. Interleukin-18 enhances a Th2 biased response and susceptibility to Leishmania mexicana in BALB/c mice. Microbes Infect. 2008;10:834–839. doi: 10.1016/j.micinf.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Buxbaum LU. Leishmania mexicana infection induces IgG to parasite surface glycoinositol phospholipids that can induce IL-10 in mice and humans. PLoS Negl Trop Dis. 2013;7:e2224. doi: 10.1371/journal.pntd.0002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LU, Denise H, Coombs GH, Alexander J, Mottram JC, Scott P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol. 2003;171:3711–3717. doi: 10.4049/jimmunol.171.7.3711. [DOI] [PubMed] [Google Scholar]
- Chatelain R, Mauze S, Coffman RL. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 1999a;21:211–218. doi: 10.1046/j.1365-3024.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- Chatelain R, Mauze S, Varkila K, Coffman RL. Leishmania major infection in interleukin-4 and interferon-gamma depleted mice. Parasite Immunol. 1999b;21:423–431. doi: 10.1046/j.1365-3024.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry AJ, Chikwe J, Smith XG, Cai M, Schwarz H, Bradley JA, et al. OX40 (CD134) blockade inhibits the co-stimulatory cascade and promotes heart allograft survival. Transplantation. 2004;78:807–814. doi: 10.1097/01.tp.0000131670.99000.54. [DOI] [PubMed] [Google Scholar]
- Fossati-Jimack L, Ioan-Facsinay A, Reininger L, Chicheportiche Y, Watanabe N, Saito T, et al. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcgamma receptor III. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol. 2013;82:155–184. doi: 10.1016/B978-0-12-407679-2.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Ishii N, Ndhlovu LC, Murata K, Sato T, Kamanaka M, Sugamura K. OX40 (CD134) and OX40 ligand interaction plays an adjuvant role during in vivo Th2 responses. Eur J Immunol. 2003;33:2372–2381. doi: 10.1002/eji.200324031. [DOI] [PubMed] [Google Scholar]
- Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, et al. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36:58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Mohammadi MR, Zeinali M, Ardestani SK, Kariminia A. Identification of novel Leishmania major antigens that elicit IgG2a response in resistant and susceptible mice. Korean J Parasitol. 2006;44:43–48. doi: 10.3347/kjp.2006.44.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram JC, Coombs GH, Alexander J. Cysteine peptidases as virulence factors of Leishmania. Curr Opin Microbiol. 2004;7:375–381. doi: 10.1016/j.mib.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Oghumu S, Lezama-Davila CM, Isaac-Marquez AP, Satoskar AR. Role of chemokines in regulation of immunity against leishmaniasis. Exp Parasitol. 2010;126:389–396. doi: 10.1016/j.exppara.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig SD, Pena-Rossi C, Long J, Godfrey WR, Fowell DJ, Reiner SL, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–6529. [PubMed] [Google Scholar]
- Rosas LE, Keiser T, Barbi J, Satoskar AA, Septer A, Kaczmarek J, et al. Genetic background influences immune responses and disease outcome of cutaneous L. mexicana infection in mice. Int Immunol. 2005;17:1347–1357. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Stamm LM, Satoskar AA, Ghosh SK, David JR, Satoskar AR. STAT-4 mediated IL-12 signaling pathway is critical for the development of protective immunity in cutaneous leishmaniasis. Eur J Immunol. 1999;29:2524–2529. doi: 10.1002/(SICI)1521-4141(199908)29:08<2524::AID-IMMU2524>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–2439. [PubMed] [Google Scholar]
- Tuladhar R, Natarajan G, Satoskar AR. Role of co-stimulation in Leishmaniasis. Int J Biol Sci. 2011;7:1382–1390. doi: 10.7150/ijbs.7.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol. 2004;34:1433–1440. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]