Abstract
Findings in BRCA1 mutation carriers suggest that physical activity, particularly during childhood, may be linked to a reduced risk of developing breast cancer. We investigated whether physical activity at puberty alters the expression of BRCA1 and two other tumor suppressor genes—p53 and estrogen receptor (ER)-β—in rats. In addition, the effects on ER-α expression, mammary proliferation and functional epithelial differentiation were investigated as markers of altered mammary cancer risk in rats exposed to regular physical activity at puberty. Female Sprague Dawley rat pups were randomized to voluntary exercise, sham-exercise control and non-manipulated control groups. Treadmill training (20–25 m/min, 15% grade, 30 min/day, 5 days/week) started on postnatal day 14 and continued through day 32. Third thoracic mammary glands (n = 5 per group and age) were obtained at days 32, 48 and 100 and assessed for changes in morphology through wholemounts, and at 100 days cell proliferation by using Ki67 staining, protein levels of ER-α and ER-β by immunohistochemistry, and mRNA expression levels of BRCA1, p53, ER-α and ER-β by real-time PCR. Mammary glands of rats exposed to exercise during puberty contained fewer terminal end buds (TEBs) and a higher number of differentiated alveolar buds and lobules than the sham controls. However, cell proliferation was not significantly altered among the groups. ER-α protein levels were significantly reduced, while ER-βlevels were increased in the mammary ducts and lobular epithelial structures of 100-day old rays which were voluntarily exercised at puberty, compared to sham controls. ER-β BRCA1 and p53 mRNA levels were significantly higher in the mammary glands of 100-day-old exercised versus sham control rats. Pubertal physical activity reduced mammary epithelial targets for neoplastic transformation through epithelial differentiation and it also up-regulated tumor suppressor genes BRCA1, p53 and ER-β and reduced ER-α/ER-β ratio in the mammary gland. It remains to be determined whether the up-regulation of BRCA1, and perhaps p53, explains the protective effect of childhood physical activity against breast cancer in women who carry a germline mutation in one of the BRCA1 alleles.
Keywords: Prepubertal physical activity, BRCA1, p53, Estrogen receptor, Terminal end bud
Introduction
Numerous epidemiological studies have shown that engagement in physical activity during certain periods of life has a protective effect against sporadic breast cancer development: women who exercised during adolescence, menopause or throughout their life exhibit a reduction in breast cancer risk [1]. A meta-analysis of 19 case-control and four cohort studies indicated a dose-dependent, inverse relationship between physical activity during adolescence and breast cancer susceptibility [2]. Interestingly, physical activity during adolescence also significantly delayed breast cancer development in women with germline mutations in the breast cancer 1 (BRCA1) gene [3]. The effects of physical activity on the breast might be related to a reduction in circulating hormone and growth factor levels, including estrogens, leptin and insulin-like growth factors [4–6]. Further, hormonal status may modify the effects of physical activity as the reduction in risk is more strongly evident in postmenopausal women, while inconsistent in pre-menopausal women [7–9].
BRCA1 and BRCA2 tumor suppressor genes are the most important breast cancer susceptibility genes identified thus far in familial breast cancer [10]. Although these genes are practically never mutated in sporadic breast cancer, BRCA1 has been shown to be down-regulated frequently [11, 12], suggesting that BRCA1 haploinsufficiency potentially could increase sporadic breast cancer risk. Conversely, increased BRCA1 expression has been associated with increased mammary gland differentiation in rodents [13, 14], which in turn is linked to reduced mammary cancer risk [15]. BRCA1 has many roles in the breast: it is involved in the maintenance of genomic integrity, DNA replication and repair, chromatin remodeling, and it also functions as a transcriptional regulator [16, 17]. In cell-based assays, wild-type BRCA1 inhibits proliferative signaling induced by activation of estrogen receptor (ER)-α by blocking the receptor’s transactivation function [18]. Moreover, BRCA1 and ER-α have been shown to interact directly at the protein level [19]. These studies indicate that BRCA1 is a negative regulator of ER-α.
p53, another tumor suppressor gene, is one of the most commonly mutated genes in human cancers. Alterations to the p53 protein contribute to neoplastic transformation through several mechanisms including changes in DNA repair, apoptosis, cell cycle regulation, angiogenesis and metastasis [20]. Germline mutations of p53 markedly elevate the risk of early-onset (<45 years) breast cancer in association with Li-Fraumeni disease [21], and sporadic p53 mutations account for nearly 20% of breast carcinomas [22]. p53 has been shown to interact directly with BRCA1 [23], and loss of the p53 protein is a critical event in the pathogenesis of tumors in BRCA1 mutation carriers [24]. p53 is also a negative regulator of ligand-activated ER-α transactivation, by directly binding to several domains within ER-α and blocking the receptor’s binding to estrogen response elements [25].
The rodent mammary gland model provides important parallels to the growth and development of the human breast and breast cancer pathology [26]. In adult rats, the mammary gland is comprised of stromal and adipose tissues surrounding a ductal network of parenchyma. The main duct, which connects to the nipple at one end, branches out radially into lobular units at the other end. Each unit further arborizes into smaller ductules and ends in terminal lobes. The most immature form of the terminal lobes is the terminal end buds (TEBs), which attain their maximum number at 21 days [27]. Under the influence of each estrus cycle, beginning around 32–36 days in rats, the TEBs begin to cleave three to five times, differentiating into alveolar buds (ABs), which further differentiate into lobules. The mammary gland undergoes additional, unique maturation during pregnancy and lactation. It finally regresses during menopause and thereafter (for review, see [15]). The rodent TEBs and their human equivalent, the terminal ductal lobular unit (TDLU), are significant in that they are maximally susceptible to carcinogens, given their high population of rapidly dividing cells [27]. In rodents, TEBs are the structures that give rise to 7,12-dimethybenz[a]anthracene (DMBA)-induced malignant mammary tumors [27]. In women, most breast tumors arise within TDLUs [28].
Maturation of the mammary gland involves a complex interplay of signaling factors, among which are the estrogens and their receptors. To date, two major ER isoforms have been identified in the mammary glands which mediate the action of estrogens: ER-α and ER-β ER-α induces proliferation of the mammary tissue, although how this happens is not clear since the proliferating mammary cells do not contain this receptor [29]. ER-β is believed to be a potent attenuator of ER-α trans-activation in the mammary gland, presumably through its dominant negative effect as a heterodimeric partner of ER-α [30]. Data from a number of tissue culture assays [31], microarray analyses [32, 33], and, in particular, ER-β null mice [34], generally support a role as a cell proliferation inhibitor for ER-β.
In the present study, we investigated, by utilizing a rat model, whether prepubertal physical activity influences mammary gland morphology and/or the expression of BRCA1 and p53 as well as the ER-α and/or ER-β levels.
Materials and methods
Animals
The AMC Cancer Research Center Institutional Animal Care and Use Committee approved all animal research and the experiments were performed following the National Institutes of Health guidelines for the proper and humane use of animals in biomedical research. Pregnant female Sprague-Dawley rats were obtained from Harlan Teklad (Organ, WI, USA) on gestation day 14 and housed singly at 72°F, 50% humidity, on a 12 h light/dark cycle. Semipurified AIN93G diet and distilled water were provided ad libitum. Pups were weaned at postnatal day 21, and housed in groups of three female rats per cage.
Physical activity exposure
Female pups were stratified by weight into three groups; exercise (n = 32), sham-exercise (n = 31), and out of room control (n = 30). The exercise and sham exercise were initiated at postnatal day 14 and terminated at day 32, consisting of a total of 14 training sessions. Training was conducted during the rats’ dark cycle under red light. Exercise training consisted of motorized treadmill running (17.5–20 m/min, 30 min/day, 15% grade, 5 days/week). Rats were gradually acclimated to running by increasing speed and duration. Within 7 days, all rats were completing the full 30 min of exercise. Mild electric shock was available, but was not used. Sham exercise consisted of placing rats on a stationary treadmill set at 15% grade for the same duration as the exercise rats. The out of room control rats were housed in a separate room from the treadmills and exercise and sham rats. They were not handled 5 days per week like the exercise and sham rats. Standard animal husbandry practices were maintained.
Mammary gland morphology by whole mount analysis
Third thoradic mammary glands were obtained from five rats per group and age, at days 32, 48 and 100. The harvested glands were stretched on a microscopic slide and placed into a mammary gland fixative solution which contained 25% glacial acetic acid (EM Science, Gibbstown, NJ) and 75% ethanol (Fisher Scientific Co.) for overnight. Mammary glands were then washed in 70% ethanol for one day and stained in carmine aluminum overnight. The next day, the glands were washed again by leaving them to 70% ethanol overnight, followed by additional washing in 95% and 100% ethanol, and then placed in xylene (Fisher Scientific Co.). Glands were covered with Permount (Fisher Scientific Co.) and coverslipped.
Mammary wholemounts were analyzed blindly by two independent investigators using an Olympus Vanox-S microscope (Olympus America, Inc.). The structures of the mammary glands were evaluated in a double-blind manner. The presence of alveolar buds (ABs) in 3-week-old pups and lobulo-alveolar units (LAUs) in 8-week-old rats was evaluated using a five-point scale (0, absent; 1, low; 2, low-moderate; 3, moderate; 4, moderate-high; 5, high). We have validated and successfully used this scale in our earlier studies [35]. The total number of TEBs per mammary gland, and the length of the epithelial tree (along a straight line from the nipple, through the lymph node, to the point most farther away from it), was also determined.
Real-time PCR to determine mRNA expression of ER-α, ER-β, BRCA1 and p53
Total RNAs were extracted from mammary glands of 100-day-old rats exposed to physical activity, sham activity or no activity. Frozen tissue samples were placed in 1″ × 1″ plastic bags, pulverized on dry ice, transferred to 35 ml conical Oakridge tubes (Nalgene, Rochester, NY), and weighed. Tissues were homogenized in 1 ml of TRIzol Reagent (Invitrogen Corporation, Carlsbad, CA) per 50 mg of tissue, using a PowerGen 35 hand-held homogenizer (Fisher Scientific, Pittsburgh, PA), with RNAse-free disposable OMNI-Tips (Fisher Scientific) for 30 s. From this point, procedures were followed according to manufacturer’s instructions for TRIzol Reagent. The quantity and quality of RNA was measured by comparing the optical density rations (OD260/OD280) obtained using a Beckman DU640 Spectrophotometer (Beckman, Fullerton, CA).
Total RNA was processed using the RNeasy mini kit (Qiagen, Inc., Valencia, CA) as per manufacturer’s instructions. cDNA was reverse transcribed from 100 µg/ml of total input RNA using Taqman Reverse Transcription Reagents as described by the manufacturer (Applied Bio-systems, Foster City, CA). Next, PCR products were generated from the cDNA samples using the Taqman Universal PCR Master Mix and Assays-on-Demand (Applied Biosystems) for the appropriate target gene. The 18S Assay-on-Demand was used as an endogenous control. All assays were run on 384-well plates so that each cDNA sample was run in triplicate for the target gene and the endogenous control. Real-time PCR was performed on an ABI Prism 7900 Sequence Detection System and the results assessed by relative quantitation of gene expression using the ΔΔCT method.
Immunohistochemistry to determine changes in protein levels
Formalin-fixed tissue sections (5 µm) obtained from the 3rd thoracic mammary glands of five 100-day-old rats per group were deparaffinized in xylene, hydrated through graded alcohol and incubated with 3% H2O2 for 10 min to block endogenous peroxidases. Non-specific binding was blocked with normal rabbit serum from the Vectastain Elite ABC Kit (Vector Laboratories, Inc.) for 20 min., blocked, incubated with ER-α antibody (1:100) (MC-20, Santa Cruz Biotechnology, CA) or ER-β antibody (1:250) (PA-313, Affinity Bioreagents, Inc, CO), washed, treated with biotinylated goat anti-serum to mouse IgG followed by incubation with Streptavidin-peroxidase conjugate (Dako Cytomation The Ark Kit, Carpinteria, CA). Antigen-antibody complexes were visualized by 3′,3-diaminobezidine and counterstained with hematoxylin stain, dehydrated and mounted. Control slide was incubated with normal mouse serum. The percentage of positive cells was determined by calculating the number of cells that had positive staining (only darked stained cells were counted) per 1000 cells per mammary gland structure (lobules or ducts). Slides were blindly evaluated with help of the Metamorph software.
Cell proliferation
The same formalin-fixed tissues used to determine protein levels were used for cell proliferation assays. Sections (5 µm) were heated overnight at 57°C, rehydrated in graded alcohol, and re-heated in microwave in high power for 10–15 min in 0.01 M citrate buffer (pH 6.0) for antigen retrieval. After cooling the slides in room temperature and washing with PBS, the sections were incubated for 10 min in 3% H2O2 to denature any endogenous peroxidase. Sections were washed again with PBS, exposed to Ki67 antibody (Goat polyclonal IgG, SC-9857, Santa Cruz), and incubated with 20% rabbit serum for over night at 4°C. Sections were then washed and treated with biotinylated rabbit antiserum to goat IgG (Vector laboratories) for 30 min followed by a 30 min incubation with streptavidin–peroxidase conjugate (Vectastin ABC Kit, Vector Laboratories). Antigen antibody complex was visualized by incubation with 3,3′diaminobenzidine (Vector laboratories). Finally, sections were treated with Gills haematoxyline, stained, dehydrated through graded alcohol, and mounted. To determine the level of cell proliferation, the number of cells showing Ki67 staining was assessed per 1,000 cells in TEBs, lobules and ducts.
Statistical analyses
Results for the data obtained on mammary gland morphology (number of TEBs, density of LAUs, length of epithelial tree) were analyzed with SigmaStat software using one-way analysis of variance (ANOVA), separately at each age studied. Data generated using immunohistochemistry (percent cells positive per 1,000 lobular cells and 1,000 ductal cells per gland) also were analyzed using one-way ANOVA. Where appropriate, between-group comparisons were done using Tukey’s multiple comparisons test. Due to the variability in mRNA levels, data obtained using real time PCR were analyzed with non-parametric Mann-Whitney rank test, comparing sham controls to exercised rats. The differences were considered significant if the P-value was less than 0.05. All probabilities were two-tailed.
Results
Weight gain and vaginal opening
The pups were weighed two times a week, starting on the first day of exercise training. The weight gain during the training in the out of room contrals (ORG) (mean ± SEM; 48.6 ± 1.6 g), sham exercise controls (SHAM) (51.2 ± 1.5 g) and exercised rats (EX) (50.1 ± 1.4 g) was similar. Vaginal opening also occurred at the same age in all three groups (data not shown).
Mammary gland morphology
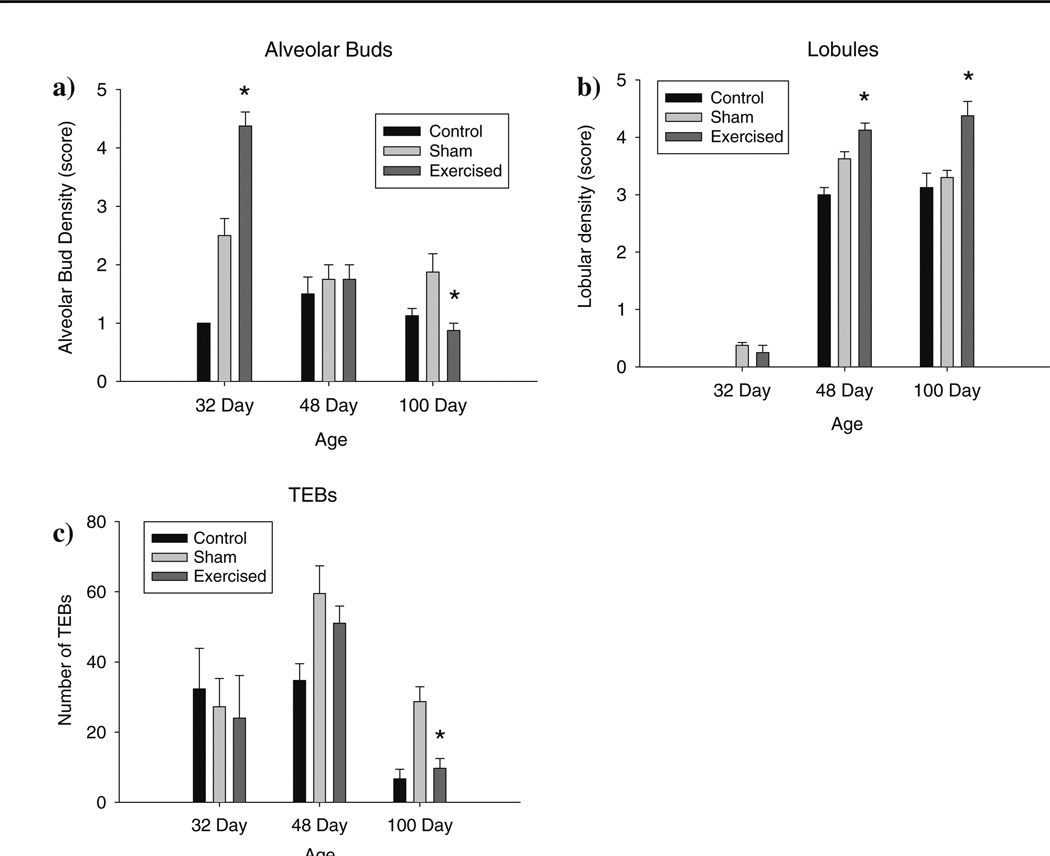
Comparisons of the morphological features of the 3rd thoracic mammary gland showed significantly increased epithelial density of both the alveolar and lobular structures among rats exposed to exercise during prepuberty versus sham-controls. This difference was evident at all three developmental time points (32, 48, 100 days). Alveolar density in the exercise group was significantly higher in the glands obtained from rats at day 32 but not at days 48 or 100 (F(4,26) =16.39, P < 0.001) (Fig. 1a); at the two older ages highest number of alveolar buds was seen in the sham controls. The density of the lobular structures was higher in the 48- and 100-day old exercised rats than in the sham controls (F(2,26) = 55.89, P < 0.001) (Fig. 1b).
Fig. 1.
Mammary gland morphology, assessed in mammary wholemounts of the 3rd thoracic mammary gland, of 32, 48 and 100 old rats exposed daily to voluntary treadmill activity between postnatal days 14 and 32. When compared to the sham exercise group, (a) alveolar density is significantly increased in the 32-day-old rats in the exercise group (* P < 0.001); (b) lobular density is significantly higher in 48- and 100-day old rats in the exercise group (* P < 0.001); and (c) the number of TEBs is significantly reduced in the 100-day-old rats (* P < 0.002). Mammary glands from 5 rats per group and age were used
The number of TEBs in the exercised rats was reduced, with a significant reduction seen in the 3rd thoracic mammary glands obtained from 100 -day-old rats, when compared to those in sham-controls (one-way ANOVA at 100 days: F(2,11) = 12.87, P < 0.002). Similar findings were obtained in the mammary glands of 32- and 48-day-old rats, but the differences failed to reach statistical significance (Fig. 1c).
Cell proliferation
Cell proliferation was assessed only in the mammary glands obtained from 100-day-old rats. At this age, not differences among the groups were seen (data not shown).
Gene expression
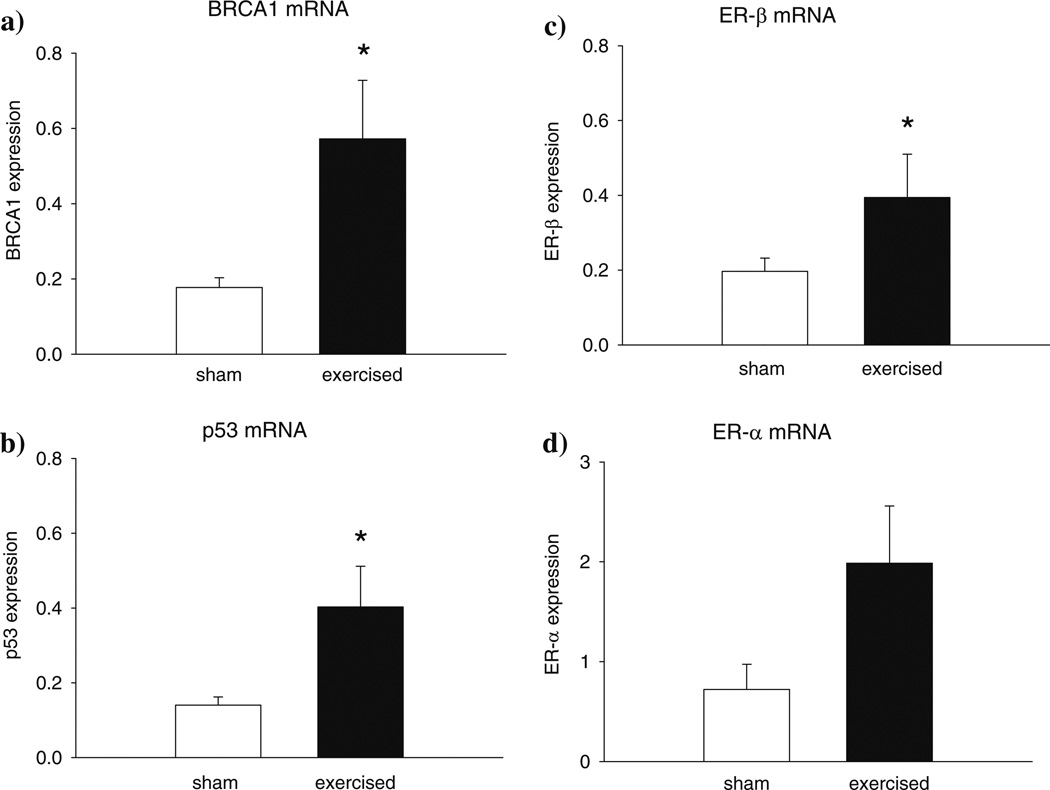
Expression levels of BRCA1 (P < 0.029), p53 (P = 0.056) and ER-β (P < 0.029) were significantly up-regulated in the 100 –day-old mammary glands of rats exposed to exercise, as compared to their sham controls (Fig. 2a– c). No differences in the expression of ERα were noted between the exercised and sham control rats (Fig. 2d). Expression of these four genes was not statistically significant in the mammary glands of the non-manipulated control rats and the sham controls (data not shown).
Fig. 2.
Expression of (a) BRCA1 (* P < 0.029), (b) p53 (* P < 0.056) and (c) ER-β (* P < 0.029) mRNAs were significantly up-regulated in the mammary glands of 100-day-old rats exposed to voluntary treadmill activity during prepuberty, when compared to sham control rats. No changes in the mRNA levels of (d) ER-α were seen. Medians and expression levels at 25% and 75% points are shown, n = 4–5 per group. Expression was determined by real time PCR, and data were quantitated using the ΔΔCT method
Protein expression
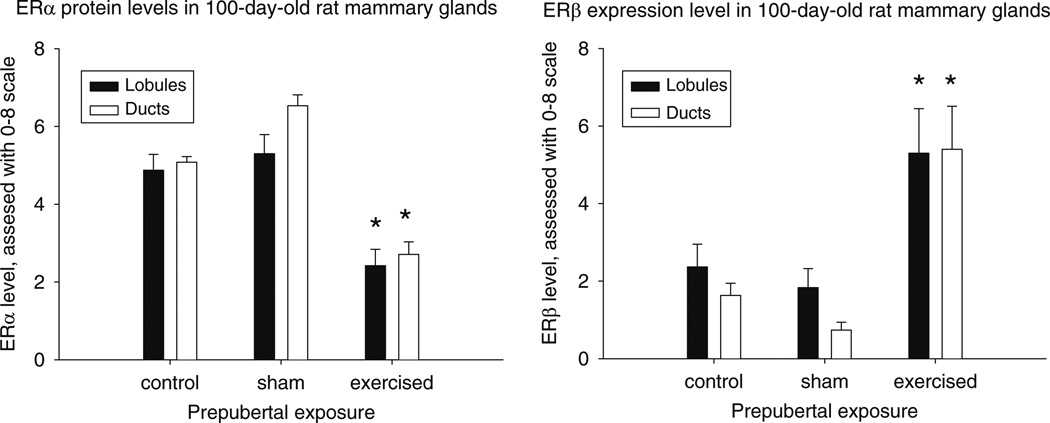
ER-α levels, based on immunohistochemistry, were significantly reduced in both the lobules (P < 0.003) (F(2,10) = 11.52, P < 0.003) and ducts (P < 0.001) (F(2,10) = 54.20, P < 0.001) of mammary glands taken from the exercised rats at 100 days, when compared to the sham controls (Fig. 3a). Conversely, ER-β levels were significantly elevated approximately three-fold in lobules (P < 0.028) (F(2,12) = 5.18, P < 0.024) and up to six-fold in ducts (P < 0.001) (F(2,12) = 13.52, P < 0.001) in the mammary glands of 100 –day-old rats exposed to physical activity before puberty versus the controls (Fig. 3b).
Fig. 3.
Protein levels of ER-α and ER-β in the mammary glands of 100-day-old rats exposed to voluntary treadmill activity during prepuberty, when compared to sham control rats. Data for non-manipulated controls are also shown. Expression was determined using immunohistochemistry, and percentile of positive cells per 1,000 cells per structure (lobules or ducts) per gland (n = 4–5 per group) was counted. Protein levels of ER-α were significantly lower and those of ER-β higher in the glands of exercised rats than in the sham controls: * P < 0.05–0.001
Discussion
To corroborate evidence from human studies which associate exercise during childhood years to reduced risk of obtaining breast cancer [2], this animal study was designed to identify changes in the mammary glands of rats exposed to voluntary physical activity before puberty onset. The rats were voluntarily running in a treadmill from the time the pups begin to consume food pellets on postnatal day 14 (instead of just nursing) until puberty onset on postnatal day 32. Vaginal opening, characterizing puberty onset, on average takes place on postnatal day 32–34 in Sprague Dawley rats [35]. The study provides one of the first insights into the biological mechanisms that may mediate the protective effect of being physically active during childhood against breast cancer.
The mammary glands of female rats which voluntarily exercised during prepuberty were significantly more differentiated and contained fewer of the TEBs than the glands of the sham-control rats. Given that TEBs—these correspond to TDLUs in humans [28]—give rise to the most of the malignant tumors, while differentiated tissue is refractory to a carcinogenic insult [27], this mammary gland profile is predictive of a reduced risk for breast cancer. Hence, exercise during childhood may confer protection against breast cancer by promoting differentiation of the mammary epithelial tree. No changes in mammary epithelial cell proliferation were noted by prepubertal exercise, but proliferation was assessed only at the age of 100 days when the mammary gland is fully mature and exhibits relatively few proliferating cells.
At the gene and protein expression level, we found that early life exercise reduced the expression of ER-α and increased the expression of ER-β in the mammary glands of adult rats. Since activation of ER-α induces proliferation of mammary epithelial cells, while ER-β inhibits the effects of ER-α and induces differentiation [30, 31], the effects of exercise before puberty onset on these two receptors is expected to cause differentiation in the epithelial tree. This is what we observed took place in the mammary epithelium. Further, ER-α is one of the transcription factors which stimulates TEB formation [36]. Thus, the increased ratio of ER-β to ER-α proteins in the lobules and ducts might have contributed to the lower number of TEBs present in the mammary glands of rats exposed to treadmill exercise before puberty onset.
BRCA1 and p53 may also be involved in affecting mammary gland morphology. Selective disruption of Brca1 in the mammary glands of Brca1 f/f:MMTV-Cre mutant mice leads to impaired TEB differentiation upon an exposure to estradiol and progesterone [37], and abberant ductal development during pregnancy, lactation and involution [38]. Overexpression of this tumor suppressor, in turn, stimulates lobulo-alveolar development [13], indicating increased epithelial differentiation. Mice lacking p53 exhibit delayed mammary epithelial involution [39], implicating that this tumor suppressor also plays a role in mammary gland morphology. It remains to be determined whether the up-regulation of BRCA1 and p53 mRNA in the mammary glands of adult rats exposed to voluntary physical activity before puberty onset participated in affecting their mammary gland development.
The significantly elevated expression of BRCA1 and p53 in the mammary glands of 100-day-old rats exposed to exercise prepubertally provides potentially additional molecular markers that may be prognostic of long-term reduced breast cancer risk by exercise. Thus far, genetic identification of germline mutations in the BRCA1 and BRCA2 genes have been used as predictors of inherited breast cancer risk. Li-Fraumeni disease which is characterized by germline p53 mutation also increases the risk of early onset breast cancer. Although somatic mutations are generally extremely rare for BRCA1, but relative common for p53, both are often down-regulated in sporadic breast cancer, perhaps due to promoter methylation of BRCA1 [40], upstream silencing of the p53 regulator, Hoxa5a [41], and frequent loss of heterozygosity for both tumor suppressor genes [42]. Our data support the evidence that increased expression of BRCA1 and p53 protect the normal breast tissue from tumor development, since exercise during childhood that is known to reduce breast cancer risk [2], both induced morphological differentiation and up-regulation of BRCA1 and p53.
In the context of prepubertal exercise, BRCA1, p53 and ER-β may provide protection against sporadic breast cancer development through a multitude of pathways and/or mechanisms. Their functions could also converge. Given the striking evidence that BRCA1 functions as a direct binding transcriptional co-regulator of p53 [23, 24] and ER-α (which heterodimerizes with ER-β) [18], these proteins may be in a multimeric protein complex(es) that is specific to breast epithelium. If so, a reduction in breast cancer risk by exercise during prepuberty may induce sufficient levels of transcripts and subsequent protein levels to form functional complexes that confer protection.
Conclusion
In rats, voluntary exercise before puberty onset decreases the number of targets for malignant transformation and promotes breast differentiation, and when determined in adult mammary gland increases the ER-β: ER-α ratio and up-regulates the expression of BRCA1 and p53. These biological changes might be associated with reduced breast cancer risk in individuals who were physically activity during prepubertal period, and might lead to reduced breast cancer risk also in women who have inherited a mutated BRCA1 gene that pre-disposes them to this disease.
Acknowledgments
We thank Dr. Elizabeth Cho-Fertick who provided medical writing services, funded by National Cancer Institute (U54 CA00100971 for L.H.-C.). The study was funded by grants from Prevent Cancer Foundation (K W.) and National Cancer Institute (U54 CA00100971, L.H.-C.).
Footnotes
Authors’ contributions The work described in the manuscript was designed to test a hypothesis proposed by Dr. Leena Hilakivi-Clarke who provided overall direction for the study. Drs. Kim Westerlind and Robert Strange performed the animal study and provided tissues for the analysis, Dr. Mingyue Wang did most of the gene and protein expression experiments, together with Drs. Bin Yu, Galam Khan and Dipti Patil. Graduate student Kelly Boeneman processed the mammary glands and performed morphological assessment.
Contributor Information
M Wang, Department of Oncology, Georgetown University, Washington, DC, USA.
B Yu, Department of Oncology, Georgetown University, Washington, DC, USA.
K Westerlind, Division of Endocrinology, University of Colorado Health Sciences Center, Denver, CO, USA.
R Strange, Division of Medical Oncology, University of Colorado Health Sciences Center, Denver, CO, USA.
G Khan, Department of Oncology, Georgetown University, Washington, DC, USA.
D Patil, Department of Oncology, Georgetown University, Washington, DC, USA.
K Boeneman, Department of Oncology, Georgetown University, Washington, DC, USA.
L Hilakivi-Clarke, Email: clarkel@georgetown.edu, Department of Oncology, Georgetown University, Washington, DC, USA.
References
- 1.Friedenreich CM, Courneya KS, Bryant HE. Influence of physical activity in different age and life periods on the risk of breast cancer. Epidemiology. 2001;12:604–612. doi: 10.1097/00001648-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lagerros YT, Hsieh SF, Hsieh CC. Physical activity in adolescence and young adulthood and breast cancer risk: a quantitative review. Eur J Cancer Prev. 2004;13:5–12. doi: 10.1097/00008469-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 3.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 4.Hulver MW, Houmard JA. Plasma leptin and exercise: recent findings. Sports Med. 2003;33:473–482. doi: 10.2165/00007256-200333070-00001. [DOI] [PubMed] [Google Scholar]
- 5.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 6.Tworoger SS, Missmer SA, Eliassen AH, Barbieri RL, Dowsett M, Hankinson SE. Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women : Exercise and premenopausal hormones. Cancer Causes Control. 2007;18:743–752. doi: 10.1007/s10552-007-9017-5. [DOI] [PubMed] [Google Scholar]
- 7.Margolis KL, Mucci L, Braaten T, Kumle M, Trolle LY, Adami HO, et al. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women’s Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:27–32. [PubMed] [Google Scholar]
- 8.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 9.Steindorf K, Schmidt M, Kropp S, Chang-Claude J. Case-control study of physical activity and breast cancer risk among premenopausal women in Germany. Am J Epidemiol. 2003;157:121–130. doi: 10.1093/aje/kwf181. [DOI] [PubMed] [Google Scholar]
- 10.Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25:5898–5905. doi: 10.1038/sj.onc.1209879. [DOI] [PubMed] [Google Scholar]
- 11.Dobrovic A, Simpfendorfer D. Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 1997;57:3347–3350. [PubMed] [Google Scholar]
- 12.Matros E, Wang ZC, Lodeiro G, Miron A, Iglehart JD, Richardson AL. BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res Treat. 2005;91:179–186. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino A, Yee CJ, Campbell M, Woltjer RL, Townsend RL, van der MR, Shyr Y, Holt JT, Moses HL, Jensen RA. Effects of BRCA1 transgene expression on murine mammary gland development and mutagen-induced mammary neoplasia. Int J Biol Sci. 2007;3:281–291. doi: 10.7150/ijbs.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquis ST, Rajan JV, Wynshaw-Boris A, Xu J, Yin GY, Abel KJ, et al. The developmental pattern of BRCA1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995;11:17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- 15.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11:931s–936s. [PubMed] [Google Scholar]
- 16.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–5863. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 17.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 18.Fan S, Wang J-A, Yuan R, Ma Y, Meng Q, Erdos MR, et al. BRCA1 inhibition of estrogen receptor signalling in transfected cells. Science. 1999;284:1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- 19.Fan S, Xian Ma Y, Wang C, Yuan R, Meng Q, Wang J-A, et al. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene. 2001;20:77–87. doi: 10.1038/sj.onc.1204073. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 21.Varley JM, McGown G, Thorncroft M, Santibanez-Koref MF, Kelsey AM, Tricker KJ, et al. Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res. 1997;57:3245–3252. [PubMed] [Google Scholar]
- 22.Kleihues P, Schauble B, Zur HA, Esteve J, Ohgaki H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150:1–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Arizti P, Fang L, Park I, Yin Y, Solomon E, Ouchi T, et al. Tumor suppressor p53 is required to modulate BRCA1 expression. Mol Cell Biol. 2000;20:7450–7459. doi: 10.1128/mcb.20.20.7450-7459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuyer M, Berns EM. Is TP53 dysfunction required for BRCA1-associated carcinogenesis? Mol Cell Endocrinol. 1999;155:143–152. doi: 10.1016/s0303-7207(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S, Wasylyk B. Physiological and pathological consequences of the interactions of the p53 tumor suppressor with the glucocorticoid, androgen, and estrogen receptors. Ann N Y Acad Sci. 2004;1024:54–71. doi: 10.1196/annals.1321.005. [DOI] [PubMed] [Google Scholar]
- 26.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- 27.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–137. [PubMed] [Google Scholar]
- 28.Cardiff RD. Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia. 1998;3:3–5. doi: 10.1023/a:1018714016205. [DOI] [PubMed] [Google Scholar]
- 29.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 30.Paech K, Webb P, Kuiper GG, Gustafsson JA, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors Er alpha and ER beta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proc Natl Acad Sci USA. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 33.Lin CY, Strom A, Li KS, Kietz S, Thomsen JS, Tee JB, et al. Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafsson JA, Warner M. Estrogen receptor beta in the breast: role in estrogen responsiveness and development of breast cancer. J Steroid Biochem Mol Biol. 2000;74:245–248. doi: 10.1016/s0960-0760(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 35.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman ME. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci USA. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 37.Jones LP, Tilli MT, Assefnia S, Torre K, Halama ED, Parrish A, et al. Activation of estrogen signaling pathways collaborates with loss of Brca1 to promote development of ERalpha-negative and ERalpha-positive mammary preneoplasia and cancer. Oncogene. 2007 doi: 10.1038/sj.onc.1210674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 39.Jerry DJ, Kuperwasser C, Downing SR, Pinkas J, He C, Dickinson E, et al. Delayed involution of the mammary epithelium in BALB/c-p53null mice. Oncogene. 1998;17:2305–2312. doi: 10.1038/sj.onc.1202157. [DOI] [PubMed] [Google Scholar]
- 40.Locke I, Kote-Jarai Z, Fackler MJ, Bancroft E, Osin P, Nerurkar A, et al. Gene promoter hypermethylation in ductal lavage fluid from healthy BRCA gene mutation carriers and mutation-negative controls. Breast Cancer Res. 2007;9:R20. doi: 10.1186/bcr1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strathdee G, Sim A, Soutar R, Holyoake TL, Brown R. HOXA5 is targeted by cell-type-specific CpG island methylation in normal cells and during the development of acute myeloid leukaemia. Carcinogenesis. 2007;28:299–309. doi: 10.1093/carcin/bgl133. [DOI] [PubMed] [Google Scholar]
- 42.Meng ZH, Ben Y, Li Z, Chew K, Ljung BM, Lagios MD, et al. Aberrations of breast cancer susceptibility genes occur early in sporadic breast tumors and in acquisition of breast epithelial immortalization. Genes Chromosomes Cancer. 2004;41:214–222. doi: 10.1002/gcc.20089. [DOI] [PubMed] [Google Scholar]