Abstract
Sleep occupies roughly one-third of our lives, yet the scientific community is still not entirely clear on its purpose or function. Existing data point most strongly to its role in memory and homeostasis: that sleep helps maintain basic brain functioning via a homeostatic mechanism that loosens connections between overworked synapses, and that sleep helps consolidate and re-form important memories. In this review, we will summarize these theories, but also focus on substantial new information regarding the relation of electrical brain rhythms to sleep. In particular, while REM sleep may contribute to the homeostatic weakening of overactive synapses, a prominent and transient oscillatory rhythm called “sharp-wave ripple” seems to allow for consolidation of behaviorally relevant memories across many structures of the brain. We propose that a theory of sleep involving the division of labor between two states of sleep–REM and non-REM, the latter of which has an abundance of ripple electrical activity–might allow for a fusion of the two main sleep theories. This theory then postulates that sleep performs a combination of consolidation and homeostasis that promotes optimal knowledge retention as well as optimal waking brain function.
Sleep is clearly a basic human drive, yet we do not fully understand its purpose or function. One could argue that quiet but conscious rest could be just as efficient as sleep for recuperating certain parts of the body and would be less dangerous, since the brain would not be closed to outside inputs. From the evolutionary point of view, then, unconscious sleep must offer an unseen advantage to the brain.
In attempting to understand the neural implications of sleep and neural activity during sleep, the field has focused on the view–well supported by data –that sleep benefits memory and general neural function. In more recent years this claim has been split into two subdomains: 1) a hypothesis centered on homeostasis, wherein sleep reverses the overelaboration and exhaustion of neural networks brought about by prolonged waking states; and 2) a hypothesis that sleep consolidates important memories for long-term storage. In sleep theory, as in neuroscience, much attention has recently been focused on synaptic connections, which carry information between neurons. Yet at the level of the synapse, these two theories seem to conflict: while the homeostatic theory states that synapses, in general, are weakened, the consolidation theory states that selected synaptic connections should be strengthened during sleep as a way to consolidate memory.
We seek here to summarize the major con cepts in the neuroscience of sleep (and refer the interested reader to a more comprehensive review of the relationship between sleep and memory).1 We propose that electrical brain rhythms are key physiological features that allow the brain to carry out all aspects of the tasks of sleep and that offer important insight into those tasks. We also seek to determine whether these two apparently opposing views on sleep might be reconciled.
Before proceeding to examine the relationship between sleep and brain rhythms, it is worth reviewing some aspects of brain structure and function that are pertinent to the topic. Our current understanding of the brain is that the basic currency of computation is a collection of electrical signals transferred from one cell to another. This occurs via action potentials (electrical signals within neurons that are triggered after neurons have received sufficient excitatory input) and highly adaptable chemical synaptic contacts (specialized junctions between neurons that allow information to pass between them). The action potential signals are generated by individual neurons at rates ranging from one per minute to tens or even hundreds per second. They are large enough in amplitude to be measured from outside the neuron, and extracellular recordings are often used by neuroscientists as measures of information transmission by a given neuron or population of neurons. The synaptic connections among neurons are relatively sparse and are often structured rather than random, creating functional “circuits”; and perhaps resultantly, volleys of action potentials are often generated by coordinated populations of neurons in a cohesive manner.
All of this complexity must be harnessed and organized somehow. This is partly accomplished through the spatial segregation of neurons into subdivisions of the brain (often referred to as nuclei or simply regions) such as the hippocampus, the thalamus, or the neocortex. Each region and its interactions are thought to handle specific neural tasks: controlling breathing rhythms, enabling visual perception, handling emotions, or navigating places and memories. To orchestrate these spatially distinct and seemingly task-specific regions, the brain employs a temporal organizational scheme using periodic electrical oscillations (regular fluctuations in electrical potential occurring simultaneously in many neurons, which are measurable even from outside the brain) to achieve two fundamental operations.
The first is perpetual local-global communication, where by the results of local computations are broadcast to widespread brain areas so that multiple structures are simultaneously informed about any given local effect.2 Relatedly, in the reverse direction, global brain activity is made available to individual local circuits by electrical oscillations; this is often referred to as “top-down” control.3 The second fundamental feature of the brain is its persistent activity; that is, the ability of an input to induce and maintain a long-lasting activity trace long after the input has already vanished, even during sleep.4 Electrical oscillations appear to facilitate these functions via their capacity to coordinate groups of neurons and to divide information into transmittable chunks.
The collective electrical activity of the neurons of the brain is such that signals from large populations of neurons can be recorded, either with high fidelity from electrodes inside the tissue of the brain (local field potential recording, or LFP), or in an attenuated form from outside the head (through electroencephalography, or EEG). Both during sleep and in waking states, the LFP and EEG show perpetually changing activity (see Figure 1A). Sometimes large-amplitude slow oscillations are predominant, while at other times small-amplitude fast oscillations are present, but most often many rhythms coexist simultaneously.
Figure 1. Electrical Oscillations in the Brain.
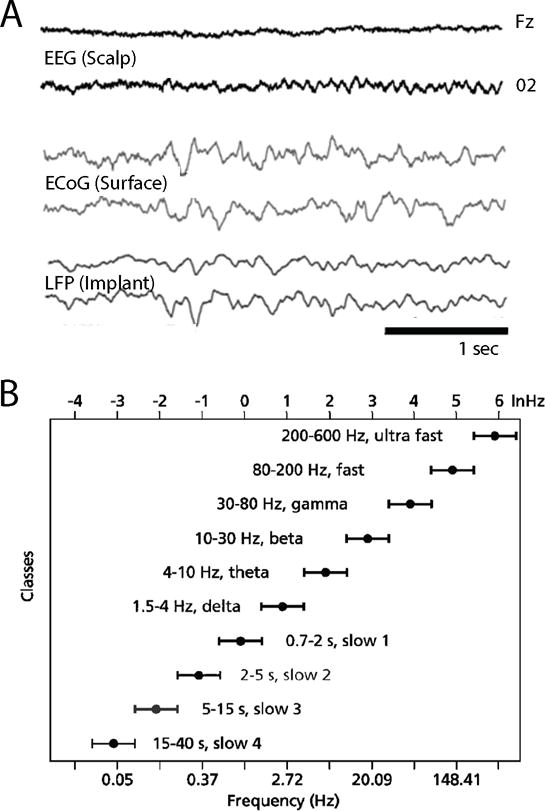
(A) Recordings of brain waves occurring over approximately three seconds. Each line is a recording from one electrode with abcissa representing time and ordinate representing voltage. Top two lines are recorded from outside the skull (EEG), the middle two lines are recordings from inside the skull but on the brain surface (ECoG; electrocorticography), and the bottom two are recorded from electrodes inside the brain. (B) Illustration of the families, or “bands,” of oscillatory rhythms in the brain; each is labeled with a horizontal bar. Note that a system of rhythms is formed with a logarithmic relationship among the constituent oscillations. Source: (A) courtesy of Gregory Worrell of the Mayo Clinic and Scott Makeig of the University of California, San Diego; (B) from György Buzsáki and Andreas DraguhnNeuronal Oscillations in Cortical Networks,” Science 304 (2004): 1926–1929.
Neuronal oscillations have been found to exist in the brains of all animals. In mammals, electrical signals over a broad range of frequencies from as low as one wave every forty seconds (0.025 Hz) to as high as six hundred waves per second (600 Hz) have been recorded. In addition, perhaps the best documented but least emphasized fact about brain dynamics is that spectral features of the EEG and LFP are similar in all mammals, independent of brain size. Every known EEG pattern of the human brain is present in all other mammals investigated to date. Furthermore, the correlations of various families of frequencies with aspects of overt behavior and cognition both within and across species have led to the idea of frequency bands: groups of oscillation frequencies in the brain that act as single functional entities (for example, all frequencies from 5–8 Hz may act similarly; Figure 1B). Scientists have classified at least ten mutually interacting oscillation bands that are mainly defined by their behavioral correlations. The rhythms constantly interact with each other and form a linear progression on a natural logarithmic scale (Figure 1B again).5 The fact that these oscillations are highly organized and evolutionarily conserved leads to hypotheses about their function: oscillations may enable neurons to form “assemblies” and synchronize enough to effectively propagate information in neural networks. Second, an even more oscillation-centric interpretation is that synchronization of various brain nuclei is the embodiment of perception, and that since oscillations would be essential to synchronization, they are the key to perception.6
Most forms of brain rhythms result from rhythmic inhibitory synaptic transmission (inputs from neurons with net inhibitory effects on the other neurons around them) onto bulk neuronal populations including the information-carrying excitatory neurons.7 The rhythmic inhibitory volleys from these cell populations provide windows of alternating reduced (inhibited) and enhanced excitability and offer natural temporal frames for grouping or temporally “chunking” neuronal activity into what appear to be functionally related groups of action potentials. The neurons generating these grouped action potentials are called cell assemblies and constitute the basic units of information processing. This stop-start parsing function of neuronal oscillators (and their hierarchical cross-frequency coupling organization, de tailed be -low) can support “syntactical” rules for neural communication that are known to both sender and receiver, making communication more straightforward than if areas of the brain had to interpret long uninterrupted messages or stochastic patterns of action potentials.
In addition to instantaneously organizing neurons and inducing them to fire action potentials together, oscillations may play broader and more task-specific organizational roles. Functionally, many different oscillations often co-occur in the same brain state and can interact with each other either within the same brain structure or across anatomical regions. The nature of these interactions of oscillations is hierarchical: the phase of the slower oscillation modulates the power of the faster ones, a mechanism known as cross-frequency coupling.8 An illustration of the effect of brain rhythms on neural communication is the interaction between (5–8 Hz) “theta” rhythm in the hippocampus and the higher-frequency (30–90 Hz) “gamma” rhythm in the neocortex.9 With theta oscillations, the hippocampus can temporally coordinate and re-route inputs from other cortical regions (during exploratory behavior, for example) so that the incoming information from disparate regions arrives at approximately the same time and at the phase when the receiver hippocampus is most able to process it.10
Oscillations also appear to participate in another major task of the brain: learning new information in order to effectively shape future action. Brains, small and large, are predictive devices that exploit the recurrence of events to learn and use effective actions for various future situations. Learning and memory allow the brain to evolve and adapt to the constantly changing realities brought into our lives by new places, new social acquaintances, new decisions, new positions, and new roles.
One of the basic tenets of modern neuroscience is that learning and memory are accomplished by the creation or alteration of synaptic connections between neurons. Synchronization of neuronal activity, as occurs during oscillations, can play a key role in the formation of new connections, physically connecting neurons (each carrying information about a different aspect of the world) in order to allow storage of new associations between the elements of the world represented by those neurons. The ability of synapses to strengthen or weaken communication among neurons as a result of experience is called plasticity, and it is a cardinal mechanism for adaptation and survival.
In summary, neuronal oscillations are a syntactical structure that is essential to the brain’s basic functions of information transmission and computation.11 Oscillations may also make learning possible by precipitating coordinated changes in internal circuits as life is experienced. With this background, we turn to sleep, focusing on how brain rhythms seem to allow all the tasks of sleep to be accomplished.
Over the last few decades, brain researchers have used empirical evidence to attempt to define the relationships between sleep, learning, and memory. Initial studies showed that although all memories decay with time, they do so more slowly during sleep than during waking. As in -creasing numbers of studies with larger sample sizes began to show similar results, researchers widely accepted that new experiences may interfere with earlier memories, and that sleep may be a “temporary shelter” in which memories can persist better than during waking.12
Later views, however, began to incorporate the knowledge that sleep is not a singular entity but rather is composed of two distinct electrochemical substates known as slow-wave sleep (SWS or non-REM) and rapid eye movement (REM) sleep, and that these physiologically distinct states may play separate roles in memory. During a given episode of sleep, these states appear in a cyclical and relatively stereotyped pattern (Figure 2). Sleep begins with a light form of SWS, progresses to deeper SWS (during which time it is more difficult to awaken the individual and the slow-wave electrical activity is more powerful), retreats back to shallow SWS, and finally concludes with REM sleep before beginning a new cycle. As mentioned, SWS is characterized by large slow waves, which occur at 0.5 to 4 Hz and are quite distinct from waking rhythms (Figure 2, bottom right); in contrast, local field potential recordings of REM sleep look very similar to those of the waking state, with smaller-amplitude gamma waves dominating the neocortex and theta-nested gamma waves (gamma-wave packets occurring at regular portions or phases of theta cycles) in the hippocampus. Chemically, SWS and REM states are also distinct: SWS correlates with a clear decrease in the activity of brain systems secreting the neuromodulators serotonin, histamine, and acetylcholine; but REM sleep involves the selective reinstatement of waking-like acetylcholine system activity. Additionally, SWS and REM each involve the activation of unique regions in the brain stem.
Figure 2. Stages of Sleep.
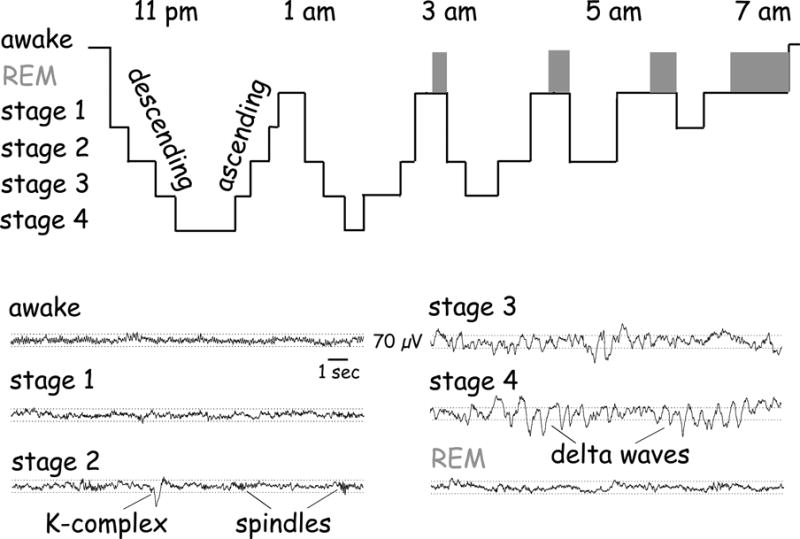
Top panel is a graph of the depth of sleep (depth greater and arousability less toward bottom of the graph) showing a cyclic alternation between SWS and REM sleep. At bottom are example tracings for each state. Note the difference in brain wave amplitude and frequency across states. Source: György Buzsaki, Rhythms of the Brain (New York: Oxford University Press, 2006).
REM sleep initially grabbed the most attention in the scientific community, and a number of researchers found strong correlations between pre-sleep learning and subsequent REM sleep duration.13 Furthermore, increased REM sleep during human nighttime sleep predicted better performance in later procedural tasks.14 Animals such as rats also showed greater memory retention and behavioral performance on memory-requiring tasks after sleep with increased REM.15 Deprivation of REM sleep in animals seemed to impair memory for complex tasks, but not simpler tasks.16 In humans, the story is not as clear, with only certain complex tasks and procedure-related tasks yielding consistent positive associations with REM.17 Moreover, REM sleep increases in individuals with major depressive disorder, but at least in geriatric populations, memory is clearly impaired rather than improved during the depressive episode.18 It is not clear whether the REM increase causes the memory change in this case, but it is notable that successful pharmacologic treatment of depression decreases or almost entirely eliminates REM.
REM is associated with some of the most recognizable and fascinating aspects of the experience of sleep, however. In experiments where human subjects were awakened during sleep, REM was associated with the most vivid, bizarre dreams, while SWS was associated with more realistic-seeming dreams.19 Additionally, in a study where people were awakened during REM, they made more associations between ideas and were more able than usual to solve complex anagrams and other problems.20 On the other hand, SWS has emerged as a critical part of sleep that is capable of changing synaptic weights and that has been tied to a greater degree to “declarative” memories: memories of consciously declarable facts and events.
A simple yet persuasive model of sleep suggests that during the day the brain processes information and coordinates perception and action, while nighttime serves for proper maintenance of the entire system. In 1982, psychopharmacologist Alexander Borbély formally proposed that sleep has a homeostatic function: the regulation of a component he called “S,” which builds with waking (causing “sleep pressure”) and is relieved or dissipated during sleep.21 Support for this view can be found in experiments showing that the magnitude of slow waves is larger at the start of sleep and weaker later in sleep; it also becomes larger yet with sleep deprivation, as if it responded to the degree of need for sleep.22 Additional support comes from data showing that when a particular region of brain is used intensely prior to sleep, slow- wave amplitude is selectively larger in that region.23 Sleep may play an even more general function by effectively removing potentially neurotoxic waste products that accumulate in the central nervous system during waking states.24
Recently, the hypothetical S-process was linked with synaptic connections via an influential theory stating that synaptic connectivity is the factor that builds as waking experience prolongs.25 Under this theory, synaptic connections are constantly built as new associations are made during the course of waking. As more and more associations are made, the brain may build too many, running the risk of be -coming less functional. Sleep might scale back synapses to allow preserved brain functioning. The homeostatic model specifically states that sleep weakens each individual synapse by a universal proportion across all synapses, although it does not attribute specific roles for REM and non-REM stages. Using advanced microscopic and molecular biological techniques to directly view the anatomical synaptic connections in mice, neuroscientist Giulio Tononi and his colleagues in fact did demonstrate that sustained waking results in more synapses and sustained sleep brings with it decreasing numbers of synapses.26 However, they also demonstrated that some synapses were actually created during sleep – a result that requires explanation.
Somewhat at odds with the theory that sleep performs a universal synapse-reducing role is the view that sleep specifically participates in memory consolidation. The conflict comes from the notion that to consolidate memories, synapses active during waking learning experience must be selectively kept active or even enhanced, which is in apparent opposition to the synapse-weakening homeostatic hypothesis.
Over the past two decades, numerous experiments have been performed that support a “two-stage model” of memory consolidation: during sleep–that is, after waking acquisition–memories are not wiped away or simply made to decay less slowly, but are often actually improved, molded, and shaped.27 Procedural memories, such as learned finger-tapping rhythms, can be sped up or even abstracted from one hand to another during sleep.28 Imagination-based mental practice of movements or observation of others can also lead to improvements after sleep.29 All of this supports the idea that new skills can be built, gained, or incorporated into the proper brain sites by sleep, which appears to be performing an active and constructive role. Additionally, people may rearrange their knowledge after a session of sleep, as evidenced by their committing novel errors in a systematic fashion that demonstrates they have internalized a general concept rather than the precise details of a given set of pre-sleep events.30 Finally, there appears to be some emotional or even conscious control over which memories will be improved during sleep: telling human subjects that they will receive rewards for retaining certain information seems to be enough to cause memory for that information to be the most bolstered by sleep.31
The seemingly opposing views of synaptic homeostasis and consolidation may be better approached if the synaptic perspective is supplemented with an electrical rhythm–based approach to categorizing and studying sleep. Oscillatory rhythms can be used to divide sleep into SWS and REM. Furthermore, in animal models, advanced neuroscientific tools can aid in exploring neural activity in novel ways, such as recording action potential activity from many neurons simultaneously during behavior and sleep.
The key player in the memory consolidation process is the sharp-wave ripple (SPW-R): a brief (50–150 ms) electrical rhythm generated by an intrinsic self-organizing process in the hippocampus, which apparently provides a perfect mechanism for the precise consolidation of waking experience.32 This brief rhythm is cross-frequency-coupled with other rhythms such as slow waves and sleep spindles, and it represents the most synchronous physiological pattern in the mammalian brain: 10 to 18 percent of all neurons in the hippocampus and highly interconnected regions (subiculum and entorhinal cortex) discharge during these events. SPW-RS occur during “offline” brain states, such as waking immobility and SWS; apparently, they also represent a counterpart to the theta oscillation, which is present during movement, active waking learning, and REM. SPW-RS have been hypothesized as an ideal mechanism for transferring information and inducing synaptic changes,33 especially since artificially induced SPW-R–like patterns can strengthen synapses even in brain slices in vitro.34 Another piece of evidence supporting SPW-RS’ key role in information transfer is the fact that sequences of neuronal assemblies present during waking behavior are replayed (and at higher speed) during subsequent SPW-R events (see Figure 3).35
Figure 3. Schematic Replay of Waking Neuronal Activity during Sleep.
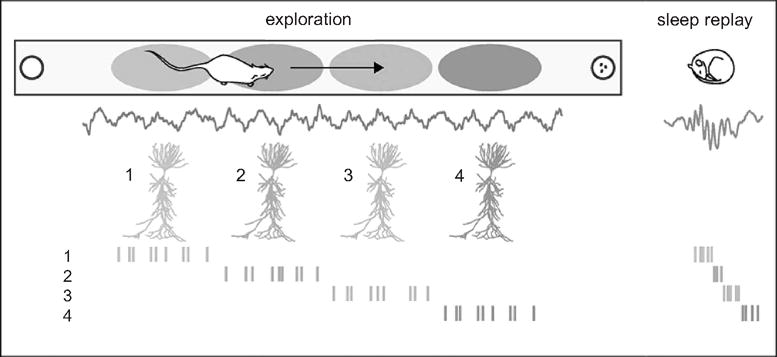
At left: during waking states, experience in the environment leads to certain sequences of neuronal firing, as in this example where a rat has a number of neurons fire in a sequence corresponding to places the animal has visited. At right: fast replay of the same firing sequence of neuronal activation during a sharp wave ripple in sleep. Experiments have shown that replay after waking experience is greater than prior to waking experience. Source: adapted with permission from Gabrielle Girardeau and Michaël ZugaroHippocampal Ripples and Memory Consolidation,” Current Opinion in Neurobiology 21 (3) (2011): 452–459.
The replay phenomenon during SPE-RS may be a direct mechanism for consolidation, given mounting evidence showing that the more often two neurons fire together (as in SPW-RS), the stronger the synapse between them becomes.36 This theory states that when neurons fire repeatedly with a particular timing relative to each other, the synapses between them will strengthen, knitting them together into a cohesive representation of a given percept. This may be precisely what happens when the brain replays waking activity patterns through SPW-RS occurring in slow-wave sleep. Direct evidence for SPW-RS’ causal role in memory consolidation comes from studies in which SPW-RS were selectively eliminated after learning.37 Such targeted interference did not affect other aspects of sleep but produced a large impairment in learning.
While SPW-Rs can emerge solely from a self-organized process, they are often biased to occur by other brain-wide oscillations, including sleep states. SPW-RS are modulated by thalamo-cortical sleep-spindle oscillations (12–16 Hz);38 both SPW-RS and spindles are modulated by slow oscillations;39 and finally, each of these three rhythms is modulated by the ultraslow (0.1 Hz) oscillation.40 Each of these rhythms predominates in certain regions of the brain and their co-modulation may allow for a coordination of the regions they reside in to accomplish a complex task: two-stage memory consolidation. Specifically, we currently believe that memories initially stored in the hippocampus during waking, and later, during sleep consolidation, are transferred to other regions such as the cerebral cortex for more permanent storage (though they leave a trace in the hippocampal system).41 So it may be that during SWS, slow waves, which are known to originate in the cortex, entrain the hippocampus and SPW-RS to transmit information when the cortex is ready to receive it.42 This would be a direct (though anatomically opposite) sleeping analog to the waking theta rhythm of the hippocampus entraining the cortex so that the cortex sends information when the hippocampus is ready to receive it. Recent evidence supports this scenario with data indicating that synaptic connections in the cortex can be formed particularly well during the receptive phase of the slow-wave oscillation.43
In a set of creative experiments, neuroscientist Jan Born and colleagues were able to demonstrate the importance of slow-wave activity by experimentally enhancing slow oscillations in sleeping people using transcranial electrical stimulation outside the scalp.44 They showed that in -creased power of slow oscillations leads to increased memory gain upon waking. It remains to be demonstrated whether sleep spindles and slow oscillations contribute to memory consolidation by their own mechanisms or by their entrainment of hippocampal SPW-RS.45
To summarize, selective and time-compressed reactivation of learning-induced firing patterns is present during sleep. Selective interference with the key rhythms underlying the replay of spike sequences impairs memory performance, whereas enhancement of the relevant oscillatory patterns improves memory performance. It should be pointed out, however, that while the experiments discussed above demonstrate the vital importance of SPW-RS and other rhythms, they do not provide direct information about the mechanism by which those rhythms bring about change, since artificial perturbations affect the dynamic of neuronal interactions at many levels.
An obvious limitation of both the homeostasis and consolidation models is that they are mute on the role of REM sleep and do not account for the complex choreography of the cyclic SWS-REM sleep process. One place for insight is the temporal dynamics of these processes during sleep: while the homeostasis model assumes that synaptic weights generally undergo a monotonic decrease over the entire duration of sleep, consolidation experiments demonstrate that compressed sequence replay of waking firing patterns vanishes after thirty to sixty minutes.46 Remarkably, this time corresponds to the onset of the first REM episode in rodents. Thus, it is possible that REM and SWS play different but complementary roles in the sleep-memory process. Recent research suggests that this may indeed be the case.
While prior evidence showed that overall firing rates of both excitatory and inhibitory neurons decreased steadily during sleep (as predicted by the homeostasis model), new findings show that most of the rate decrease could be accounted for by a number of brief REM episodes (see Figure 4).47 It is assumed that firing rate may correlate with overall neuronal synaptic connectivity. These experiments show that firing rates actually increase slightly during sws, which occupies the majority of sleep, but that during brief REM bouts there are dramatic drops in firing rate that cumulate over multiple REM episodes. Thus, while several hours of waking is necessary to reach the hypothesized saturation of synaptic weights and firing rates, short REM episodes with electrophysiological characteristics of the waking brain can paradoxically bring about rapid downscaling of global firing rates. These results bring us back to the critical importance of both SWS and REM and their interaction for allowing sleep to carry out its full purpose of both consolidation and homeostatic synaptic downscaling.
Figure 4. Complementary Roles for SWS and REM in Neuronal Physiology.
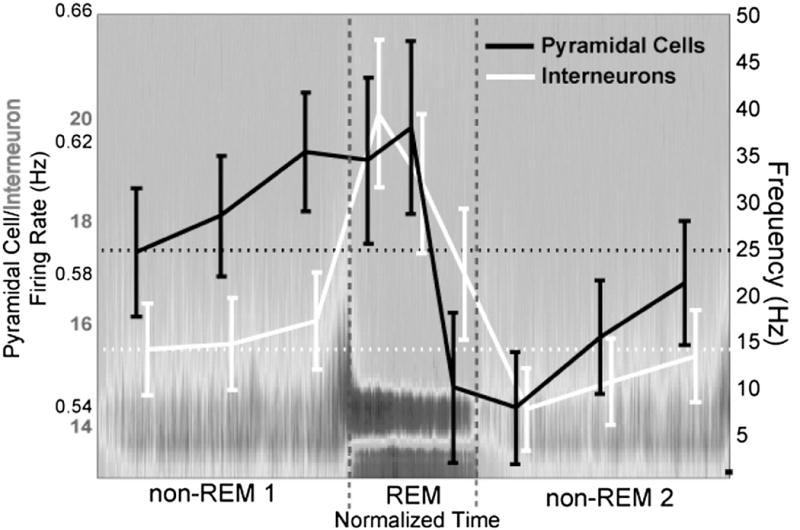
In two types of neurons studied (pyramidal cells and inhibitory interneurons), SWS led to a slight increase of spiking activity and possibly synaptic connectivity (rising slopes), while during REM sleep, action potential generation and possibly synaptic connectivity decreased (falling slopes). Source: A. D. Grosmark, K. Mizuseki, E. Pastalkova, K. Diba, and G. Buzsakirem Sleep Reorganizes Hippocampal Excitability,” Neuron 75 (2012): 1001–1007.
The data presented above instruct us that an approach oriented toward measuring and understanding the electrical rhythms of the brain gives a fuller picture of the function and mechanisms of sleep. We have proposed a possible fusion of the two dominant models of sleep through the division of labor by REM and sws. In its simplest form, SWS may be in charge of consolidation and selective enhancement of learning-related neuronal firing patterns, whereas REM may be responsible for homeostatic downscaling of rates and synaptic weights. Sleep always begins with sws; thus, consolidation and strengthening of important synapses come first, followed by a hypothetically “evenhanded” downscaling of synapses by rem. This would ensure that the most important synapses are not lost in a population of relatively similarly-sized synapses. The process then can swing back to SWS to consolidate the content modified by REM before preparing for another iteration of the cycle.
Alternatively, one can point out the overlapping aspects of the models. The consolidation model is de facto homeostatic because selective enhancement of learning-related connectivity can occur only at the expense of other synapses. This is due to the fact that brain-wide synaptic weights (and, by extension, brain-wide firing rates) must remain constant; otherwise, epileptic activity would result.48 Therefore, any increase must be accompanied by a compensatory decrease.
New findings may also need to be considered when evaluating the theories discussed here. First, only a small fraction of neurons active during SPW-R events are the same as those that were active during prior learning, and many more sequences occur than could be accounted for by the sequences observed during waking behavior.49 Several experiments show that the spike content of SPW-Rs does not exclusively relate to the activity during the most recently experienced situation or to the most frequent neuronal sequences of the preceding waking period.50 Finally, new evidence is accumulating that suggests spw-rs have a constructive (and not just post-hoc) role in learning: it has been observed that groups of neurons that fire in a specific sequence during a novel running behavior will have actually fired in that same order (though compressed in time) in sleep before the new behavior oc curs.51 This suggests that the brain may use pre-constructed network structures during behavior, and that sleep plays a role in the pre-behavior activity of those network constructs.
It is, of course, likely that the model presented above is overly simplistic and misses many details. Additionally, certain synapses and neurons may have varying roles in network function that may accordingly cause them to be treated differently by sleep, and many assumptions about the nature of these systems may need to be re-addressed in light of new data. However, it is worth seriously considering the theory that sleep is a general tool used by mammals to tune their entire neural system to be able to properly acquire, select, and store information. If experience is gained during waking, then the connections formed by the registration of that experience are not only passively sheltered by sleep, but are amplified, re-organized, and generalized (by consolidation); additionally, the slate is wiped mostly clean and many synapses reset for the next day of learning (by synaptic down-scaling).
Without sleep, the waking brain system might be encumbered by having to take on additional roles, such as preventing the formation of too many connections and selecting only the most useful to remain. It seems nature has found that allowing the waking state to be fully dedicated to tasks such as perception, learning, and motor control is most adaptive as long as sleep will come a few hours later to clean and selectively reorganize the system. Thus, the system of adaptation, learning, and memory may owe its efficiency to sleep’s balancing effects.
Additional evidence for sleep’s crucial role in proper adaptive functioning comes from cases of sleep deprivation. Acute sleep deprivation can lead to seizures, impaired cognition, poor memory, mood lability, irritability, and even frank psychosis with disorganized thinking and poor ability to accurately perceive reality.52 Indeed, there is a well-documented but poorly understood link between many neuropsychiatric disorders (including major depressive disorder, anxiety disorders, and bipolar disorder) and sleep, and there is an emerging consensus that sleep disorganization might be causally related to the cognitive-affective problems associated with these disorders.
In summary, sleep is a pair of special modes in the brain: sws and rem. Both of these states are closed to outside inputs and work in tandem to prepare and clean the learning and memory system while retaining important information for later use–all so that the brain can be maximally focused on learning and adapting to its surroundings during the next period of awake activity.
Biographies
BRENDON O. WATSON is a clinical psychiatrist and a research fellow at Weill Cornell Medical College at Cornell University and is doing postdoctoral research work at the Buzsáki Lab at the New York University School of Medicine. His research interests include sleep mechanisms and emotional processing in animal models and his clinical interests include affective and personality disorders. He has published in such journals as Dialogues in Clinical Neuroscience, Frontiers in Neuroscience, Frontiers in Neural Circuits, and Neuron.
GYÖRGY BUZSÁKI is the Biggs Professor of Neural Sciences at the New York University School of Medicine. His laboratory’s research goal is to investigate syntactical structures that enable internal communication within the brain. He is among the top 1 percent of the most cited authors in neuroscience, the recipient of the 2011 Brain Prize, and the author of Rhythms of the Brain (2006). He also sits on the editorial board of numerous journals, including Science and Neuron.
Contributor Information
Brendon O. Watson, Clinical psychiatrist and a research fellow at Weill Cornell Medical College at Cornell University and is doing post doctoral research work at the Buzsáki Lab at the New York University School of Medicine.
György Buzsáki, Biggs Professor of Neural Sciences at the New York University School of Medicine..
References
- 1.Rasch Björn, Born Jan. About Sleep’s Role in Memory. Physiological Reviews. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehaene Stanislas, Changeux Jean-Pierre. Experimental and Theoretical Approaches to Conscious Processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]; Tononi G, Edelman GM. Consciousness and Complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 3.Varela Francisco, Lachaux Jean-Philippe, Rodriguez Eugenio, Martinerie Jacques. The Brainweb: Phase Synchronization and Large-Scale Integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]; Engel Andreas K, Fries Pascal, Singer Wolf. Dynamic Predictions: Oscillations and Synchrony in Top-Down Processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 4.Buzsáki György. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 5.Buzsáki György, Draguhn Andreas. Neuronal Oscillations in Cortical Networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 6.Gray CM, Singer W. Stimulus-Specific Neuronal Oscillations in Orientation Columns of Cat Visual Cortex. Proceedings of the National Academy of Sciences. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsáki G, Leung LW, Vanderwolf CH. Cellular Bases of Hippocampal EEG in the Behaving Rat. Brain Research. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]; Buzsáki György, Chrobak James J. Temporal Structure in Spatially Organized Neuronal Ensembles: A Role for Interneuronal Networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]; Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-Based Rhythms: Experimental and Mathematical Observations on Network Dynamics. International Journal of Psychophysiology. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]; Logothetis Nikos K. The Underpinnings of the BOLD Functional Magnetic Resonance Imaging Signal. The Journal of Neuroscience. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragin Anatol, et al. Gamma (40–100 Hz) Oscillation in the Hippocampus of the Behaving Rat. The Journal of Neuroscience. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schroeder Charles E, Lakatos Peter. Low-Frequency Neuronal Oscillations as Instruments of Sensory Selection. Trends in Neurosciences. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buzsáki G, Watson BO. Brain Rhythms and Neural Syntax: Implications for Efficient Coding of Cognitive Content and Neuropsychiatric Disease. Dialogues in Clinical Neuroscience. 2012;14:345–367. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirota Anton, et al. Entrainment of Neocortical Neurons and Gamma Oscillations by the Hippocampal Theta Rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirota Anton, Buzsáki György. Neural Syntax: Cell Assemblies, Synapsembles, and Readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzsáki, “Neural Syntax: Cell Assemblies, Synapsembles, and Readers.”
- 12.Rasch and Born, “About Sleep’s Role in Memory”; Ellenbogen Jeffrey M, Payne Jessica D, Stickgold Robert. The Role of Sleep in Declarative Memory Consolidation: Passive, Permissive, Active or None? Current Opinion in Neurobiology. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Fishbein William, Kastaniotis Chris, Chattman Dennis. Paradoxical Sleep: Prolonged Augmentation Following Learning. Brain Research. 1974;79:61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- 14.Fischer Stefan, Hallschmid Manfred, Elsner Anna Lisa, Born Jan. Sleep Forms Memory for Finger Skills. Proceedings of the National Academy of Sciences. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wetzel W, Balschun D, Janke S, Vogel D, Wagner T. Effects of CLIP (Corticotropin-Like Intermediate Lobe Peptide) and CLIP Fragments on Paradoxical Sleep in Rats. Peptides. 1994;15:237–241. doi: 10.1016/0196-9781(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 16.Pearlman Chester A. Effect of Rapid Eye Movement (Dreaming) Sleep Deprivation on Retention of Avoidance Learning in Rats. Fort Belvoir, Va: Defense Technical Information Center; 1969. http://archive.rubicon-foundation.org/xmlui/handle/123456789/8608. [PubMed] [Google Scholar]
- 17.Rasch and Born, “About Sleep’s Role in Memory.”
- 18.Reynolds Charles F, III, et al. EEG Sleep in Elderly Depressed, Demented, and Healthy Subjects. Biological Psychiatry. 1985;20:431–442. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 19.Hobson JA, Pace-Schott Edward F. The Cognitive Neuroscience of Sleep: Neuronal Systems, Consciousness, and Learning. Nature Reviews Neuroscience. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 20.Cai Denise J, Mednick Sarnoff A, Harrison Elizabeth M, Kanady Jennifer C, Mednick Sara C. REM, not Incubation, Improves Creativity by Priming Associative Networks. Proceedings of the National Academy of Sciences. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borbély Alexander A. A Two-Process Model of Sleep Regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 22.Vyazovskiy Vladyslav V, et al. Cortical Firing and Sleep Homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda Tadanobu, Yasuda Kyo, Brown Richard A, Krueger James M. State-Dependent Effects of Light-Dark Cycle on Somatosensory and Visual Cortex EEG in Rats. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2005;289:R1083–R1089. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- 24.Xie Lulu, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tononi Giulio, Cirelli Chiara. Sleep Function and Synaptic Homeostasis. Sleep Medicine Reviews. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]; Vyazovskiy Vladyslav V, Harris Kenneth D. Sleep and the Single Neuron: The Role of Global Slow Oscillations in Individual Cell Rest. Nature Reviews Neuroscience. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maret Stephanie, Faraguna Ugo, Nelson Aaron B, Cirelli Chiara, Tononi Giulio. Sleep and Waking Modulate Spine Turnover in the Adolescent Mouse Cortex. Nature Neuroscience. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto Hiroyuki, Hensch Takao K. Reciprocal Interaction of Sleep and Synaptic Plasticity. Molecular Interventions. 2003;3:404–417. doi: 10.1124/mi.3.7.404. [DOI] [PubMed] [Google Scholar]; Benington Joel H, Frank Marcos D. Cellular and Molecular Connections between Sleep and Synaptic Plasticity. Progress in Neurobiology. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 28.Gais et al. “Early Sleep Triggers Memory for Early Visual Discrimination Skills.”
- 29.Van Der Werf Ysbrand D, Van Der Helm Els, Schoonheim Menno M, Ridderikhoff Arne, Van Someren Eus JW. Learning by Observation Requires an Early Sleep Window. Proceedings of the National Academy of Sciences. 2009;106:18926–18930. doi: 10.1073/pnas.0901320106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Debarnot Ursula, Creveaux Thomas, Collet Christian, Doyon Julien, Guillot Aymeric. Sleep Contribution to Motor Memory Consolidation: A Motor Imagery Study. Sleep. 2009;32:1559–1565. doi: 10.1093/sleep/32.12.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roediger Henry L, McDermott Kathleen B. Creating False Memories: Remembering Words not Presented in Lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:803–814. [Google Scholar]
- 31.Fischer S, Born J. Anticipated Reward Enhances Offline Learning During Sleep. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:1586–1593. doi: 10.1037/a0017256. [DOI] [PubMed] [Google Scholar]
- 32.Buzsáki György. Two-Stage Model of Memory Trace Formation: A Role for ‘Noisy’ Brain States. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 33.Buzsáki György, Chrobak JJ, Buzsáki G. Selective Activation of Deep Layer (V–VI) Retro-hippocampal Cortical Neurons During Hippocampal Sharp Waves in the Behaving Rat. The Journal of Neuroscience. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsáki György, Haas Helmut L, Anderson Edmund G. Long-Term Potentiation Induced by Physiologically Relevant Stimulus Patterns. Brain Research. 1987;435:331–333. doi: 10.1016/0006-8993(87)91618-0. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MA, McNaughton BL. Reactivation of Hippocampal Ensemble Memories During Sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]; Karlsson Mattias P, Frank Loren M. Awake Replay of Remote Experiences in the Hippocampus. Nature Neuroscience. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buzsáki György, Lopes da Silva Fernando. High Frequency Oscillations in the Intact Brain. Progress in Neurobiology. 2012;98(3):241–249. doi: 10.1016/j.pneurobio.2012.02.004. http://www.ncbi.nlm.nih.gov/pubmed/22449727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebb Donald O. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 37.Karlsson and Frank, “Awake Replay of Remote Experiences in the Hippocampus”; Girardeau Gabrielle, Benchenane Karim, Wiener Sidney I, Buzsáki György, Zugaro Michaël B. Selective Suppression of Hippocampal Ripples Impairs Spatial Memory. Nature Neuroscience. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 38.Siapas Athanassios G, Wilson Matthew A. Coordinated Interactions Between Hippocampal Ripples and Cortical Spindles During Slow-Wave Sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]; Sirota Anton, Csicsvari Jozsef, Buhl Derek, Buzsáki György. Communication Between Neocortex and Hippocampus During Sleep in Rodents. Proceedings of the National Academy of Sciences. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steriade M, Contreras D, Dossi R Curró, Nuñez A. The Slow (< 1 Hz) Oscillation in Reticular Thalamic and Thalamocortical Neurons: Scenario of Sleep Rhythm Generation in Interacting Thalamic and Neocortical Networks. The Journal of Neuroscience. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton Sirota et al., “Communication Between Neocortex and Hippocampus During Sleep in Rodents”; Mölle Matthias, Yeshenko Oxana, Marshall Lisa, Sara Susan J, Born Jan. Hippocampal Sharp Wave-Ripples Linked to Slow Oscillations in Rat Slow-Wave Sleep. Journal of Neurophysiology. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]; Sullivan David, et al. Relationships Between Hippocampal Sharp Waves, Ripples, and Fast Gamma Oscillation: Influence of Dentate and Entorhinal Cortical Activity. The Journal of Neuroscience. 2011;31:8605–8616. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzsáki, “Two-Stage Model of Memory Trace Formation: A Role for ‘Noisy’ Brain States.”
- 42.Sirota Anton, Buzsáki György. Interaction Between Neocortical and Hippocampal Networks via Slow Oscillations. Thalamus & Related Systems. 2005;3:245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hahn Thomas G, Sakmann Bert, Mehta Mayank R. Differential Responses of Hippocampal Subfields to Cortical Up-Down States. Proceedings of the National Academy of Sciences. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruskal Peter B, Li Lucy, MacLean Jason N. Circuit Reactivation Dynamically Regulates Synaptic Plasticity in Neocortex. Nature Communications. 2013;4 doi: 10.1038/ncomms3574. [DOI] [PubMed] [Google Scholar]
- 44.Marshall Lisa, Helgadóttir Halla, Mölle Matthias, Born Jan. Boosting Slow Oscillations During Sleep Potentiates Memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 45.Destexhe Alain, Sejnowski Terrence. Thalamocortical Assemblies: How Ion Channels, Single Neurons, and Large-Scale Networks Organize Sleep Oscillations. Oxford: Oxford University Press; 2001. p. 452. [Google Scholar]
- 46.Wilson and McNaughton, “Reactivation of Hippocampal Ensemble Memories during Sleep.”
- 47.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G. REM Sleep Reorganizes Hippocampal Excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Racine Ronald J. Modification of Seizure Activity by Electrical Stimulation: II. Motor Seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 49.Foster David J, Wilson Matthew A. Reverse Replay of Behavioural Sequences in Hippocampal Place Cells During the Awake State. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]; Diba Kamran, Buzsáki György. Forward and Reverse Hippocampal Place-Cell Sequences During Ripples. Nature Neuroscience. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nádasdy Zoltán, Hirase Hajime, Czurkó András, Csicsvari Jozsef, Buzsáki György. Replay and Time Compression of Recurring Spike Sequences in the Hippocampus. The Journal of Neuroscience. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupret David, O’Neill Joseph, Pleydell-Bouverie Barty, Csicsvari Jozsef. The Reorganization and Reactivation of Hippocampal Maps Predict Spatial Memory Performance. Nature Neuroscience. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carr Margaret F, Jadhav Shantanu P, Frank Loren M. Hippocampal Replay in the Awake State: A Potential Substrate for Memory Consolidation and Retrieval. Nature Neuroscience. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gupta Anoopum S, van der Meer Matthijs AA, Touretzky David S, Redish A David. Hippocampal Replay is Not a Simple Function of Experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta et al., “Hippocampal Replay is Not a Simple Function of Experience”; Dragoi G, Tonegawa S. Preplay of Future Place Cell Sequences by Hippocampal Cellular Assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West Louis Jolyon, Janszen Herbert H, Lester Boyd K, Cornelisoon Floyd S., Jr The Psychosis of Sleep Deprivation. Annals of the New York Academy of Sciences. 1962;96:66–70. doi: 10.1111/j.1749-6632.1962.tb50101.x. [DOI] [PubMed] [Google Scholar]


