Abstract
DTI studies in multiple sclerosis (MS) reveal white matter (WM) injury that occurs with disease progression. In the present study we aimed to elucidate the relationship of microstructural WM damage in patients with varying periods of disease duration. DTI scans were acquired from 90 MS patients and 25 healthy controls. Patients were grouped to short (<1 year), moderate (1 up to 6 years) and long (6–10 years) disease duration periods. Statistical analyses of the fractional anisotropy (FA) data were performed using tract-based spatial statistics (TBSS). Whole-brain skeletal FA measurements showed a significant decrease between healthy controls and the short MS disease duration group, as well as between moderate disease duration and long disease duration groups, but failed to show a significant difference between short and moderate disease duration groups.
Voxelwise analysis revealed clusters of diffuse FA reductions in 40 WM tracts when comparing healthy controls and MS short disease duration group, with the point of maximal significant difference located in the left inferior longitudinal fasciculus.
Comparing short with long disease duration groups, progressive FA reduction was demonstrated across 30 WM tracts, with the point of maximal significant difference migrating to the body of the corpus callosum. A non-linear pattern of WM microstructure disruption occurs in RRMS. Alterations are seen early in the disease course within 1 year from onset, reach a plateau within the next 5 years, and only later additional WM changes are detected. An important period of a possible therapeutic window therefore exists within the early disease stage.
Keywords: Multiple sclerosis, White matter, Diffusion-tensor imaging (DTI), Fractional anisotropy (FA), Tract-based spatial statistics (TBSS)
Abbreviations: MS, multiple sclerosis; WM, white matter; FA, fractional anisotropy; TBSS, tract-based spatial statistics; EDSS, expanded disability status scale; RRMS, relapsing–remitting multiple sclerosis; CIS, clinically-isolated syndrome; ILF, inferior longitudinal fasciculus
Highlights
-
•
A non-linear pattern of WM microstructure disruption occurs in patients with RRMS.
-
•
WM disruption is identified within 1 year from disease onset.
-
•
FA reduction is similar in patients with early and moderate disease duration periods.
-
•
Different patterns of WM disruption occur in patients with longer disease duration.
1. Introduction
White matter (WM) injury is the cardinal pathological process occurring early in multiple sclerosis (MS). Disruptions of WM tracts are significant predictors for physical disability and cognitive impairment in MS, and hence are important to be quantified at different stages of the disease course (Samann et al., 2012). WM damage can be detected and measured by brain magnetic resonance imaging (MRI), which demonstrates focal and macroscopic inflammatory lesions by T2 and FLAIR as well as axonal loss by T1 black holes. However, injuries outside these lesions are not easily detected by conventional MRI techniques. Therefore, there is a need to apply innovative methods that may provide additional insight into the ongoing pathological processes. Diffusion tensor imaging (DTI) is a nonconventional MRI technique that is sensitive to the magnitude and direction of water diffusion within tissues and therefore can provide integrity assessment and measurements of WM tract damage even in the presumed normal appearing WM (Giorgio et al., 2010).
DTI allows for calculation of the fractional anisotropy (FA), which is a quantitative parameter for evaluating the direction-dependent diffusivity of water molecules along the WM tracts. FA ranges from 0 to 1 and is an indirect marker for WM integrity, as decreased FA reflects greater demyelination of fibers (Commowick et al., 2008; Roosendaal et al., 2009). This is in accordance with a pathological study demonstrating that FA is affected by myelin content in post-mortem MS brains (Schmierer et al., 2007). Aligning FA data from multiple subjects can be performed by tract-based spatial statistics (TBSS), an automated observer-independent method. TBSS allows group-wise comparisons of DTI data by analyzing it in a voxel-wise fashion and minimizes registration errors, and is an advantageous approach as it enables that maximal FA values for each subject will be directly compared at each point even if the fiber centers are not perfectly aligned (Smith et al., 2007). TBSS has already been validated as an effective way to evaluate the FA in MS, and several studies reported widespread WM damage in patients with clinically isolated syndrome (CIS), RRMS and primary progressive MS (Bodini et al., 2009; Raz et al., 2010a; Roosendaal et al., 2009). The significance of TBSS analysis of DTI data from MS patients was established by studies that demonstrated that decreased FA correlated with increased disability by EDSS (Liu et al., 2012), lower cognitive performance and specifically for processing speed, visual and verbal working memory (Yu et al., 2012b). However, TBSS studies assessing patients at different stages of disease course are scarce. Therefore, the aim of the present study was to investigate how changes in WM tracts relate to MS disease duration, as clarifying this relationship could give better insight on the microstructure changes that occur at different stages of the disease course. To this end, we investigated how structural WM disruptions based on fractional anisotropy (FA) data and tract-based spatial statistics (TBSS) analysis were affected by MS disease duration within a cross-sectional study design in MS patients with short, moderate and long disease duration periods. We first investigated the relationship of whole-brain skeletal FA measurements between healthy subjects and MS patients with short disease duration of up to 1 year from onset as we hypothesized that disruption of WM tracts will be identified already at the this early disease stage. Further analyses were performed grouping patients by 5 year intervals to moderate (from 1 year and up to 6 years duration) and long (from 6 to 10 years inclusive) disease duration periods to assess the implications of WM disruption as the disease progresses. In addition, we performed voxelwise analysis to recognize clusters of FA reductions in various WM tracts and to identify associations between regional WM changes that are detected in the early phase of MS and if these are similar in pattern in patients with moderate and long disease duration periods.
2. Materials and methods
2.1. Study design
Retrospective, cross-sectional in a tertiary referral center.
2.2. Subjects
In total, MRI data obtained from 100 MS patients and 30 healthy subjects were analyzed. Data from some subjects were excluded from analyses because of neurological comorbidity (N = 7), too many artifacts or noise in the raw MRI data (N = 4), or absence of complete MRI data (N = 4).
Consequently, 90 MS patients and 25 healthy subjects remained in the study. All MS subjects met McDonald diagnostic criteria (Polman et al., 2011) and were recruited from the MS database registry at the Sheba MS Center, Tel-Hashomer. Enrolled patients fulfilled the following inclusion criteria: (1) Age above 18 years; (2) RRMS disease course; (3) MRI examination performed on 3.0 T including DTI protocol; (4) no relapse or corticosteroid use within 4 weeks before study entry to avoid transient confounding effects on MRI; and (5) absence of another major medical disorder. Clinically stable patients were defined as patients without relapses in the last year prior to be enrolled in the study Patients were divided according to disease duration periods: short, up to 1 year from clinical onset, moderate, 1 year and up to 6 years from clinical onset, and long, 6–10 years from clinical onset. Disease duration was calculated since the onset of the first symptomatology suggestive of MS. All subjects underwent comprehensive neurological examination and disability was scored by the Expanded Disability Status Scale (EDSS) by MS specialist neurologists. Data for age-matched normal healthy subjects were obtained from the hospital radiology MRI Unit database, which includes MRI data of healthy volunteers recruited from the local community and hospital staff. None of the healthy volunteers suffered from any neurological disease or took central nervous system active medications. The study protocol was approved by the Medical Ethical Review Committee of Sheba Medical Center, Tel-Hashomer, Israel and all subjects gave written informed consent.
2.3. MRI acquisition protocol
All subjects underwent the same brain MR imaging protocol, performed on 3.0-T MR scanner (Signa, GE) using high-resolution 8 channel head coil. Data were obtained using the following sequences: (1) 3D-FSPGR (1 × 1 × 1 mm voxel, TE = 2 ms, TR = 6 ms), (2) T2-FSE (slice thickness 2.6 mm, TE = 102 ms, TR = 3500 ms), (3) FLAIR (slice thickness 2.6 mm, TE = 122 ms, TR = 9502 ms, TI = 2375 ms), (4) 2D-FSPGR with contrast GD-DTPA (slice thickness 2.6 mm, TE = 2 ms, TR = 250 ms). Axial DTI data were acquired along 31 independent directions using a single shot echo-planar imaging sequence, (TE = 76 ms, TR = 14,000 ms, b = 1000 s/mm2, FOV 256 × 256 mm, matrix 128 × 128). Two additional images without diffusion weighting (b = 0 s/mm2) were acquired. Axial images were acquired by contiguous slices with 2.6 mm thickness. The slices were positioned to run parallel to a line that joins the anterior commissure–posterior commissure plane. During image acquisition the same image resolution and the same localizer were used for T2 Flair and DTI series, to obtain similar axial slice positions.
2.4. Image analysis and post-processing
Specifications of the methods for DTI analysis were previously described in detail (Smith et al., 2007). In brief, DICOM files of the DTI acquisition were converted into a single multivolume NIFTI file (Neuroimaging Informatics Technology Initiative file) and transferred to a linux-based workstation. Analysis process with FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) was initiated with eddy-current correction (compensating for distortions and for simple head motion) and followed by automatic brain extraction. Diffusion tensors were then reconstructed by fitting a diffusion tensor model at each voxel of the diffusion images and FA values were generated. Whole-brain voxelwise differences between subjects were carried out using TBSS (Smith et al., 2004; Smith et al., 2006). First, FA data of all subjects were transformed into 1 × 1 × 1 mm3 MNI152 common space by means of nonlinear registration. Then, the transformed FA images were averaged to generate a mean FA image which was subsequently skeletonized, representing tracts common to all of the subjects. In order to prevent inclusion of non-skeleton voxels, each subject's aligned FA map was mapped onto the “mean FA skeleton” using a lower threshold of FA of 0.2 in order to exclude gray matter voxels. The approach of carefully tuned non-linear registration, followed by a creation of a mean FA skeleton intends to face the cross-subject spatial variability effect (Douaud et al., 2007). FA values were extracted for whole-brain analysis and for major WM tracts according to the ICBM-DTI-81 WM labels atlas, which includes 48 labels created by hand segmentation of a standard-space average of diffusion MRI tensor maps from 81 healthy subjects (Oishi et al., 2008). In addition, two fiber tracts for the inferior longitudinal fasciculi from the JHU-White Matter Tractography atlas were included for greater spatial coverage (Mori et al., 2009).
2.5. Statistical analysis
Region-of-interest (ROI) analysis was performed to initially evaluate the magnitude of FA decreases within predetermined regions. ROI analysis was calculated by splitting the 4D post-threshold skeletonized volume of each subject's WM tract and repackaging them into group 4D files. In order to achieve accurate inference of multiple comparisons over space, voxelwise analysis was performed by the randomize permutation algorithm from the FSL library, with 5000 permutations and a cluster significance level of p < 0.05 using threshold-free cluster enhancement (TFCE) (Keihaninejad et al., 2012). TFCE through the randomize script in TBSS was utilized to establish the volume of significant changes in the skeleton on both whole-brain and regional level, using the aforementioned atlases. Relationship between FA and clinical parameters was evaluated by Spearman's rank correlation test.
3. Results
Ninety RRMS patients, 66 females, mean age ± SE: 37.6 ± 1.0, and 25 healthy controls, 14 females, mean age ± SE: 35.1 ± 2.2 years were included in this study. As expected, patients in the short disease duration group were younger and with lower neurological disability as measured by the EDSS. The rate of patients treated by immunomodulatory drugs (IMDs) was 65.7% in the total sample population, and was similar between the disease duration groups. In the short group, 65.4% were treated with IMDs, and similarly 65.2% and 66.6% were treated in the moderate and long disease duration groups, respectively. In the short duration group, 70.6% were treated for more than 3 months, whereas in the moderate and long disease duration groups, 100% and 91.7% of treated patients, respectively, received IMD treatment for more than 3 months prior to the MRI examination. These similar rates of treatment between patients enabled us to compare the impact of DTI variables between groups. The pertinent demographic information is shown in Table 1.
Table 1.
Demographic and clinical variables of the studied populations.
| Healthy subjects | All MS patients | MS patients by disease duration groups |
|||
|---|---|---|---|---|---|
| Short (<1 y) | Moderate (1–5.9 y) | Long (6–10 y) | |||
| N | 25 | 90 | 26 | 46 | 18 |
| Female/male | 14/11 | 66/24 | 18/8 | 35/11 | 13/5 |
| Age | 35.1 ± 2.2 (22.6–58.0) | 37.6 ± 1.0 (20.1–68.7) |
35.0 ± 1.7 (20.1–51.7) |
37.2 ± 1.3 (21.5–59.0) |
42.2 ± 2.6 (28.2–68.7) |
| Disease duration | 4.2 ± 0.2 (0.0–9.9) |
0.5 ± 0.1 (0.0–0.9) |
4.5 ± 0.1 (1.0–5.9) |
8. 7 ± 0.3 (6.0–9.9) |
|
| EDSS | 2.0 ± 0.1 (0.0–6.5) |
1.6 ± 0.3 (0.0–5.5) |
1.9 ± 0.2 (0.0–6.5) |
2.7 ± 0.4 (1.0–6.5) |
|
| Age at onset | 33.4 ± 9.1 (18.8–58.8) |
34.5 ± 1.7 (19.6–51.2) |
32.8 ± 1.3 (18.8–55.6) |
33.5 ± 2.5 (19.9–58.8) |
|
| % IMD Tx |
65.7 | 65.4 | 65.2 | 66.6 | |
| IMD, N (% of treated) | |||||
| Interferons | 36 (61.0) | 8 | 20 | 8 | |
| Glatiramer acetate | 12 (20.3) | 9 | 4 | 3 | |
| IVIg | 3 (5.1) | 5 | 1 | ||
| Natalizumab | 1 (1.7) | 1 | |||
| IMD Tx duration > 3 months N (%) |
53/59 (89.8) |
12/17 (70.6) | 30/30 (100) | 11/12 (91.7) | |
| Clinically stable, N (%) | 0 | 33 (71.7) | 14 (77.8) | ||
EDSS = Expanded Disability Status Scale; IMD = immunomodulatory drug; Tx = treatment; N = number.
3.1. Whole-brain mean skeleton FA
Whole-brain mean skeleton FA measurements were significantly decreased in RRMS patients with short disease duration as compared with healthy controls (0.443 ± 0.023 vs. 0.465 ± 0.023, p < 0.001), as well as in patients with long disease duration as compared with patients in the moderate disease duration group (0.427 ± 0.020 vs. 0.443 ± 0.022, p < 0.01), but failed to show significant difference in mean FA values between short and moderate disease duration groups (p = 0.95), Fig. 1. Correlations of FA values in various ROIs with disease variables, e.g., disease duration and neurological disability by the EDSS for the whole group of MS patients are shown in Table 2. Decrease in FA values correlated with disease duration while no correlations were found with disability. Graphical presentation of the magnitude of these FA reductions with disease duration is demonstrated in Fig. 2 and Supplementary Movies 1 and 2.
Fig. 1.
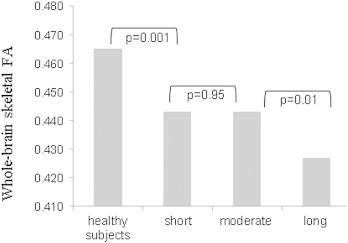
Mean whole-brain skeletal FA value comparison between study groups.
Table 2.
Number of voxels in WM tracts with significant reduced FA values between groups.
| WM tracts | Disease duration groups |
|||
|---|---|---|---|---|
| Short vs. healthy subjects | Moderate vs. short | Long vs. moderate | Long vs. short | |
| L cerebral peduncle | 331 | 0 | 169 | 380 |
| R cerebral peduncle | 342 | 0 | 95 | 354 |
| L cingulum cingulate | 238 | 0 | 306 | 414 |
| R cingulum cingulate | 288 | 0 | 327 | 322 |
| L cingulum hippocampus | 13 | 0 | 5 | 2 |
| R cingulum hippocampus | 11 | 0 | 10 | 136 |
| L ant corona radiata | 1314 | 0 | 822 | 1122 |
| R ant corona radiata | 1199 | 0 | 472 | 895 |
| L post corona radiata | 726 | 0 | 452 | 517 |
| R post corona radiata | 779 | 0 | 508 | 681 |
| L sup corona radiata | 1004 | 0 | 747 | 625 |
| R sup corona radiata | 975 | 0 | 560 | 747 |
| Body, corpus callosum | 2888 | 0 | 2813 | 3104 |
| Genu, corpus callosum | 1211 | 0 | 1364 | 1354 |
| Splenium, corpus callosum | 2134 | 0 | 1494 | 2152 |
| L corticospinal tract | 0 | 0 | 0 | 88 |
| R corticospinal tract | 41 | 0 | 0 | 44 |
| L external capsule | 1174 | 0 | 562 | 727 |
| R external capsule | 1110 | 0 | 509 | 638 |
| Fornix | 124 | 0 | 153 | 158 |
| L inf cerebellar peduncle | 0 | 0 | 0 | 122 |
| R inf cerebellar peduncle | 0 | 0 | 0 | 102 |
| L ant internal capsule | 193 | 0 | 0 | 266 |
| R ant internal capsule | 72 | 0 | 0 | 283 |
| L post internal capsule | 376 | 0 | 108 | 344 |
| R post internal capsule | 117 | 0 | 296 | 519 |
| L retrolenticular internal capsule | 686 | 0 | 657 | 630 |
| R retrolenticular internal capsule | 617 | 0 | 528 | 567 |
| L medial lemniscus | 0 | 0 | 0 | 100 |
| R medial lemniscus | 0 | 0 | 0 | 83 |
| Mid cerebellar peduncle | 1 | 0 | 0 | 853 |
| Pontine crossing tract | 0 | 0 | 0 | 88 |
| L post thalamic/optic radiation | 1060 | 0 | 990 | 1042 |
| R post thalamic/optic radiation | 1085 | 0 | 813 | 1071 |
| L sagittal striatum | 472 | 0 | 280 | 250 |
| R sagittal striatum | 543 | 0 | 168 | 362 |
| L stria terminalis | 280 | 0 | 297 | 302 |
| R stria terminalis | 274 | 0 | 226 | 251 |
| L sup cerebellar peduncle | 0 | 0 | 0 | 21 |
| R sup cerebellar peduncle | 1 | 0 | 0 | 15 |
| L sup front-occipital fasciculus | 83 | 0 | 0 | 27 |
| R sup front-occipital fasciculus | 0 | 0 | 0 | 31 |
| L sup longitudinal fasciculus | 958 | 0 | 648 | 973 |
| R sup longitudinal fasciculus | 1086 | 0 | 883 | 1130 |
| L uncinate fasciculus | 53 | 0 | 8 | 39 |
| R uncinate fasciculus | 40 | 0 | 0 | 1 |
| L inf longitudinal fasciculus | 1768 | 0 | 982 | 1467 |
| R inf longitudinal fasciculus | 1167 | 0 | 948 | 1050 |
There are diffuse changes between controls and short disease group, virtually no change between short and moderate disease groups (0 voxels in every region), and diffuse areas of change between long and moderate disease groups. Long and short disease groups were compared in order to demonstrate the magnitude of the widespread FA reduction.
Fig. 2.
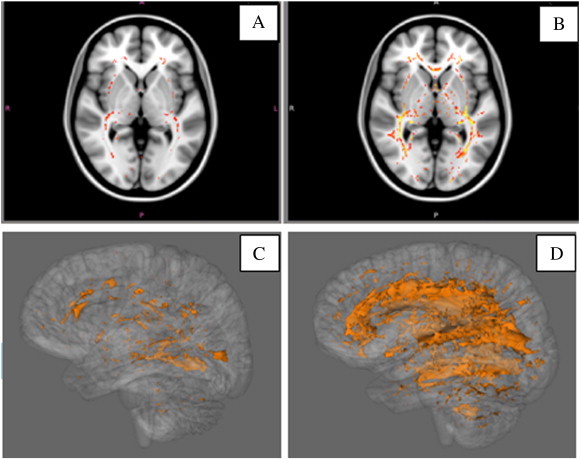
Comparison of FA differences in WM tracts between healthy subjects and MS patients according to disease duration. Significant clusters of reduced FA (p < 0.05) are shown in red (axial) and orange (3-dimensional) overlaid on a template image brain. A, C, Comparison between healthy subjects and MS patients with short disease duration (<1 year). B, D, Comparison between healthy subjects and MS patients with long disease duration (>6 years).
Whole-brain mean skeleton FA measurements were significantly decreased in RRMS patients with short disease duration as compared with healthy controls (0.443 ± 0.023 vs. 0.465 ± 0.023, p < 0.001), as well as in patients with long disease duration as compared with patients in the moderate disease duration group (0.427 ± 0.020 vs. 0.443 ± 0.022, p < 0.01), but failed to show significant difference in mean FA values between short and moderate disease duration groups (p = 0.95), Fig. 1. Correlations of FA values in various ROIs with disease variables, e.g., disease duration and neurological disability by the EDSS for the whole group of MS patients are shown in Table 2. Decrease in FA values correlated with disease duration while no correlations were found with disability. Graphical presentation of the magnitude of these FA reductions with disease duration is demonstrated in Fig. 2 and Supplementary Movies 1 and 2.
3.2. TFCE voxelwise analyses
Additional in-depth voxelwise analyses with TFCE to accurately control for multiple comparisons over space are shown in Table 3. The results strengthen the whole-brain mean skeleton FA findings. There were diffuse changes between age-matched healthy subjects and RRMS patients with short disease, virtually no significant change between short and moderate disease duration groups (0 voxels in every region), and diffuse areas of changes between long and moderate disease duration groups. Long and short disease duration groups were compared in order to demonstrate the magnitude of the widespread FA reduction. This voxelwise analysis revealed 31% diffuse FA reduction spanning a skeletal area of 60,784 of 195,350 total voxels in 40 major WM tracts when comparing healthy controls and the short disease duration group. The point of maximal significant difference was observed in the left inferior longitudinal fasciculus. FA reduction of additional 22% was shown in 30 major WM tracts when comparing the long and moderate duration groups, affecting 42,212 of 192,208 skeletal voxels, with the point of maximal significant difference migrating to the body of the corpus callosum.
Table 3.
Correlation between FA values in various ROIs with MS disease related variables.
| ROI | Disease duration | Age |
|---|---|---|
| Fornix | r = −0.31 p = 0.002 | r = −0.24 p = 0.02 |
| L cerebral peduncle | r = −0.29 p = 0.004 | r = −0.20 p = 0.05 |
| R cerebral peduncle | r = −0.30 p = 0.03 | r = −0.24 p = 0.02 |
| Genu, corpus callosum | r = −0.23 p = 0.023 | |
| Splenium, corpus callosum | r = −0.31 p = 0.003 | |
| R corticospinal tract | r = −0.44 p = 0.000 | |
| R medial lemniscus | r = −0.24 p = 0.021 | |
| L medial lemniscus | r = −0.31 p = 0.003 | |
| R inf cerebellar peduncle | r = −0.34 p = 0.001 | |
| R pos internal capsule | r = −0.26 p = 0.013 | |
| L pos internal capsule | r = −0.23 p = 0.031 | |
| L sup corona radiata | r = −0.27 p = 0.01 | |
| R sup corona radiata | r = −0.24 p = 0.021 | |
| L pos corona radiata | r = −0.28 p = 0.007 | |
| L pos thalamic/optic radiation | r = −0.26 p = 0.01 | |
| R pos thalamic/optic radiation | r = −0.30 p = 0.003 | |
| R sup long fasciculus | r = −0.25 p = 0.01 | |
| R tapetum | r = −0.34 p = 0.001 | |
| L tapetum | r = −0.35 p = 0.001 |
EDSS did not correlate with any WM tract.
ROI = region of interest; FA = fractional anisotropy; EDSS = Expanded Disability Status Scale; R = right; L = left; inf = inferior; pos = posterior; sup = superior.
4. Discussion
DTI has proven to be an effective tool for providing a non-invasive in-vivo investigation into WM tissue alterations unseen through conventional MRI (Yu et al., 2012a).
In our current study, we used a region-of-interest approach to investigate the temporal nature of injury to WM tracts in RRMS patients in relation to disease duration. The strengths of our findings are related to the robust methodology combining a high-resolution imaging protocol with both voxelwise and ROI approaches. This approach allowed us to characterize regional tract damage that occurred in large patient sample groups with varying disease duration.
We have confirmed the early occurring WM alterations also through voxelwise analysis, which further established the decrease in FA values between patients with short, moderate and long disease duration periods. The diffuse changes throughout the cerebral WM tracts were significant even in patients with very short disease duration of less than one year as compared with age-matched controls. These findings are in agreement with previous studies that showed decreased in FA in MS patients with short disease duration. Raz et al. compared 34 MS patients within 3 months from the onset of first symptomatology to 16 healthy controls, identifying and demonstrating widespread changes in FA, especially in the corticospinal tracts and the corpus callosum (Raz et al., 2010b). Similarly, we have demonstrated that early FA changes occurred in the corticospinal tracts and in the corpus callosum; however the most significant change with the onset of disease was observed in the left inferior longitudinal fasciculus (ILF) that connects the occipital lobe with the anterior part of the temporal lobe, running laterally and inferiorly to the optic radiation fibers. The ILF is associated with visual content organization and the establishment of its emotional significance and plays an important role in social tasks requiring recognition of face emotion expression (Kleinhans et al., 2008). Sensory deficits are typical of MS at onset and we have previously shown the occurrence of cortical thinning of several sensory-related regions in the early disease stage (Achiron et al., 2013b). As the ILF is known to be associated with visual comprehension and visual memory (Chanraud et al., 2010), our findings suggest that the impairment in its structural integrity may affect the capacity of MS patients to appropriately and in-time comprehend visual cues, even if no significant neurological disability is demonstrated. Disruption of the ILF was already reported to occur in MS patients with average disease duration of 3.6 years (Roosendaal et al., 2009). Our results show that not only are these changes the most significant among the WM alterations, but that they also occur earlier than previously reported.
Further comparison of RRMS patients with short disease duration (up to 1 year) with the moderate disease duration (between 1 and 6 years) showed no significant differences between WM skeletons on both whole-brain skeletal FA measurements and in-depth TFCE voxelwise analyses. The finding that short and moderate disease duration groups did not show any FA differences is of interest. This suggests that WM disruption reaches a plateau during this 5 year interval. Similarly, changes in FA were not detected over a period of a 1 year follow-up in clinically-isolated MS patients (Raz et al., 2010b), and no significant FA changes were identified over time in RRMS patients by repeated MRI scans within 5 years from onset (Giorgio et al., 2010). This may be related to the concept of “brain reserve,” whereby the disease-induced insult is not significant enough to result in structural changes within the WM tracts, reflecting the capacity to withstand deteriorating processes and the ability to endure or recover quickly from the insult. The idea of this reserve against brain damage stems from repeated observations showing no apparent relationship between the degree of brain pathology or damage and the clinical manifestations of that damage (Stern, 2002). In both the early and moderate disease duration groups, patients bear greater capacity to withstand acquired white matter insults. Related to this observation, a recent study reported that both patients with radiologically-isolated syndrome (RIS) and relapsing–remitting MS had altered integrity of WM tracts as compared to normal matched controls. However, in measuring WM tract integrity, RIS patients lacked functional reorganization in key brain networks, suggesting a model of “functional reserve” which may become upregulated, with an adaptive or maladaptive role, only at a later stage. (Giorgio et al., 2015)
In contrast, the comparison between the moderate (1–6 years) and the long disease duration (6–10 years) groups showed diffuse and significant changes involving almost all major WM tracts, with FA reduction of 22% affecting 42,212 of 192,208 skeletal voxels, with the point of maximal significant difference migrating from the ILF to the body of the corpus callosum which is widely known to be involved in MS. Accordingly, FA reductions appear to exhibit a non-linear time course with two “accelerations”. An acute, diffuse WM change within the first year following the initial clinical event, and another more gradual decline occurred as the disease approaches the 10-year mark. We hypothesize that in the early stage, the sharp decline in FA relates to diffuse alterations in the normal-appearing WM, whereas the more gradual decrease over a longer disease duration relates to subsequent Wallerian degeneration and axonal disruption. This progression signifies a point-of-no-return, whereby the accumulating WM damage spreads exponentially and therefore makes the first 6 years an important time-frame for immunomodulatory drug treatment. This possibility of a therapeutic window stands for the period of time following MS onset whereby early treatment may favorably affect long-term disease outcome, and has been also suggested by the results of our previous study where we demonstrated that cognitive impairment in MS patients was significant only at disease duration greater than 5 years (Achiron et al., 2013a).
Other studies also reported DTI changes in RRMS patients within this time-frame. Roosendaal et al. (2009), studying regional DTI differences in 30 RRMS patients with a mean disease duration of 3.6 years as compared with 31 age-matched controls, demonstrated FA decrease in various brain regions including the fornices, the left corona radiata, the inferior longitudinal fasciculus in both hemispheres, both optic radiations, and parts of the corpus callosum; Giorgio et al. (2010) used TBSS cross-sectional analysis in 45 RRMS patients with 3 years of disease duration and demonstrated decreased FA in the splenium of the corpus callosum and along the pyramidal tract; Kern et al. (2011) evaluated 25 MS patients with a mean disease duration of 4.8 years in comparison to 16 controls and demonstrated a decrease in FA in the transcallosal hand motor fibers and corticospinal tracts.
The therapeutic window opportunity is further intensified by the fact that the WM damage accumulates microscopically but is not apparent clinically, as we did not find correlation with neurological disability. This is in accordance with previous study that also did not find correlations between mean FA values and EDSS (Raz et al., 2010a).
Some limitations apply to this work. First, the cross-sectional nature of the study limited our ability to monitor the evolution of WM distraction on an individual basis. A longitudinal study will enable us to verify the results by following the same patients overtime. Second, we had relatively small sample size particularly for the long (6–10 years) disease duration group, and third we were not able to synchronize the MRI data to different IMDs' treatment history and current treatment regimens and thus we did not analyze potential medication effects on WM pathology. This was related to the diversity in the type of treatment (e.g., different IMDs), difference in treatment duration (e.g., different time-frames for each IMD) and treatment sequence order (e.g., difference in the choice of the first, second and third lines of IMD).
It is of importance to mention that we have focused on global aspects of the microstructural WM damage in patients with varying periods of disease duration future studies will need to focus on more homogenous groups of patients to identify local properties of WM damage over time as well as to examine other diffusion metrics such as axial and radial diffusivities that may add additional useful information about the processes occurring in MS.
In conclusion, utilizing DTI with TBSS analyses, we have demonstrated the occurrence of widespread injury to WM tracts in RRMS patients. We have subsequently shown that this structural damage appears in the very early stage of the disease with less than a year from onset. These alterations reach a plateau within the next 5 years, and only later as the pathological process accumulates, additional WM changes are detected. An important therapeutic window therefore exists within the early disease stage.
The following are the supplementary data related to this article
Whole brain comparison between healthy subjects and MS patients with short disease duration (<1 year). Significant clusters of reduced FA (p < 0.05) are shown in red and orange overlaid on a template image brain with a skeletonized WM tract (green) mask.
Whole brain comparison between healthy subjects and MS patients with long disease duration (>6 years). Significant clusters of reduced FA (p < 0.05) are shown in red and orange overlaid on a template image brain with a skeletonized WM tract (green) mask.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.04.020.
Conflicts of interest
There are no conflicts of interest, financial or otherwise, related to the submitted work.
References
- Achiron A., Chapman J., Magalashvili D., Dolev M., Lavie M., Bercovich E., Polliack M., Doniger G.M., Stern Y., Khilkevich O., Menascu S., Hararai G., Gurevich M., Barak Y. Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLOS One. 2013;8(8):e71058. doi: 10.1371/journal.pone.0071058. 23936485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Chapman J., Tal S., Bercovich E., Gil H., Achiron A. Superior temporal gyrus thickness correlates with cognitive performance in multiple sclerosis. Brain Struct. Funct. 2013;218(4):943–950. doi: 10.1007/s00429-012-0440-3. 22790785 [DOI] [PubMed] [Google Scholar]
- Bodini B., Khaleeli Z., Cercignani M., Miller D.H., Thompson A.J., Ciccarelli O. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum. Brain Mapp. 2009;30(9):2852–2861. doi: 10.1002/hbm.20713. 19172648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S., Zahr N., Sullivan E.V., Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol. Rev. 2010;20(2):209–225. doi: 10.1007/s11065-010-9129-7. 20422451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commowick O., Fillard P., Clatz O., Warfield S.K. Detection of DTI white matter abnormalities in multiple sclerosis patients. Med Image Comput. Comput. Assist. Interv. 2008;11(1):975–982. doi: 10.1007/978-3-540-85988-8_116. 18979840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(9):2375–2386. doi: 10.1093/brain/awm184. 17698497 [DOI] [PubMed] [Google Scholar]
- Giorgio A., Palace J., Johansen-Berg H., Smith S.M., Ropele S., Fuchs S., Wallner-Blazek M., Enzinger C., Fazekas F. Relationships of brain white matter microstructure with clinical and MR measures in relapsing–remitting multiple sclerosis. J. Magn. Reson. Imaging. 2010;31(2):309–316. doi: 10.1002/jmri.22062. 20099343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Stromillo M.L., De Leucio A., Rossi F., Brandes I., Hakiki B., Portaccio E., Amato M.P., De Stefano N. Appraisal of brain connectivity in radiologically isolated syndrome by modeling imaging measures. J. Neurosci. 2015;35(2):550–558. doi: 10.1523/JNEUROSCI.2557-14.2015. 25589750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keihaninejad S., Ryan N.S., Malone I.B., Modat M., Cash D., Ridgway G.R., Zhang H., Fox N.C., Ourselin S. The importance of group-wise registration in tract based spatial statistics study of neurodegeneration: a simulation study in Alzheimer's disease. PLOS One. 2012;7(11):e45996. doi: 10.1371/journal.pone.0045996. 23139736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern K.C., Sarcona J., Montag M., Giesser B.S., Sicotte N.L. Corpus callosal diffusivity predicts motor impairment in relapsing–remitting multiple sclerosis: a TBSS and tractography study. Neuroimage. 2011;55(3):1169–1177. doi: 10.1016/j.neuroimage.2010.10.077. 21056674 [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. 18234695 [DOI] [PubMed] [Google Scholar]
- Liu Y., Duan Y., He Y., Yu C., Wang J., Huang J., Ye J., Parizel P.M., Li K., Shu N. Whole brain white matter changes revealed by multiple diffusion metrics in multiple sclerosis: a TBSS study. Eur. J. Radiol. 2012;81(10):2826–2832. doi: 10.1016/j.ejrad.2011.11.022. 22172535 [DOI] [PubMed] [Google Scholar]
- Mori S., Oishi K., Faria A.V. White matter atlases based on diffusion tensor imaging. Curr. Opin. Neurol. 2009;22(4):362–369. doi: 10.1097/WCO.0b013e32832d954b. 19571751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Zilles K., Amunts K., Faria A., Jiang H., Li X., Akhter K., Hua K., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P.C., Mazziotta J., Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43(3):447–457. doi: 10.1016/j.neuroimage.2008.07.009. 18692144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O'Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. 21387374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E., Cercignani M., Sbardella E., Totaro P., Pozzilli C., Bozzali M., Pantano P. Gray- and white-matter changes 1 year after first clinical episode of multiple sclerosis: MR imaging. Radiology. 2010;257(2):448–454. doi: 10.1148/radiol.10100626. 20858849 [DOI] [PubMed] [Google Scholar]
- Raz E., Cercignani M., Sbardella E., Totaro P., Pozzilli C., Bozzali M., Pantano P. Clinically isolated syndrome suggestive of multiple sclerosis: voxelwise regional investigation of white and gray matter. Radiology. 2010;254(1):227–234. doi: 10.1148/radiol.2541090817. 20019140 [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Geurts J.J., Vrenken H., Hulst H.E., Cover K.S., Castelijns J.A., Pouwels P.J., Barkhof F. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44(4):1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. 19027076 [DOI] [PubMed] [Google Scholar]
- Sämann P.G., Knop M., Golgor E., Messler S., Czisch M., Weber F. Brain volume and diffusion markers as predictors of disability and short-term disease evolution in multiple sclerosis. A.J.N.R. Am. J. Neuroradiol. 2012;33(7):1356–1362. doi: 10.3174/ajnr.A2972. 22383242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K., Wheeler-Kingshott C.A., Boulby P.A., Scaravilli F., Altmann D.R., Barker G.J., Tofts P.S., Miller D.H. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35(2):467–477. doi: 10.1016/j.neuroimage.2006.12.010. 17258908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. 16624579 [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. 15501092 [DOI] [PubMed] [Google Scholar]
- Smith S.M., Johansen-Berg H., Jenkinson M., Rueckert D., Nichols T.E., Miller K.L., Robson M.D., Jones D.K., Klein J.C., Bartsch A.J., Behrens T.E. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat. Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. 17406613 [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8(3):448–460. 11939702 [PubMed] [Google Scholar]
- Yu H.J., Christodoulou C., Bhise V., Greenblatt D., Patel Y., Serafin D., Maletic-Savatic M., Krupp L.B., Wagshul M.E. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59(4):3713–3722. doi: 10.1016/j.neuroimage.2011.10.053. 22062194 [DOI] [PubMed] [Google Scholar]
- Yu H.J., Christodoulou C., Bhise V., Greenblatt D., Patel Y., Serafin D., Maletic-Savatic M., Krupp L.B., Wagshul M.E. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59(4):3713–3722. doi: 10.1016/j.neuroimage.2011.10.053. 22062194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole brain comparison between healthy subjects and MS patients with short disease duration (<1 year). Significant clusters of reduced FA (p < 0.05) are shown in red and orange overlaid on a template image brain with a skeletonized WM tract (green) mask.
Whole brain comparison between healthy subjects and MS patients with long disease duration (>6 years). Significant clusters of reduced FA (p < 0.05) are shown in red and orange overlaid on a template image brain with a skeletonized WM tract (green) mask.


